Abstract
Chalcones and their derivatives have been widely studied due to their versatile pharmacological and biological activities, such as anti-inflammatory, antibacterial, antiviral, and antitumor effects. These compounds have shown suitable antiviral effects through the selective targeting of a variety of viral enzymes, including lactate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), fumarate reductase, protein tyrosine phosphatase, topoisomerase-II, protein kinases, integrase/protease, and lactate/isocitrate dehydrogenase, among others. Chalcones and their derivatives have displayed excellent potential for combating pathogenic bacteria and fungi (especially, multidrug-resistant bacteria). However, relevant mechanisms should be further explored, focusing on inhibitory effects against DNA gyrase B, UDP-N-acetylglucosamine enolpyruvyl transferase (MurA), and efflux pumps (e.g., NorA), among others. In addition, the antifungal and antiparasitic activities of these compounds (e.g., antitrypanosomal and antileishmanial properties) have prompted additional explorations. Nonetheless, systematic analysis of the relevant mechanisms, biosafety issues, and pharmacological properties, as well as clinical translation studies, are vital for practical applications. Herein, recent advancements pertaining to the antibacterial, antiviral, antiparasitic, and antifungal activities of chalcones and their derivatives are deliberated, focusing on the relevant mechanisms of action, crucial challenges, and future prospects. Furthermore, due to the great importance of greener and more sustainable synthesis of these valuable compounds, especially on an industrial scale, the progress made in this field has been briefly discussed. Hopefully, this review can serve as a catalyst for researchers to delve deeper into the exploration and designing of novel chalcone compounds with medicinal properties, especially against pathogenic viruses and multidrug-resistant bacteria as major causes of concern for human health.
Keywords: Chalcones, Chalcone derivatives, Antiviral effects, Antimicrobial applications, Antifungal activities, Greener synthesis, Sustainable techniques
1. Introduction
Natural products and herbal medicine is one of the common strategies for several diseases such as asthma [1], hepatitis [2], parasitic, bacterial, viral infections [[3], [4], [5]], tendinitis [6], pulmonary fibrosis [7], burn injury [8], cancer [9,10], and etc. Phenolics and flavonoids compounds are one of the most responsive ingredients due to their therapeutic effects [11]. Chalcones are phenolic compounds that are a member of the flavonoid family, commonly known as ‘open-chain flavonoids’, and are synthesized through the shikimate pathway. These compounds are believed to be the precursors of flavonoid biosynthesis. Chalcones are typically α,β-unsaturated ketones composed of two aromatic rings (A and B rings) connected by an alkenone group with three carbons; however, they can also contain certain saturated ketones, frequently referred to as dihydrochalcones, wherein an alkanone group with three carbons replaces the alkenone group with three carbons [12]. A large number of natural chalcones have been described in previous studies [13], many of which have been reported to react with different biomolecules and show cytoprotective and regulatory features, making them promising candidates for treating a variety of human disorders [14]. The bioinspired synthesis of various chalcones and analysis of their biological effects have been reported in previous investigations, with their structural simplicity and therapeutic promises [13]. According to the studies, chalcones have a variety of bioactivities such as antibacterial [15], antimalarial [16], antileishmanial [17], antifibrogenic [18], anticancer [19], anti-inflammatory [20], immunomodulatory [21], cytotoxic [22], analgesic [23], and antioxidant [24] properties. Several synthesized chalcones have been reported to have possible antidiabetic effects by targeting α-amylase or α-glucosidase [25]. Previous research has indicated the anti-inflammatory activity of a few chalcones and their derivatives, showing their potential to suppress the cyclooxygenase (COX) enzyme [26,27]. Naturally occurring and synthetic chalcones exerted strong antiproliferative effects in both stomach carcinoma HGC-27 cells [28] and early-stage and advanced ovarian cell carcinoma [29]. Chalcones containing the piperazine moiety exhibit a wide range of beneficial pharmacological effects such as antihistamine [30], anti-infective [31], antioxidant, anti-inflammatory [32], and anticarcinogenic activities [33]. A recent study has been performed on 46 chalcones to evaluate their antiproliferative effects on the human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant breast (MCF-7, MDA-MB-231), liver (HepG2), ovarian (Caov-3), cervical (HeLa), erythromyeloblastoid (K-562), nasopharyngeal (CNE-1), lung (A549), T-lymphoblastoid carcinoma (CEM-SS), colorectal (HT-29), and common human embryonic kidney (HEK-293) cells [34].
