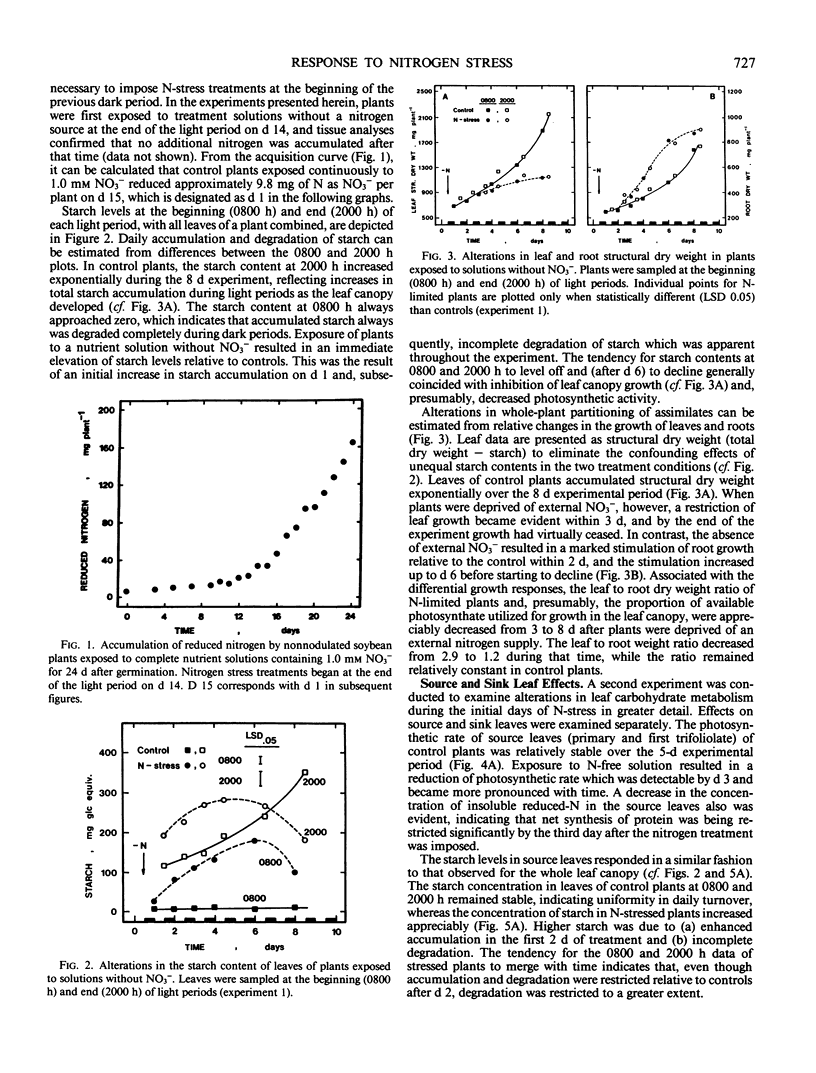
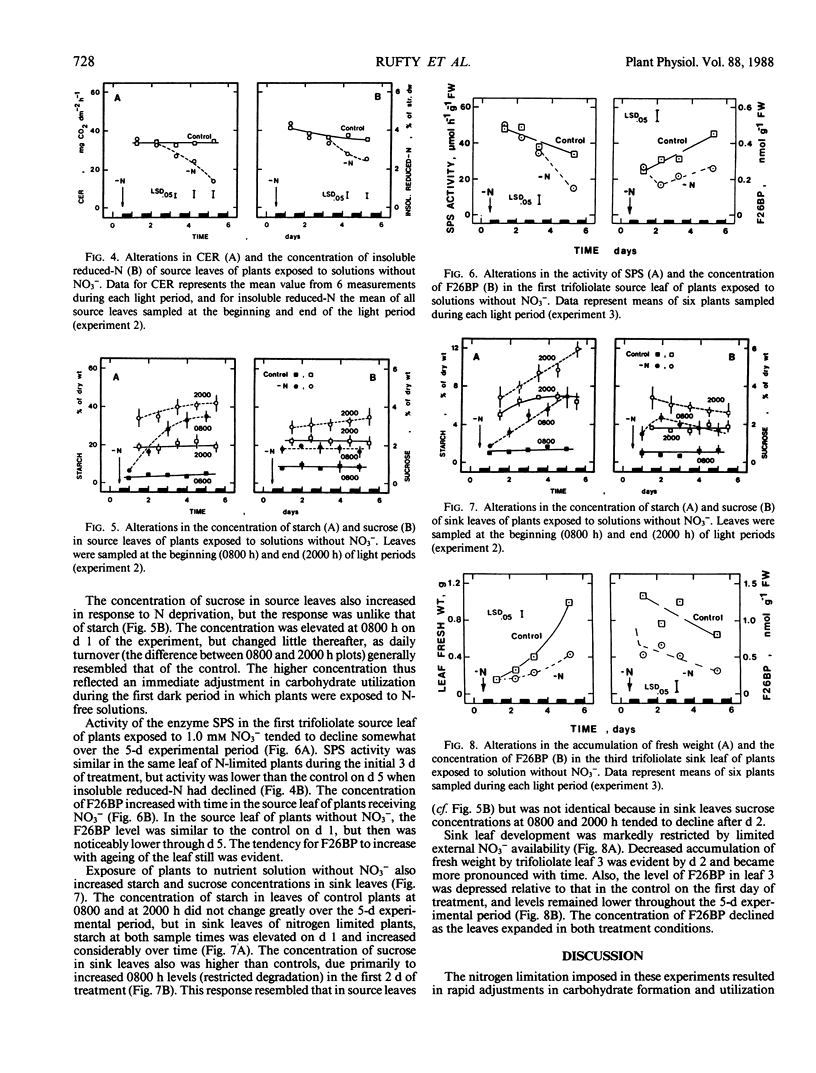
Abstract
A series of experiments was conducted to characterize alterations in carbohydrate utilization in leaves of nitrogen stressed plants. Two-week-old, nonnodulated soybean plants (Glycine max [L.] Merrill, `Ransom'), grown previously on complete nutrient solutions with 1.0 millimolar NO3−, were transferred to solutions without a nitrogen source at the beginning of a dark period. Daily changes in starch and sucrose levels of leaves were monitored over the following 5 to 8 days in three experiments. Starch accumulation increased relative to controls throughout the leaf canopy during the initial two light periods after plant exposure to N-free solutions, but not after that time as photosynthesis declined. The additional increments of carbon incorporated into starch appeared to be quantitatively similar to the amounts of carbon diverted from amino acid synthesis in the same tissues. Since additional accumulated starch was not degraded in darkness, starch levels at the beginning of light periods also were elevated. In contrast to the starch effects, leaf sucrose concentration was markedly higher than controls at the beginning of the first light period after the N-limitation was imposed. In the days which followed, diurnal turnover patterns were similar to controls. In source leaves, the activity of sucrose-P synthase did not decrease until after day 3 of the N-limitation treatment, whereas the concentration of fructose-2,6-bisphosphate was decreased on day 2. Restricted growth of sink leaves was evident with N-limited plants within 2 days, having been preceeded by a sharp decline in levels of fructose-2,6 bisphosphate on the first day of treatment. The results suggest that changes in photosynthate partitioning in source leaves of N-stressed plants resulted largely from a stable but limited capacity for sucrose formation, and that decreased sucrose utilization in sink leaves contributed to the whole-plant diversion of carbohydrate from the shoot to the root.