Abstract
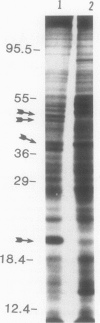
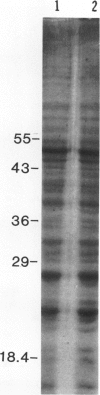
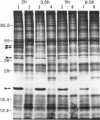
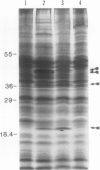
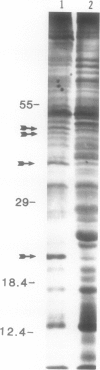
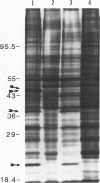
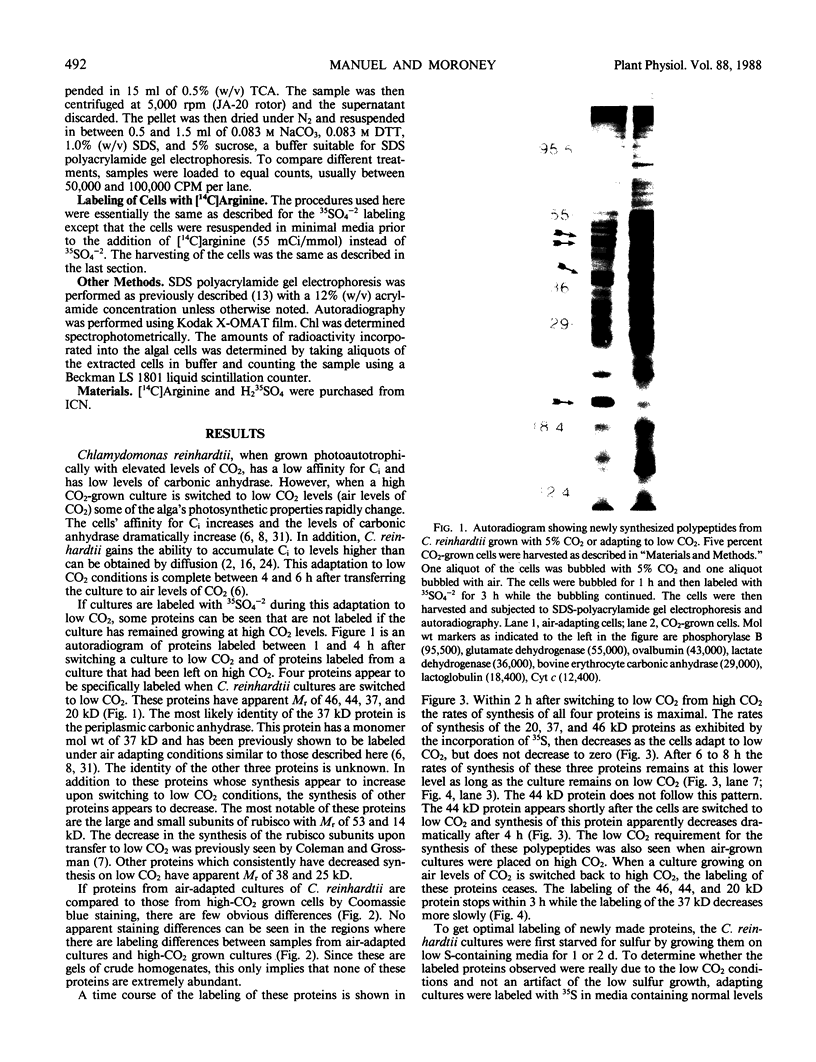
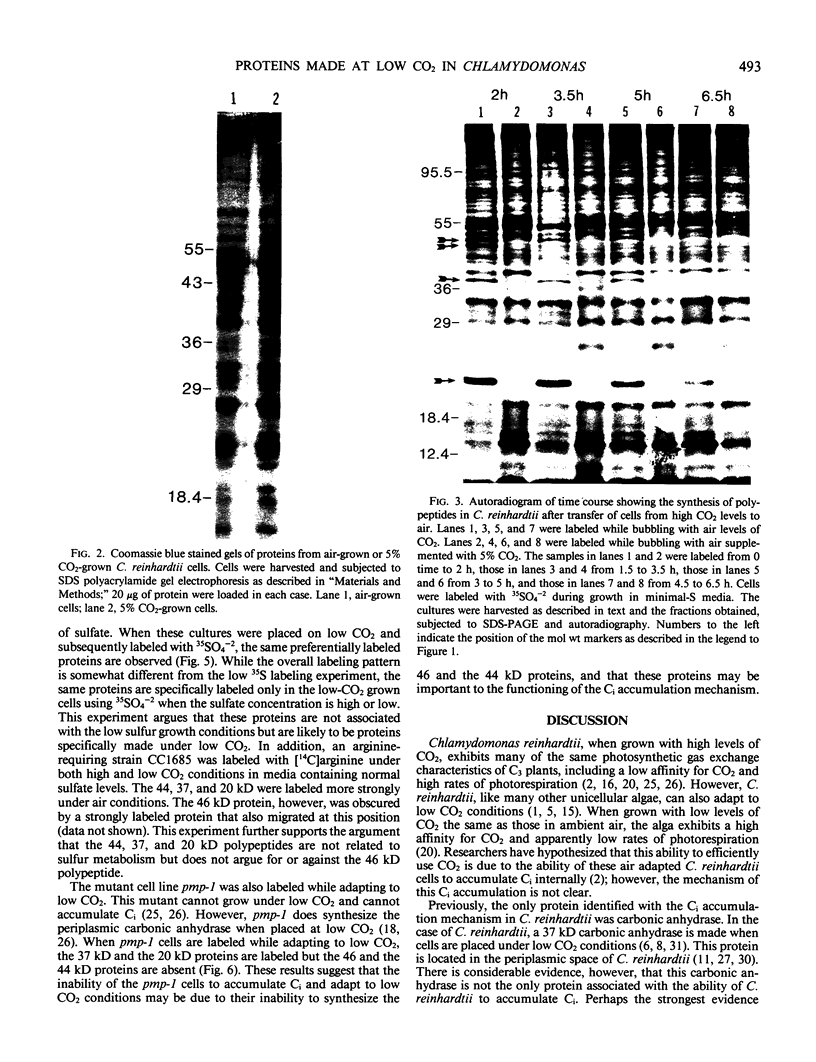
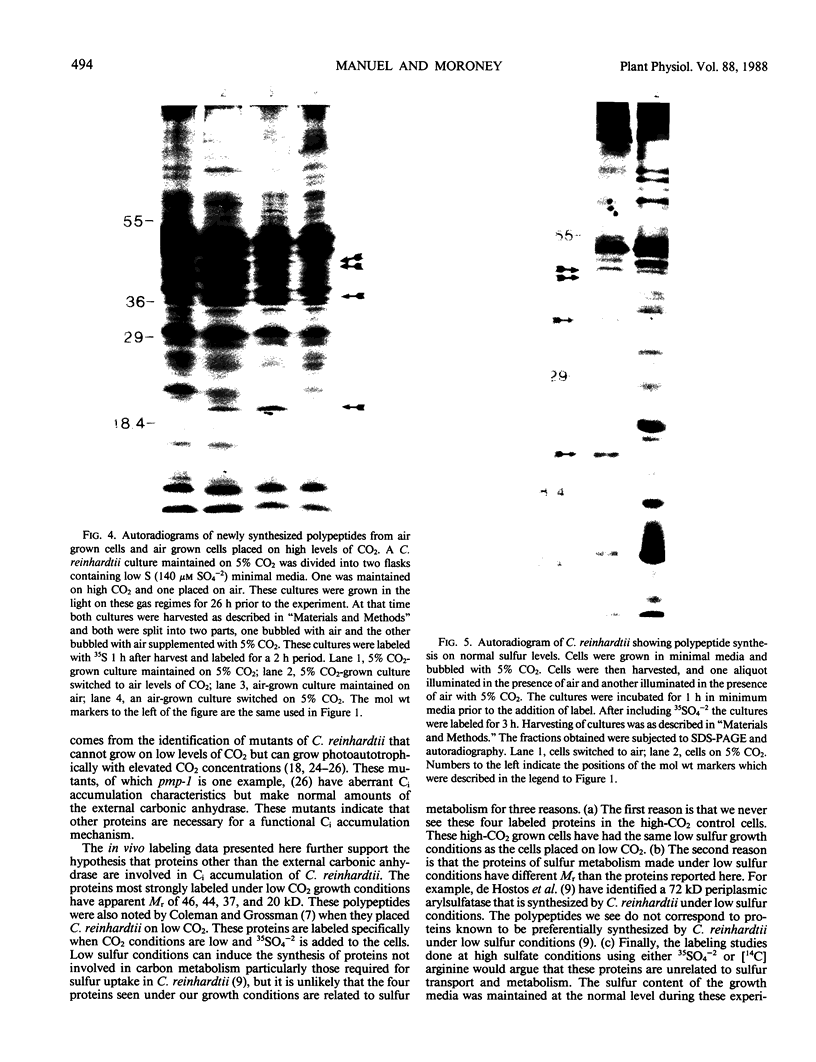
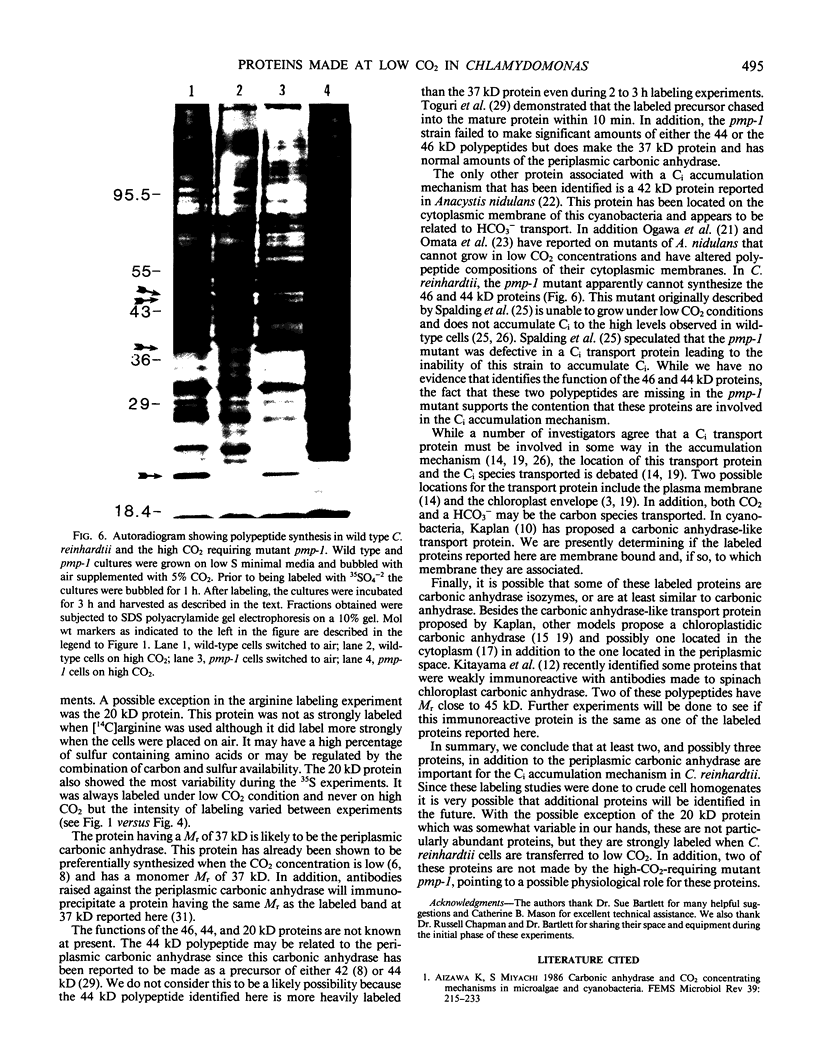
When the unicellular green alga Chlamydomonas reinhardtii is placed under low CO2 conditions it adapts by making an inorganic carbon accumulating mechanism. Algal cells were labeled with 35SO4−2 during this adaptation period and labeled proteins specific for this low CO2 adaptation were identified. Four major proteins were preferentially synthesized under low CO2 conditions and had Mr of 46, 44, 37, and 20 kilodaltons. The 37 kilodalton protein is most likely the periplasmic carbonic anhydrase previously identified as being part of the inorganic carbon accumulation mechanism of C. reinhardtii. The other three proteins have not been identified. The 46 and the 44 kilodalton proteins were not synthesized by a mutant algal strain, pmp-1, which cannot grow at low CO2 concentrations. This