Chalcones are reported to exhibit antiviral properties by targeting several viral enzymes such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH), fumarate reductase, lactate dehydrogenase, protein tyrosine phosphatase, topoisomerase-II, several protein kinases, human immunodeficiency virus (HIV integrase (IN)/protease), and lactate/isocitrate dehydrogenase, among others [35]. One of the main problems with many antiviral medications is resistance to these drugs, which can develop as a result of mutations, genetic alterations, and phenotypic modifications. Thus, the previously successful treatment will no longer be effective against the virus, which will result in failure to manage the disease, increased risk of virus transmission, and elevated fatality rates [36]. Thus, the development of novel antiviral medications is crucial, and researchers have begun to take a greater interest in chalcones due to their established antiviral function.
On the other hand, infectious diseases remain a major cause of mortality worldwide. The growth of antimicrobial resistance due to wide misuse and poor patient compliance is causing concern on a global scale. Antimicrobial resistance is a public health challenge that has significant financial and societal effects [37,38]. A serious crisis in the management of infectious illnesses is yet to emerge along with the slowing rate of the development of new antibiotics. As a result, it is crucial to discover novel antimicrobial agents with suitable therapeutic, toxicological, and pharmacokinetic characteristics [37]. The antimicrobial properties of naturally occurring and synthesized chalcones, including their effects against a number of bacterial and fungal pathogens, have been reported by several studies [12]. Herein, the most recent advancements pertaining to the antiviral and antimicrobial applications of chalcones and their derivatives are discussed, focusing on crucial challenges and future prospects. Greener techniques for synthesizing these compounds are also briefly highlighted.
2. Greener synthesis of chalcones and their derivatives
Although the main focus of this review is on the antibacterial and antiviral effects of chalcones and their derivatives, in this section, a few examples of greener techniques for synthesizing these compounds are provided. Since the industrial production of these materials is of great importance, in order to obtain optimal conditions based on green chemistry principles, additional explorations should be conducted on synthesis strategies that are environmentally friendly, simple, cost-effective, and up-scalable. In this context, green catalysts and flow chemistry are among the introduced techniques with great potential for chalcone synthesis and its derivatization; however, limitations and challenges still exist regarding the generation of complex structures and the standardization and optimization of reaction/synthesis conditions, which warrant more in-depth analyses [39]. Generally, the synthesis of chalcones is conducted based on conventional Claisen-Schmidt condensation at high temperatures, but alternative approaches using green sources of energy, such as ultrasound and microwave, have been introduced. In addition, the utilization of ionic liquids as catalysts can provide opportunities for solvent-free synthesis, thus enhancing the yield of production, shortening the operational processes, decreasing the formation of wastes, and reducing the reaction time [39].
An eco-friendly technique was reported for the synthesis of biphenyl chalcone and coumarin derivatives through Suzuki coupling using polyethylene glycol (PEG)-400 as a solvent under microwave irradiation. The salient advantages of this approach were the short reaction time (30–45 min), yields ranging from good to excellent (∼95%), and the prominent tolerance of various functional groups (Fig. 1) [40]. Moreover, the solvent-free synthesis of chalcones was performed by grinding benzaldehyde (unsubstituted, 4-methyl, 4-methoxy, 3-chloro, or 4-chloro) and acetophenone (unsubstituted, 4-methyl, 4-bromo, or 4-methoxy) in the presence of solid sodium hydroxide with a mortar and pestle. The resulting chalcones exhibited high yields and purity, although minor quantities of ketol and Michael addition products were also detected by nuclear magnetic resonance (NMR) spectroscopy [41]. These side-products could be simply eliminated by the recrystallization process. The melting point of chalcone is crucial in the solvent-free preparation technique. Accordingly, chalcones with relatively high melting points (higher than 80 °C) could be obtained in high yields, while three other chalcones that could not be synthesized in good yields had relatively low melting points [41].