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Huber S. C., Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986 Aug;81(4):1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby E. A., Mengel K. Ionic balance in different tissues of the tomato plant in relation to nitrate, urea, or ammonium nutrition. Plant Physiol. 1967 Jan;42(1):6–14. doi: 10.1104/pp.42.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Boyer J. S. Control of Leaf Expansion by Nitrogen Nutrition in Sunflower Plants : ROLE OF HYDRAULIC CONDUCTIVITY AND TURGOR. Plant Physiol. 1982 Apr;69(4):771–775. doi: 10.1104/pp.69.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Eidenbock M. P. Carbon Accumulation during Photosynthesis in Leaves of Nitrogen- and Phosphorus-Stressed Cotton. Plant Physiol. 1986 Nov;82(3):869–871. doi: 10.1104/pp.82.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M. Carbon dioxide and nitrite photoassimilatory processes do not intercompete for reducing equivalents in spinach and soybean leaf chloroplasts. Plant Physiol. 1986 Mar;80(3):676–684. doi: 10.1104/pp.80.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Huber S. C. Changes in Starch Formation and Activities of Sucrose Phosphate Synthase and Cytoplasmic Fructose-1,6-bisphosphatase in Response to Source-Sink Alterations. Plant Physiol. 1983 Jun;72(2):474–480. doi: 10.1104/pp.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Israel D. W., Volk R. J. Assimilation of NO(3) Taken Up by Plants in the Light and in the Dark. Plant Physiol. 1984 Nov;76(3):769–775. doi: 10.1104/pp.76.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Jr, Raper C. D., Jr, Huber S. C. Alterations in internal partitioning of carbon in soybean plants in response to nitrogen stress. Can J Bot. 1984;62:501–508. doi: 10.1139/b84-074. [DOI] [PubMed] [Google Scholar]
- Silvius J. E., Chatterton N. J., Kremer D. F. Photosynthate partitioning in soybean leaves at two irradiance levels: comparative responses of acclimated and unacclimated leaves. Plant Physiol. 1979 Nov;64(5):872–875. doi: 10.1104/pp.64.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]