strain does make the 37 and 20 kilodalton proteins, however. These data suggest that at least two or three proteins in addition to the periplasmic carbonic anhydrase are part of the inorganic carbon accumulation mechanism in C. reinhardtii.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba S., Mishima H., Miyachi Y. Levels of cyclic-AMP, cyclic-GMP and betamethasone in the aqueous humor following topical administration of betamethasone in rabbit eyes. Hiroshima J Med Sci. 1983 Sep;32(3):301–304. [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Berry J. A., Togasaki R. K., Grossman A. R. Identification of Extracellular Carbonic Anhydrase of Chlamydomonas reinhardtii. Plant Physiol. 1984 Oct;76(2):472–477. doi: 10.1104/pp.76.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Grossman A. R. Biosynthesis of carbonic anhydrase in Chlamydomonas reinhardtii during adaptation to low CO(2). Proc Natl Acad Sci U S A. 1984 Oct;81(19):6049–6053. doi: 10.1073/pnas.81.19.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moroney J. V., Husic H. D., Tolbert N. E. Effect of Carbonic Anhydrase Inhibitors on Inorganic Carbon Accumulation by Chlamydomonas reinhardtii. Plant Physiol. 1985 Sep;79(1):177–183. doi: 10.1104/pp.79.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Kitayama M., Togasaki R. K., Tolbert N. E. Evidence for Inorganic Carbon Transport by Intact Chloroplasts of Chlamydomonas reinhardtii. Plant Physiol. 1987 Mar;83(3):460–463. doi: 10.1104/pp.83.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Tolbert N. E. Inorganic Carbon Uptake by Chlamydomonas reinhardtii. Plant Physiol. 1985 Feb;77(2):253–258. doi: 10.1104/pp.77.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Wilson B. J., Tolbert N. E. Glycolate Metabolism and Excretion by Chlamydomonas reinhardtii. Plant Physiol. 1986 Nov;82(3):821–826. doi: 10.1104/pp.82.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaneda T., Omata T. A Mutant of Synechococcus PCC7942 Incapable of Adapting to Low CO(2) Concentration. Plant Physiol. 1987 Jul;84(3):711–715. doi: 10.1104/pp.84.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Ogawa T. Biosynthesis of a 42-kD Polypeptide in the Cytoplasmic Membrane of the Cyanobacterium Anacystis nidulans Strain R2 during Adaptation to Low CO(2) Concentration. Plant Physiol. 1986 Feb;80(2):525–530. doi: 10.1104/pp.80.