Fig. 1.
Eco-friendly synthesis of chalcone and coumarin derivatives through a Suzuki coupling reaction using microwave irradiation. Reproduced with permission from Ref. [40].
Among the proposed synthesis techniques, microwave-assisted methods have attracted the attention of many researchers due to their many benefits including rapidness, mass production capabilities, uniformity in the heating process, low processing costs, short preparation time, low energy consumption, high purity, and high production yield. For instance, microwave-irradiated solvent-free synthesis of chalcone derivatives was shown by applying a zinc nanoferrite catalyst. The main advantages of this approach were a solvent-free reaction, cost-effectiveness, environmentally friendly conditions, and favorable yields, along with reduced reaction times and easy recovery of zinc ferrite nanocatalyst (good recyclability) (Fig. 2) [42]. In addition, microwave-assisted fabrication of a chalcone compound, namely 1-(2,5-dimethylthiophen-3-yl)-3-(9-ethyl-9H-carbazol-3-yl)prop-2-en-1-one, and its polycyclic heterocyclic analogs was reported [43]. After the microwave-assisted condensation of 9-ethyl-9H-carbazole-3-carbaldehyde and 3-acetyl-2,5-dimethyl thiophene, the aforementioned chalcone was obtained, which was further cyclized to pyrazoline and pyrimidine derivatives. Among the examined compounds, the pyrazoline skeleton exhibited higher antibacterial effects (in vitro), and the antibacterial mechanism was associated with the interaction and inhibition of the glucosamine-6-phosphate synthase enzyme [43].
Fig. 2.
(A) The preparation of zinc ferrite nanoparticles. (B) The production of (E)-3-(4′-chlorophenyl)-1-phenyl prop-2-en-1-one chalcone using zinc ferrite nanocatalyst. (C) The related mechanism of (E)-chalcone synthesis over zinc ferrite magnetic nanoparticles (MNPs). Reproduced with permission from Ref. [42]. (CC BY 4.0).
Green and sustainable solvents that are renewable and abundant have been utilized for the synthesis of chalcones and their derivatives. For instance, glycerin (as a sustainable solvent) was employed to synthesize chalcones with moderate inhibitory effects against pathogenic microorganisms [44]. Glycerin solvent (also named glycerol) is considered a green solvent with the benefits of low toxicity, cost-effectiveness, wide availability, and renewability [45]. In another study, a green technique was presented for the preparation of thiophene-pyrazole hybrids via the reaction of chalcones using thiosemicarbazide hydrochloride in an aqueous medium of citrus extract (as a green solvent), leading to the production of thiophene tethered pyrazoline carbothioamides. These compounds exhibited excellent antimicrobial effects, as well as radical scavenging abilities [46].
