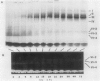
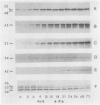
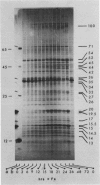
Abstract
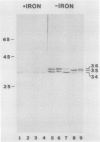
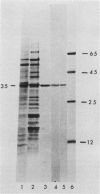
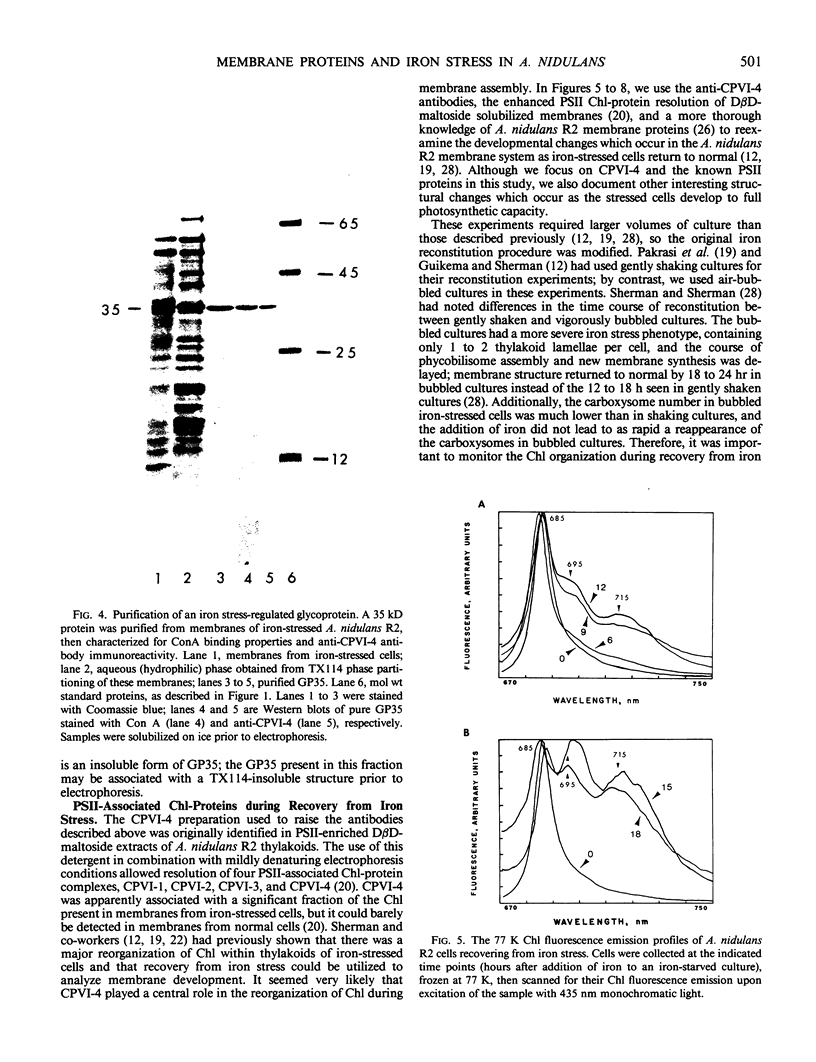
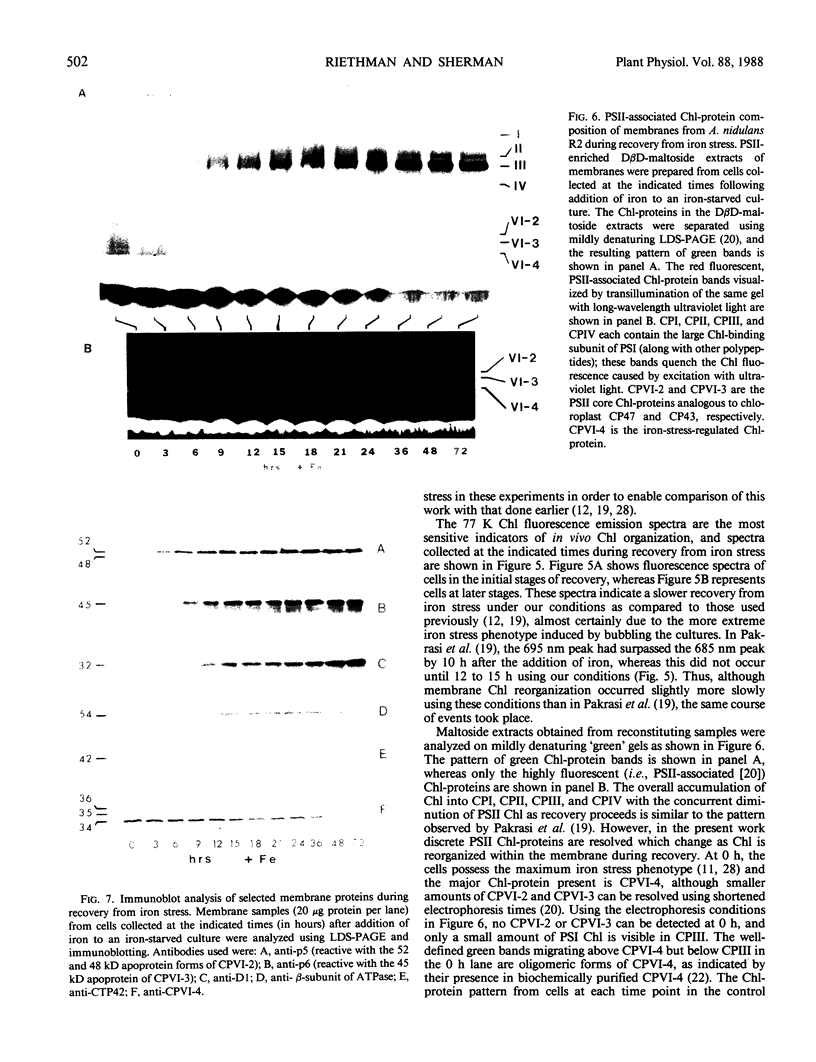
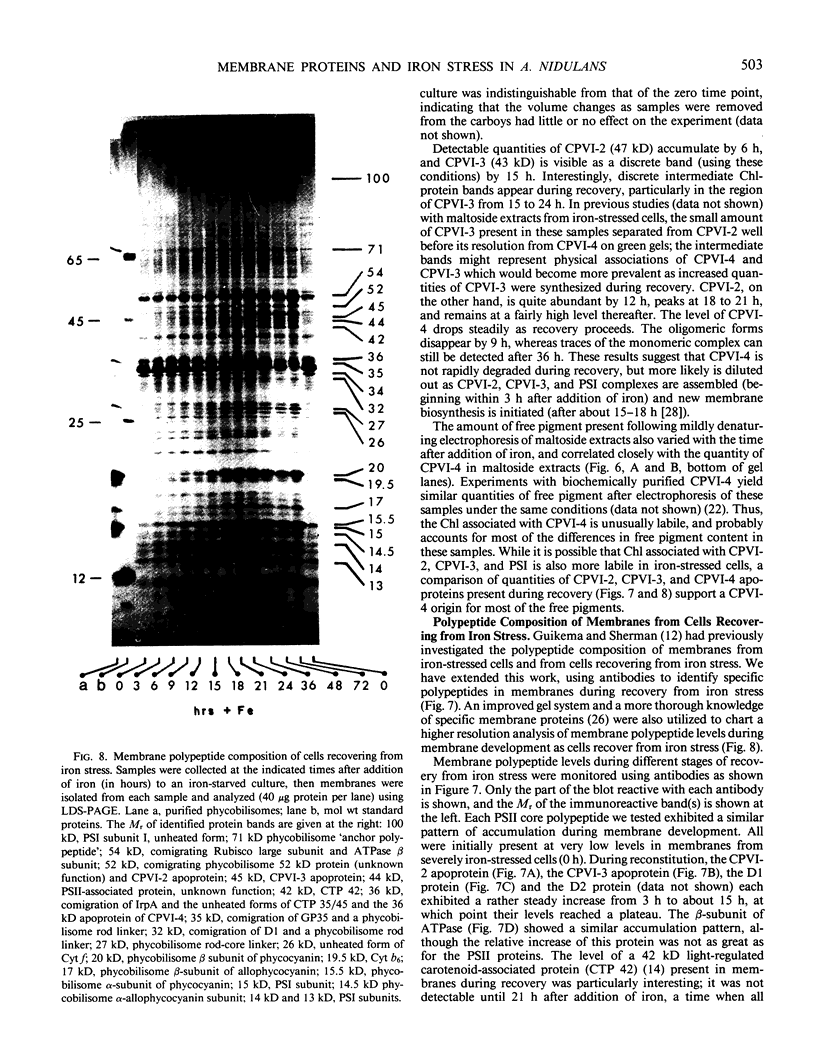
Antibodies cross-reactive with specific membrane proteins were used to investigate membrane development in Anacystis nidulans R2 during recovery from iron stress. Polyclonal antibodies prepared using the iron-regulated chlorophyll (Chl)-protein CPVI-4 (HB Pakrasi, HC Riethman, LA Sherman 1985 Proc Natl Acad Sci USA 82: 6903-6907) as antigen were characterized and used to identify three iron stress-induced polypeptides of 36, 35, and 34 kilodaltons on immunoblots of polyacrylamide gels. The 34 kilodalton protein was shown to be a component of the Chlbinding CPVI-4 complex. The 36 kilodalton protein is an unrelated, intrinsic membrane protein tightly regulated by iron (designated IrpA), whereas the 35 kilodalton immunoreactive component is an extremely abundant glycoprotein (GP35). An analysis of photosystem II (PSII)-associated Chl-proteins during recovery from iron stress demonstrates that CPVI-4 is associated with most of the Chl present in iron-starved cells, whereas the PSII core polypeptides are present in very low levels; upon recovery, CPVI-4 diminishes in abundance as the