Abstract
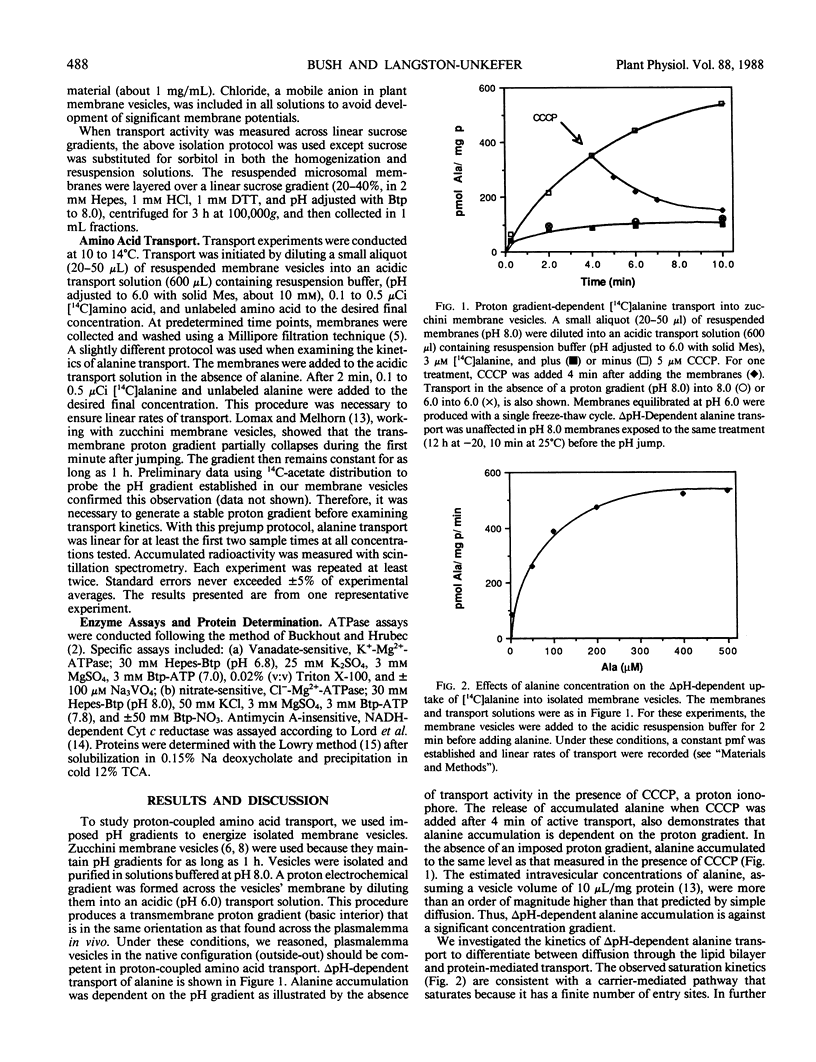
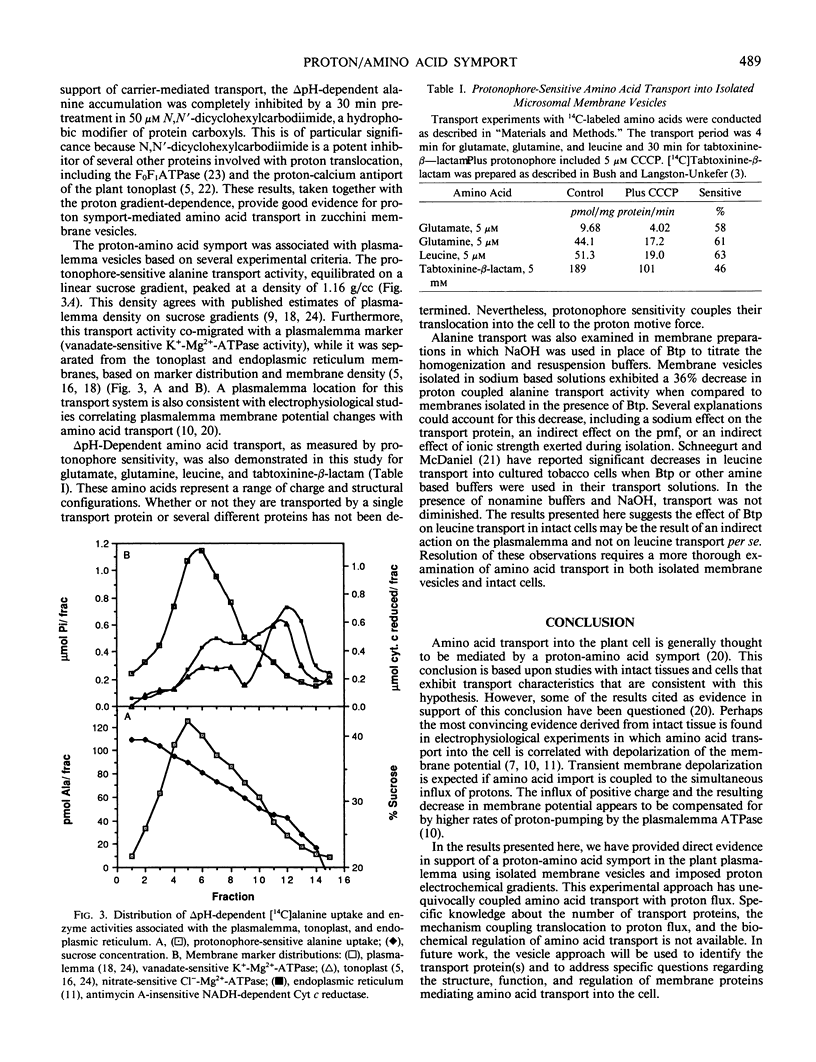
Several lines of evidence with intact tissues suggest amino acid transport is mediated by a proton-amino acid symport (L Rheinhold, A Kaplan 1984 Annu Rev Plant Physiol 35: 45-83). However, biochemical studies of proton-coupled amino acid transport in isolated membrane vesicles have not been reported. In the experiments presented here, amino acid transport was studied in membrane vesicles isolated from zucchini (Cucurbita pepo L. cv Black Beauty) hypocotyls. An imposed pH gradient (basic interior) was used to energize isolated membrane vesicles and drive amino acid transport. Proton-coupled amino acid accumulation was demonstrated for alanine, glutamate, glutamine, leucine, and tabtoxinine-β-lactam. Alanine transport into the isolated membrane vesicles was studied in detail. Alanine transport was protonophore sensitive and accumulation ratios exceeding 10 times that predicted by diffusion alone were observed. ΔpH-Dependent alanine transport exhibited saturation kinetics, suggesting translocation was mediated via a carrier transport system. In support of that conclusion, 50 micromolar N,N′-dicyclohexylcarbodiimide, a hydrophobic modifier of protein carboxyls, completely inhibited proton-coupled alanine accumulation. Transport activity, equilibrated on a linear sucrose gradient, peaked at 1.16 grams per cubic centimeter and co-migrated with a plasmalemma marker (vanadate-sensitive K+-Mg2+-ATPase). These results provide direct evidence in support of a proton-amino acid symport in the plasmalemma of higher plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush D. R., Langston-Unkefer P. J. Tabtoxinine-beta-Lactam Transport into Cultured Corn Cells : Uptake via an Amino Acid Transport System. Plant Physiol. 