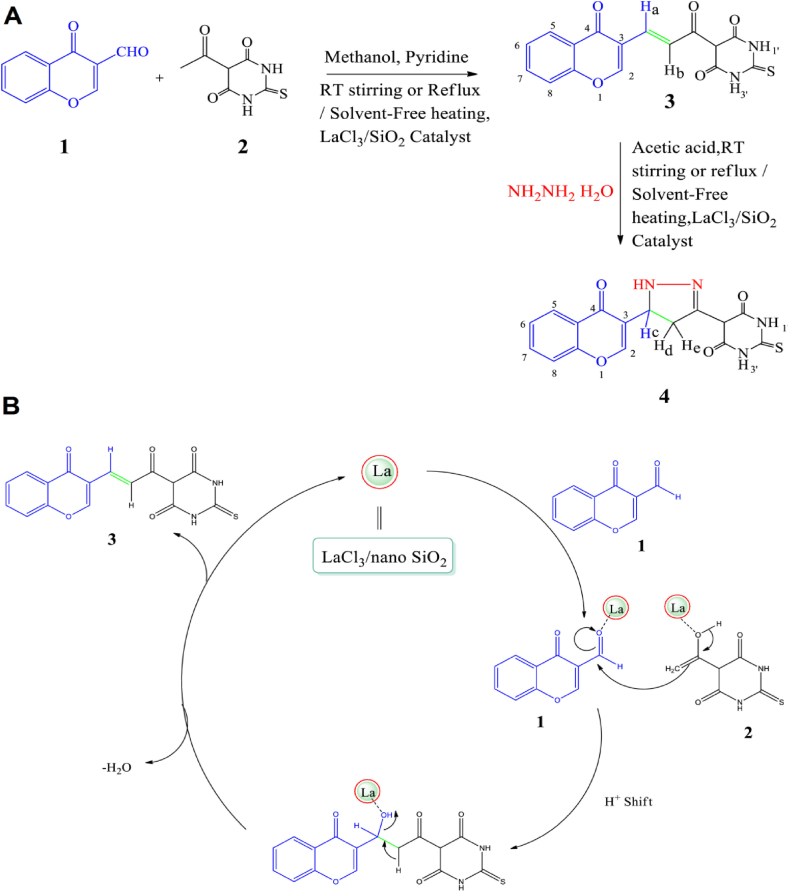
Chalcones were synthesized in an eco-friendly manner using thiamine hydrochloride (vitamin B1) as a biodegradable and cost-effective catalyst via a metal-free Claisen-Schmidt condensation process with high production yield, catalyst recoverability, and tolerance for a broad range of functional groups. This technique could be additionally applied for synthesizing 2′,4′-dihydroxy-6′-methoxy-3′, 5′-dimethyl chalcone and its derivatives as pharmaceutically important molecules [47]. In addition, in order to produce biologically significant chromonyl chalcone and pyrazoline derivatives, an eco-friendly technique was employed in which the lanthanum (III) chloride (LaCl3)/nano silicon dioxide (SiO2) catalyst was utilized under a solvent-free heating process (Fig. 3) [48]. Accordingly, chromonyl chalcone could be obtained from 3-formyl chromone and 5-acetyl thiobarbituric acid via Claisen-Schmidt condensation. These compounds exhibited efficient antibacterial and antifungal effects. The reaction was completed in a short period of time (∼15 min), had an excellent yield of production (∼90%), and was environmentally friendly without any toxic waste products [48].
Fig. 3.
(A) The preparation process of chalcone 3 and its reaction with hydrazine to provide pyrazoline 4. (B) Plausible mechanism for the formation of chalcone 3. Reproduced with permission from Ref. [48].
Solvent-free synthesis of chalcones was reported using graphene oxide-supported MnO2 catalysts prepared by a green solution combustion technique [49]. The nanocatalysts could be applied for synthesizing chalcones through Claisen-Schmidt condensation, wherein the chalcones were obtained with high yields at 110 °C under solvent-free conditions in a short period of time [49]. In another study, Ca(OH)2 and K–Ca(OH)2 catalysts were synthesized from chicken eggshell wastes in an eco-friendly manner with high efficiency, cost-effectiveness, stability, and recyclability. These green catalysts were employed for the synthesis of chalcones through the Claisen-Schmidt condensation reaction, with high yields (∼74–92%) in aqueous ethanol at room temperature [50]. Thus, one of the effective strategies for a greener production of chalcones and their derivatives is the utilization of green-synthesized nanocatalysts.
3. Antiviral function of chalcones
3.1. Chalcones and coronaviruses (CoVs)
CoVs are members of the Coronaviridae family, the Nidovirales order, and the Coronavirus genus. They are the main class of viruses that cause infections in the lungs and the gastrointestinal tract. They are enclosed viruses with a positive-sense non-fragmented single-stranded RNA. These viruses are pleomorphic with a length of 80–160 nm and possess a small genome (27–32 kb) with a distinct replication method. Four CoV genera have been identified, including Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus [51]. All known CoVs possess the same structure, which is comprised of four key proteins, namely the membrane (M), envelope (E), spike (S), and nucleocapsid (N) [51].
