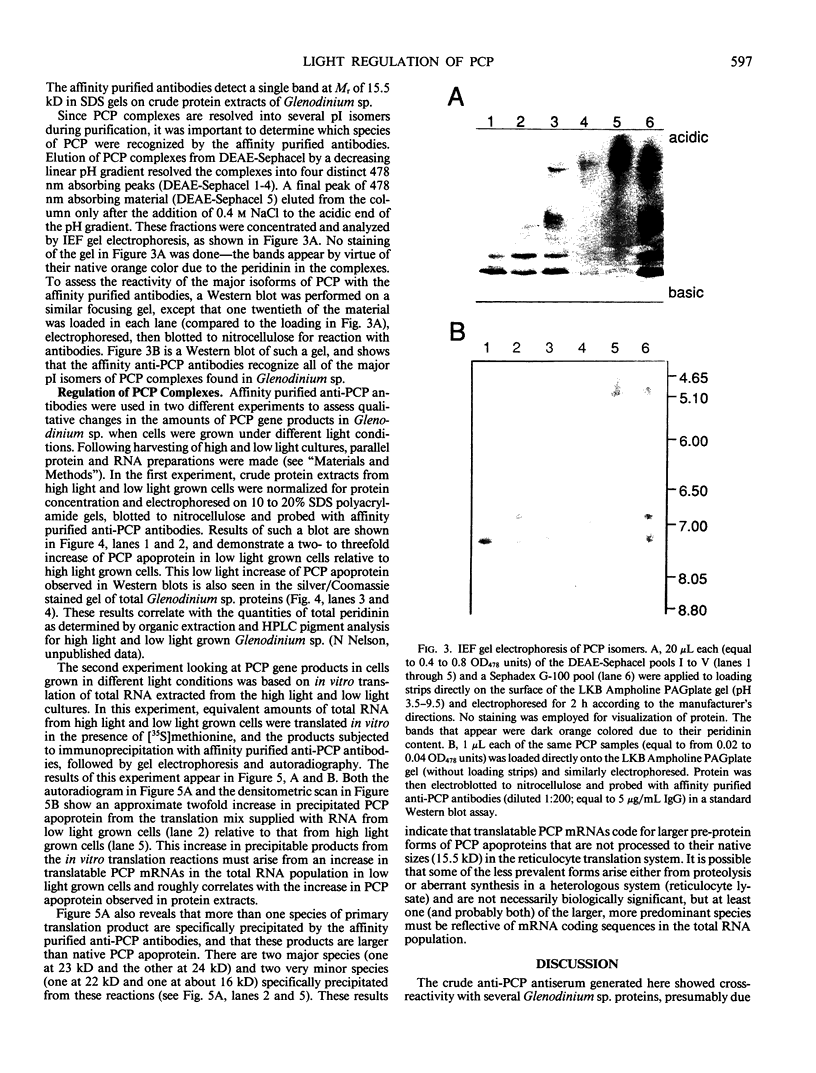
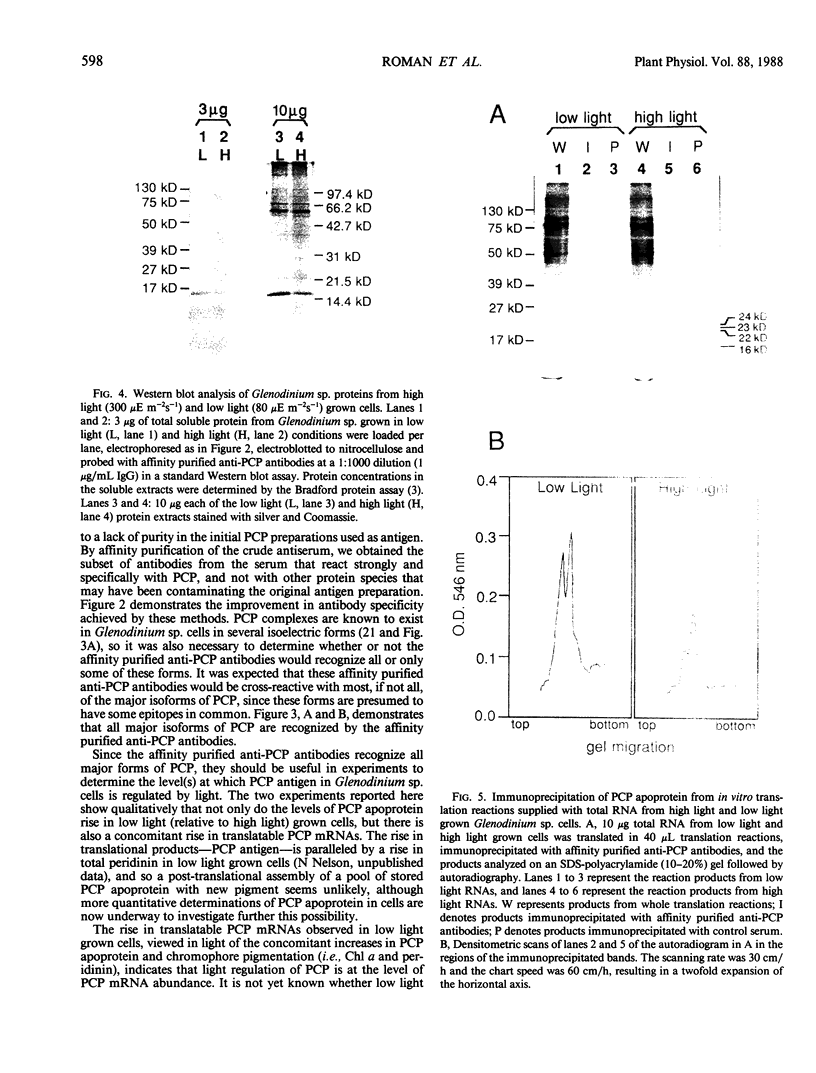
Abstract
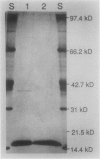
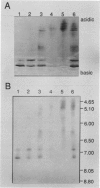
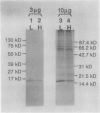
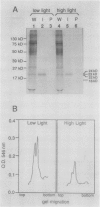
As a step toward developing the tools needed to study the molecular bases of light regulation of gene expression in dinoflagellates, light-harvesting peridinin-chlorophyll a-protein (PCP) complexes from Glenodinium sp. were purified and used to generate anti-PCP antibodies. Affinity purified anti-PCP antibodies were isolated from the crude anti-PCP antiserum resulting in improved specificity of immune reactions. The affinity purified anti-PCP antibodies were shown to react strongly and specifically with all major isoforms of PCP complexes in Glenodinium sp. cells, and were used to assess qualitative changes in the levels of PCP gene products in cells grown under different light conditions. Western blot analysis revealed a two- to three-fold increase in detectable PCP apoprotein in low light compared to high light grown cells. In vitro translation reactions supplied with total RNA from high and low light grown Glenodinium sp. cultures also showed an approximate twofold increase in translatable PCP mRNAs in low light grown cells as determined by immunoprecipitation of the primary translation products with affinity purified anti-PCP antibodies. In addition, PCP apoproteins appear to be encoded as larger pre-proteins, since the major immunoprecipitated products from in vitro translation are 23 and 22 kilodaltons, while mature PCP apoproteins are 15.5 kilodaltons. The parallel increases in PCP apoprotein and translatable PCP mRNAs indicate that light regulation of PCP complexes occurs at the level of PCP mRNA