1987 Nov;85(3):845–849. doi: 10.1104/pp.85.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. R., Sze H. Calcium transport in tonoplast and endoplasmic reticulum vesicles isolated from cultured carrot cells. Plant Physiol. 1986 Feb;80(2):549–555. doi: 10.1104/pp.80.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Evidence for amino Acid-h co-transport in oat coleoptiles. Plant Physiol. 1978 Jun;61(6):933–937. doi: 10.1104/pp.61.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langston-Unkefer P. J., Robinson A. C., Knight T. J., Durbin R. D. Inactivation of pea seed glutamine synthetase by the toxin, tabtoxinine-beta-lactam. J Biol Chem. 1987 Feb 5;262(4):1608–1613. [PubMed] [Google Scholar]
- Lomax T. L., Mehlhorn R. J. Determination of osmotic volumes and pH gradients of plant membrane and lipid vesicles using ESR spectroscopy. Biochim Biophys Acta. 1985 Nov 21;821(1):106–114. doi: 10.1016/0005-2736(85)90160-9. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Proton transport in isolated vacuoles from corn coleoptiles. Plant Physiol. 1985 May;78(1):104–109. doi: 10.1104/pp.78.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Schneegurt M. A., McDaniel C. N. Amino Acid Transport in Suspension-Cultured Plant Cells : VI. Influence of pH Buffers, Calcium, and Preincubation Media on l-Leucine Uptake. Plant Physiol. 1986 May;81(1):36–40. doi: 10.1104/pp.81.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986 Sep 15;261(26):12172–12178. [PubMed] [Google Scholar]