Severe acute respiratory syndrome (SARS) was initially observed in 2002–2003, and severe acute respiratory syndrome coronavirus (SARS-CoV) was recognized as the cause. This virus led to the first pandemic occurring in the current century, which originated in China and spread to other regions [52]. The Middle East respiratory syndrome (MERS) appeared about a decade later (2012), and a patient exhibiting severe respiratory symptoms in Saudi Arabia was found to be infected with a sixth coronavirus (Middle East respiratory syndrome coronavirus, MERS-CoV) [53]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel member of the human CoV family, which was recently detected in Wuhan, China [51].
The 3-chymotrypsin-like protease (3CLpro), which is also referred to as the main protease (Mpro), is the primary protease of SARS-CoV-2 and is necessary for viral proliferation. Because of its minimal resemblance to human genes, it is a major target for drug formulation [54]. This enzyme catalyzes the cleavage of several preserved sites in polyproteins 1 ab (PP1ab) and 1a (PP1a), which contain glutamine, a small aliphatic amino acid residue and a large hydrophobic residue. Due to the structural composition and enzymatic function of 3CLpro, it is a selective target for the discovery of new medications [55].
Several compounds and their natural or synthetic derivatives with anti-inflammatory and antiviral properties are shown to exhibit high affinity for 3CLpro [56] (for others see Table 1). Various molecules with different medicinal characteristics have been synthesized using the chalcone structure. Clinical trials have indicated that these chalcone derivatives reached acceptable plasma levels and did not induce toxicity. Therefore, chalcones have continually attracted much interest for both academic studies and commercial uses [57]. Chalcone derivatives have been reported to suppress cysteine proteases. In a study using a library of 3000 chalcone derivatives, different computational approaches were applied to identify 3CLPro inhibitors, which are one of the potential targets against coronavirus. Inhibition of 3CLPro will interrupt viral replication. The calculation of binding free energy revealed that four chalcone derivatives had high binding affinities for the 3CLPro enzyme. These compounds were then subjected to chemical absorption, distribution, metabolism, excretion, and toxicity (ADMET) studies and drug-like filters, indicating that they are not carcinogenic and have potential as therapeutic candidates [58]. In another study, 269 chalcones were examined as 3CLpro inhibitors using in silico screening models, including molecular docking, molecular dynamics simulation, binding free energy calculation, and ADME prediction. C264 and C235 were introduced as the two structures with the most potential [59]. Moreover, the interaction between the Mpro active site (7BQY) and a heterocyclic chalcone-based ligand (i.e., (E)-1-(2,4-dichlorophenyl)-3-[4-(morpholin-4-yl)phenyl]prop-2-en-1-one) was screened via molecular docking. The findings indicated that the binding affinity of the studied chalcone for Mpro is comparable to those of certified drugs remdesivir and favipiravir for SARS-CoV-2 Mpro [60].
Table 1.
Chalcone derivatives investigated against various coronaviruses.