abundance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson S. C., Triplett E. L. The synthesis and activity of tyrosinase during development of the frog Rana pipiens. Dev Biol. 1974 Oct;40(2):270–282. doi: 10.1016/0012-1606(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Lomax T. L., Grossman A. R. Genes encoding major light-harvesting polypeptides are clustered on the genome of the cyanobacterium Fremyella diplosiphon. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3924–3928. doi: 10.1073/pnas.83.11.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P. The petunia chlorophyll a/b binding protein genes: a comparison of Cab genes from different gene families. Nucleic Acids Res. 1985 Apr 11;13(7):2503–2518. doi: 10.1093/nar/13.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J. Photoregulation of plant gene expression. Biosci Rep. 1986 Feb;6(2):127–136. doi: 10.1007/BF01114998. [DOI] [PubMed] [Google Scholar]
- Fluhr Robert, Moses Phyllis, Morelli Giorgio, Coruzzi Gloria, Chua Nam-Hai. Expression dynamics of the pea rbcS multigene family and organ distribution of the transcripts. EMBO J. 1986 Sep;5(9):2063–2071. doi: 10.1002/j.1460-2075.1986.tb04467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxo F. T., Kycia J. H., Somers G. F., Bennett A., Siegelman H. W. Peridinin-Chlorophyll a Proteins of the Dinoflagellate Amphidinium carterae (Plymouth 450). Plant Physiol. 1976 Feb;57(2):297–303. doi: 10.1104/pp.57.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Kano-Murakami Y., Tanaka Y., Ozeki Y., Yamamoto N. Nucleotide sequence of cDNA encoding the small subunit of ribulose-1,5-bisphosphate carboxylase from maize. J Biochem. 1987 Oct;102(4):673–676. doi: 10.1093/oxfordjournals.jbchem.a122103. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]