relative levels of the other PSII proteins increase. The abundance of CPVI-4 in iron-stressed cells and the distribution of Chl among individual Chl-proteins during recovery suggest a possible role for CPVI-4 in the direction of membrane assembly during recovery from iron stress.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bullerjahn G. S., Sherman L. A. Identification of a carotenoid-binding protein in the cytoplasmic membrane from the heterotrophic cyanobacterium Synechocystis sp. strain PCC6714. J Bacteriol. 1986 Jul;167(1):396–399. doi: 10.1128/jb.167.1.396-399.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Clegg J. C. Glycoprotein detection in nitrocellulose transfers of electrophoretically separated protein mixtures using concanavalin A and peroxidase: application to arenavirus and flavivirus proteins. Anal Biochem. 1982 Dec;127(2):389–394. doi: 10.1016/0003-2697(82)90192-0. [DOI] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Influence of Iron Deprivation on the Membrane Composition of Anacystis nidulans. Plant Physiol. 1984 Jan;74(1):90–95. doi: 10.1104/pp.74.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Organization and Function of Chlorophyll in Membranes of Cyanobacteria during Iron Starvation. Plant Physiol. 1983 Oct;73(2):250–256. doi: 10.1104/pp.73.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K., Riethman H. C., Sherman L. A. Isolation and Characterization of a Carotenoid-Associated Thylakoid Protein from the Cyanobacterium Anacystis nidulans R2. Plant Physiol. 1987 Jul;84(3):633–639. doi: 10.1104/pp.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S., Selman B. R. Identification of the alpha and beta subunits of the chloroplast coupling factor one in Chlamydomonas reinhardi. Eur J Biochem. 1983 Dec 1;137(1-2):373–376. doi: 10.1111/j.1432-1033.1983.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Goldenberg A., Sherman L. A. Membrane Development in the Cyanobacterium, Anacystis nidulans, during Recovery from Iron Starvation. Plant Physiol. 1985 Sep;79(1):290–295. doi: 10.1104/pp.79.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., Riethman H. C., Sherman L. A. Organization of pigment proteins in the photosystem II complex of the cyanobacterium Anacystis nidulans R2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6903–6907. doi: 10.1073/pnas.82.20.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Mawhinney T. P., Sherman L. A. Phycobilisome-associated glycoproteins in the cyanobacterium Anacystis nidulans R 2. FEBS Lett. 1987 May 11;215(2):209–214. doi: 10.1016/0014-5793(87)80147-3. [DOI] [PubMed] [Google Scholar]
- Sherman D. M., Sherman L. A. Effect of iron deficiency and iron restoration on ultrastructure of Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):393–401. doi: 10.1128/jb.156.1.393-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]