| Compound(s) “Chemical name(s)” | Virus subtype | Study model(s) | Results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| “[6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]-[4-(2-piperidin-1-ylethoxy)phenyl]methanone” “N-(5-benzoyl-4 phenyl-1 3-thiazol -2-yl)-2-(4 ethylsulfonylphenyl)acetamide)” “(R)-(6-hydroxy-2-(4 hydroxyphenyl)benzo[b]thiophen-3-yl)(4-(2-(3-methylpyrrolidin-1 -yl)ethoxy)phenyl)methanone” “2,4′-Bis(benzyloxy)-3,5-dimethyl-4-hydroxy-trans-chalcone” |
SARS-CoV-2 | In silico | Bindings affinities of −66.125 (kJ/mol), −59.589 (kJ/mol), −66.728 (kJ/mol), −87.962 (kJ/mol), respectively | Inhibition of 3CLPro | [58] |
| “(E)-1-(2,4-dichlorophenyl)-3-[4-(morpholin-4-yl) phenyl]prop-2-en-1-one” | SARS-CoV-2 | In silico | Binding affinity of the chalcone with 7BQY was −7.0 kcal/mol | Inhibition of Mpro | [60] |
| Xanthoangelol E (3-prenylated chalcones) “(E)-1-[3-(2-hydroperoxy-3-methylbut-3-enyl)-2-hydroxy-4-methoxyphenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one” | SARS-CoV-2 | In vitro | IC50 values of 11.4 μM (3CLpro) and 1.2 μM (PLpro) | Inhibition of SARS-CoV PLpro and 3CLpro | [62] |
| Isobavachalcone “(E)-1-[2,4-dihydroxy-3-(3-methylbut-2-enyl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one)” Helichrysetin “(E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one” |
MERS-CoV | In silico, In vitro | IC50 of 35.85 and 67.04 μM, respectively | Inhibition of MERS-CoV 3CLpro | [63] |
| Panduratin A “(2,6-dihydroxy-4-methoxyphenyl)-[(1R,2S,6R)-3-methyl-2-(3-methylbut-2-enyl)-6-phenylcyclohex-3-en-1-yl]methanone” | SARS-CoV-2 | In vitro | Post-infection: IC50 0.81 μΜ, Pre-entry: 5.30 μΜ | Inhibition of SARS-CoV-2 replication and infectivity both before entrance and after infection | [64] |
SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2, IC50: Half maximal inhibitory concentration.
In one study, the potent antiviral activity of newly synthesized chalcone derivatives against viral RNA-dependent RNA polymerase (RdRp) in SARS-CoV-2 was evaluated in silico and confirmed by calculating the RT-PCR cycling threshold (Ct) values. According to the results of docking studies, compound 6 formed a hydrogen bond with Ser814 and had charged (negative) interactions with Asp760, Asp761, and Asp618. The binding affinity of this compound was determined to be −4370 kcal/mol (Fig. 4) [61].
Fig. 4.
Interaction of compound 6 with the active site of RNA-dependent RNA polymerase showed a high docking score of −4.127 kcal/mol for compound 6 which made a hydrogen bond with Arg403 and Gly496. Reproduced with permission from Ref. [61].
Nine chalcone derivatives were extracted from the medicinal plant Angelica keiskei and their anti-SARS-CoV effects were examined in another work. Among them, an alkylated chalcone with perhydroxyl and methoxy substituents was reported to exhibit significant suppressive effects on critical SARS-CoV enzymes, namely cysteine proteases including 3CLpro and papain-like protease (PLpro) (IC50 values of 11.4 and 1.2 μM against 3CLpro and PLpro, respectively). Chalcones are therefore promising compounds for the development of anti-SARS medicines due to their significant activity against these proteases [62].
MERS is caused by MERS-CoV, which is a zoonotic virus that spreads between livestock and humans. However, no vaccination or specialized therapy is accessible at the present. Jo et al. employed a flavonoid library to screen for agents active against MERS-CoV 3CLpro. They found that two chalcone derivatives, namely isobavachalcone and helichrysetin, were able to inhibit the activity of this enzyme. An induced-fit docking study also demonstrated that S1 and S2 sites are involved in the interaction with these compounds. This study revealed that the preferred scaffolds for binding with the MERS-CoV 3CLpro enzyme at the catalytic site are flavonol and chalcone scaffolds [63]. In addition, it was reported that the Boesenbergia rotunda extract and panduratin A, a phytochemical component found in this plant, displayed potent inhibitory effects on SARS-CoV-2 (IC50 of 3.62 μg/mL and 0.81 μΜ, respectively) with satisfactory cytotoxicity results (CC50: 28.06 μg/mL and 14.71 μM, respectively) [64].
3.2. Chalcones and HIV
Acquired immunodeficiency syndrome (AIDS) is caused by two subtypes of HIV: HIV-1 and HIV-2. They are comparable in several ways, such as intracellular replication processes, transmission routes (transfusion, sexual contact, and sharing of needles), and clinical outcomes [65]. The prevalence of HIV-1 is 24 times that of HIV-2. Effective treatment of HIV is a global health challenge, especially as concerns about drug resistance among patients grow [66]. Anti-HIV medications are allocated to several groups in terms of their methods of action, which target various phases of the life cycle of HIV. These groups include nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), capsid inhibitors, protease inhibitors (PIs), IN inhibitors, and entry inhibitors [67].
