Abstract
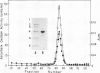
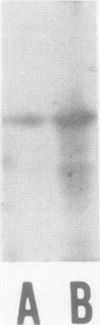
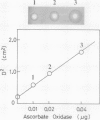
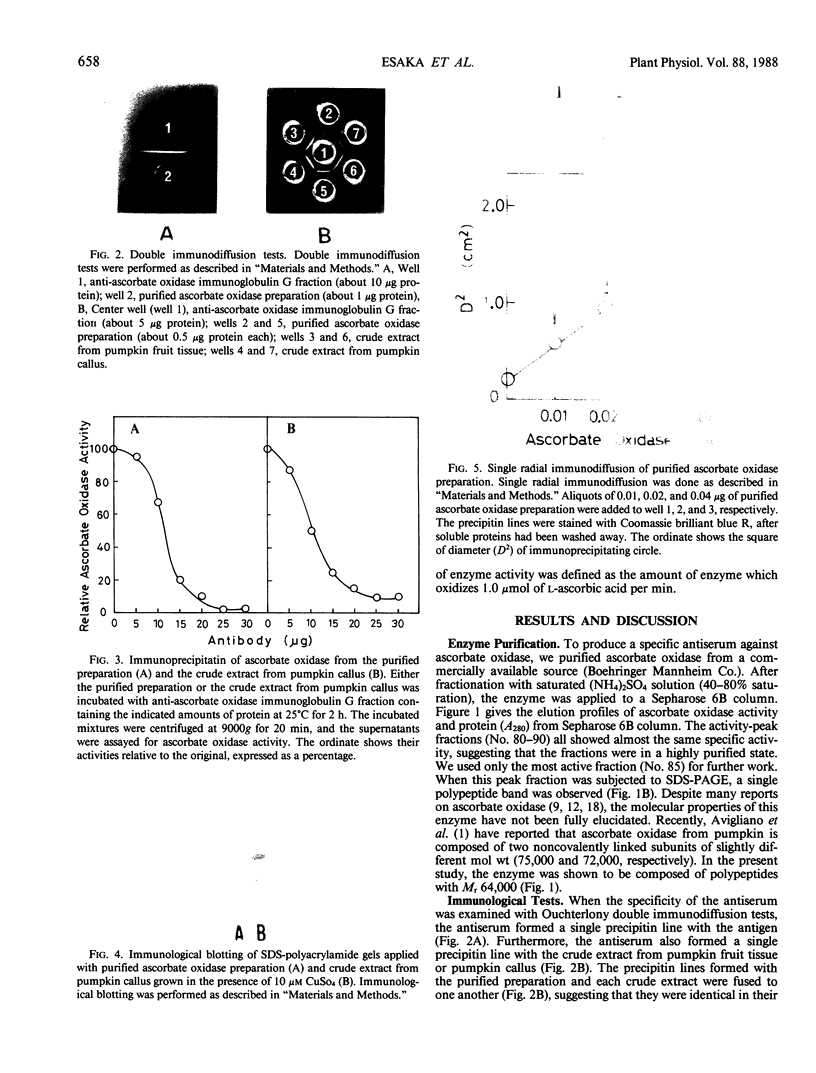
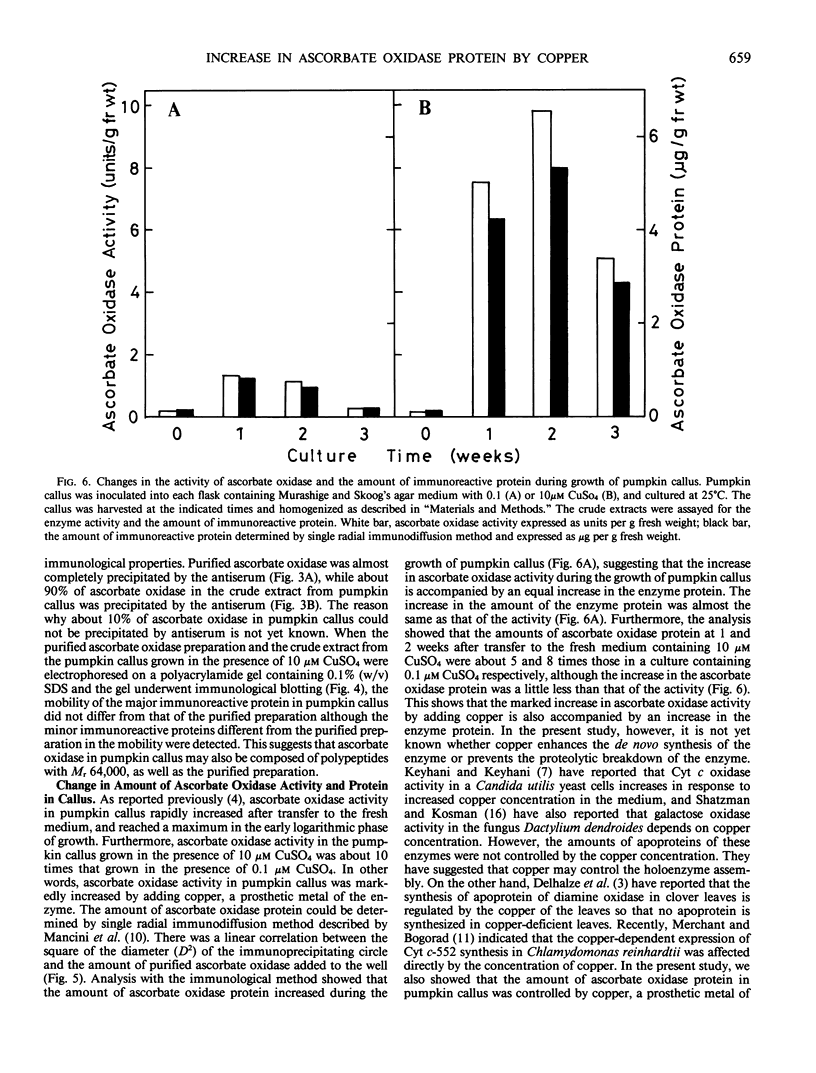
Ascorbate oxidase from pumpkin (Cucurbita sp.) was purified from a commercially available preparation. A single polypeptide band with Mr 64,000 was detected after sodium dodecylsulfate-polyacrylamide gel electrophoresis of the purified enzyme. In double immunodiffusion tests, antiserum against the purified preparation formed a single precipitin line with the crude extract from pumpkin fruit tissue or the callus as well as with the purified preparation. Immunological blotting method showed that mol wt of ascorbate oxidase subunit in pumpkin callus was the same as that of the purified preparation. Analysis with the single radial immunodiffusion method showed that the increase in ascorbate oxidase activity during the growth of pumpkin callus correlated with an increase in the enzyme protein. Furthermore, enzyme protein in the callus grown in the presence of 10 micromolar CuSO4 for 2 weeks was about eight times that grown in the presence of 0.1 micromolar CuSO4. The synthesis of ascorbate oxidase in pumpkin callus may be induced by copper, a prosthetic metal of the enzyme, or copper may help stabilize the enzyme against proteolytic breakdown.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigliano L., Vecchini P., Sirianni P., Marcozzi G., Marchesini A., Mondovi B. A reinvestigation on the quaternary structure of ascorbate oxidase from Cucurbita pepo medullosa. Mol Cell Biochem. 1983;56(2):107–112. doi: 10.1007/BF00227210. [DOI] [PubMed] [Google Scholar]
- Deikman J., Jones R. L. Control of alpha-amylase mRNA accumulation by gibberellic Acid and calcium in barley aleurone layers. Plant Physiol. 1985 May;78(1):192–198. doi: 10.1104/pp.78.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E., Dilworth M. J., Webb J. The effects of copper nutrition and developmental state on the biosynthesis of diamine oxidase in clover leaves. Plant Physiol. 1986 Dec;82(4):1126–1131. doi: 10.1104/pp.82.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Carbonell J. Regulation of the synthesis of barley aleurone alpha-amylase by gibberellic Acid and calcium ions. Plant Physiol. 1984 Sep;76(1):213–218. doi: 10.1104/pp.76.