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Ogawa T., Marcus Y., Friedberg D., Kaplan A. Adaptation to Low CO(2) Level in a Mutant of Anacystis nidulans R(2) which Requires High CO(2) for Growth. Plant Physiol. 1987 Apr;83(4):892–894. doi: 10.1104/pp.83.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Carbonic Anhydrase-Deficient Mutant of Chlamydomonas reinhardii Requires Elevated Carbon Dioxide Concentration for Photoautotrophic Growth. Plant Physiol. 1983 Oct;73(2):268–272. doi: 10.1104/pp.73.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Reduced Inorganic Carbon Transport in a CO(2)-Requiring Mutant of Chlamydomonas reinhardii. Plant Physiol. 1983 Oct;73(2):273–276. doi: 10.1104/pp.73.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toguri T., Muto S., Miyachi S. Biosynthesis and intracellular processing of carbonic anhydrase in Chlamydomonas reinhardtii. Eur J Biochem. 1986 Aug 1;158(3):443–450. doi: 10.1111/j.1432-1033.1986.tb09773.x. [DOI] [PubMed] [Google Scholar]
- de Hostos E. L., Togasaki R. K., Grossman A. Purification and biosynthesis of a derepressible periplasmic arylsulfatase from Chlamydomonas reinhardtii. J Cell Biol. 1988 Jan;106(1):29–37. doi: 10.1083/jcb.106.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]