The US FDA has currently authorized approximately 30 anti-HIV agents for clinical use, including highly active antiretroviral therapy (HAART). The best therapeutic option for HIV/AIDS patients is HAART. The medications are so effective at managing HIV that they remove the virus from circulation and maintain a zero plasma virus load in the majority of the patients. However, the drawback of this treatment is that the medication must be taken constantly, otherwise the plasma virus load would be restored. Anti-HIV medications are also proven to have adverse effects to the extent that the patient might be compelled to stop using them. Therefore, more effective anti-HIV drugs are needed [68]. Numerous chalcone derivatives, both natural and synthetic, have been tested on HIV infections.
The main target of antiviral treatments is HIV reverse transcriptase (RT). HIV RT is a low-fidelity DNA polymerase with a high error rate. Mutations are prevalent during reverse transcription as a result of this feature. HIV has two RNA genomes, which facilitate the generation of recombinant progeny. The two main groups of drugs that block the RT enzyme are NRTIs and NNRTIs [69]. NRTIs block RT via the active site whereas NNRTIs do not alter the enzyme's active site but rather cause a conformational change in the RT enzyme, which significantly lowers its efficacy, and therefore, function as non-competitive inhibitors of this enzyme [70]. A number of quinoline-based chalcones were found to be potentially active against the RT enzyme. According to the results of bioassay, theoretical, and docking analyses, the bromo- and chloro-substituted chalcones exhibited significant inhibition against RT. The IC50 values of chalcones with the strongest activities were 0.10 and 0.11 μg/mL and their free binding energies (ΔG) were −9.30 and −9.13 kcal. It was also indicated that none of these compounds displayed cytotoxicity toward the CEM, PBM, and VERO cell lines [69]. In another study, the antiviral effects of an Indian medicinal herb known as Pongamia pinnata against HIV were investigated. Aqueous extracts from the leaves, seeds, and roots of this plant were prepared and examined for anti-HIV-1 activity. It was reported that active chalcone derivatives glabarachalcone and karanijin exhibited promising binding scores against the P24 protein and RT enzyme [68]. The P24 protein is a key capsid protein in HIV-1, which plays an essential role in the pathogenesis of the virus and its inhibition either prevents viral entry, blocks other enzymes, or disrupts virion assembly [68,71].
The IN enzyme of HIV has lately received much attention as a promising therapeutic target for the formulation of drugs against HIV. IN is one of the proteins required for HIV replication. It contributes to a critical phase of the replication cycle by inserting a viral DNA copy into the host genome [72,73]. The integration reaction by IN consists of two steps: 3′- end processing, in which two nucleotide bases are cleaved off each 3′-end, and 3′- end joining or strand transfer, which involves inserting the shorter strand into the host DNA [73,74]. Ten new compounds related to the structure of ferrocenyl chalcone difluoridoborate were synthesized in a study. These molecules were shown to display inhibitory activity against the IN 3′ processing and strand transfer steps [73].
A novel chalcone-based compound, namely (E)-3-(5-(adamantan-1-yl)-2,4-bis (methoxymethoxy) phenyl)-1-(2-hydroxy-5-methylphenyl)prop-2-en-1-one or Amt-87, has been identified, which can act as a latency-reversing agent (LRA) [75]. LRAs are chemicals that can reawaken a latent virus from its inactive form, allowing the immune system to recognize the infected cells [76]. The main impediment to HIV/AIDS elimination is the dormant HIV reservoir, which comprises incorporated but transcriptionally inactive proviruses. The development of efficient treatment strategies aimed at destroying this reservoir is currently underway. The “shock and kill” technique is one such strategy, in which the latent HIV proviruses are reactivated using LRAs in the “shock” stage. Subsequently, in the “kill” stage, HAART is used to limit the spread of new infections while also rendering the reactivated cells susceptible to viral cytopathogenicity and the host immune response. The absence of LRAs that are clinically effective has hampered the execution of the “shock and kill” technique thus far [73]. Numerous LRAs have been demonstrated to awaken dormant HIV, but their high toxicity and low efficiency have prevented their use in clinical settings. However, the chalcone derivative Amt-87 was reported to show only minimal cytotoxicity and was capable of considerably reactivating the transcription of dormant HIV proviruses [75].