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhani E., Keyhani J. Cytochrome c oxidase biosynthesis and assembly in Candida utilis yeast cells. Function of copper in the assembly of active cytochrome c oxidase. Arch Biochem Biophys. 1975 Apr;167(2):596–602. doi: 10.1016/0003-9861(75)90503-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Dawson C. R. Ascorbate oxidase. Further studies on the purification of the enzyme. J Biol Chem. 1973 Oct 10;248(19):6596–6602. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Merchant S., Bogorad L. Metal ion regulated gene expression: use of a plastocyanin-less mutant of Chlamydomonas reinhardtii to study the Cu(II)-dependent expression of cytochrome c-552. EMBO J. 1987 Sep;6(9):2531–2535. doi: 10.1002/j.1460-2075.1987.tb02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Makino N., Ogura Y. Purification and properties of ascorbate oxidase from cucumber. J Biochem. 1968 Aug;64(2):189–195. doi: 10.1093/oxfordjournals.jbchem.a128879. [DOI] [PubMed] [Google Scholar]
- OBERBACHER M. F., VINES H. M. Spectrophotometric assay of ascorbic acid oxidase. Nature. 1963 Mar 23;197:1203–1204. doi: 10.1038/1971203a0. [DOI] [PubMed] [Google Scholar]
- Polacco J. C. Nitrogen Metabolism in Soybean Tissue Culture: II. Urea Utilization and Urease Synthesis Require Ni. Plant Physiol. 1977 May;59(5):827–830. doi: 10.1104/pp.59.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARK G. R., DAWSON C. R. On the accessibility of sulfhydryl groups in ascorbic acid oxidase. J Biol Chem. 1962 Mar;237:712–716. [PubMed] [Google Scholar]
- Shatzman A. R., Kosman D. J. The utilization of copper and its role in the biosynthesis of copper-containing proteins in the fungus, Dactylium dendroides. Biochim Biophys Acta. 1978 Nov 15;544(1):163–179. doi: 10.1016/0304-4165(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W. Ascorbic acid and biological systems. Ascorbic acid and electron transport. Ann N Y Acad Sci. 1975 Sep 30;258:190–200. doi: 10.1111/j.1749-6632.1975.tb29279.x. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Interchangeable copper and iron proteins in algal photosynthesis. Studies on plastocyanin and cytochrome c-552 in Chlamydomonas. Eur J Biochem. 1978 Jun 1;87(1):9–19. doi: 10.1111/j.1432-1033.1978.tb12346.x. [DOI] [PubMed] [Google Scholar]