Another key enzyme in the replication process of HIV is HIV-1 protease. This molecule is a crucial target in the discovery of drugs against HIV. By breaking down Gag and Gag-Pol polyproteins, HIV-1 protease produces mature pathogenic virions with the ability to infect CD4+ cells. Inhibition of this enzyme's activity leads to the generation of immature virions that are not capable of infecting cells [77]. Since HIV-1 has been demonstrated to be resistant to various synthetic anti-HIV-1 protease inhibitors, there is still a need for the development of efficient drugs against this enzyme. In an investigation, the impact of the methanol extract of Boesenbergia pandurata rhizomes against HIV-1 protease was examined. The safety of this extract was established by an earlier study that indicated its minimal toxicity and lack of fatalities in rats following seven days of administration [78]. A novel cyclohexenyl chalcone called panduratin C and a few other chalcone-based compounds (panduratin A and hydroxypanduratin A) were obtained from this extract, which exhibited potent anti-HIV-1 protease activity [79]. Another study indicated that cardamonin, which is a chalcone derivative obtained from the Boesenbergia pandurata Holtt. (yellow rhizome) extract, had considerable anti-HIV-1 protease activity [80]. Studies with more details are summarized in Table 2.
Table 2.
Chalcone derivatives investigated against HIV.
| Compound(s) “Chemical name(s)” | Virus subtype | Study model(s) | Results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| Glabarachalcone “(E)-1-7(7-hydroxhy-2-2dimethyl chromen-6-yl)-3-phenylprop-2-en-1-one” | HIV-1 | In silico, In vitro | Mean inhibition against HIV gag p24: 66.9 ± 4.4% | Potent binding to RT enzyme and P24 protein , inhibition of HIV-1 gag p24 |
[68] |
| Quinoline-based chalcones | HIV-1 | In silico, In vitro | IC50 (the most active compounds): 0.10 and 0.11 μg/mL; binding free energies (ΔG): 9.30 and −9.13 kcal |
Inhibition of RT (non-nucleoside reverse transcriptase) | [69] |
| A series of ferrocenyl chalcone borates | HIV-1 | In vitro | IC50: 4–16 μM (for 3′ processing activities) and 0.7–9 μM (for strand transfer activities) | Inhibition of the IN 3′ processing and strand transfer steps | [73] |
| Amt-87 “(E)-3-(5-(adamantan-1-yl)-2,4-bis (methoxymethoxy) phenyl)-1-(2-hydroxy-5-methylphenyl)prop-2-en-1-one” | HIV-1 | In vitro | Stimulated GFP expression in J-Lat A2 cells at concentrations of 50 and 100 μg/mL | Latency-reversing agent (LRA) | [75] |
| Hydroxypanduratin A “(1R,2S,6R)-3-methyl-2-(3-methylbut-2-enyl)-6-phenylcyclohex-3-en-1-yl]-(2,4,6-trihydroxyphenyl)methanone” panduratin A “(2,6-dihydroxy-4-methoxyphenyl)-[(1R,2S,6R)-3-methyl-2-(3-methylbut-2-enyl)-6-phenylcyclohex-3-en-1-yl]methanone” | HIV-1 | In vitro | IC50: 5.6 and 18.7 μM, respectively | Suppression of HIV-1 protease | [79] |
| Cardamonin “(E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop-2-en-1-one” | HIV-1 | In vitro | IC50 of 31 μg/mL | Inhibition of HIV-1 protease | [80] |
HIV: Human immunodeficiency virus, IC50: Half maximal inhibitory concentration.
3.3. Chalcones and influenza virus
Influenza is a periodical infectious disease induced by RNA viruses that belong to the Orthomyxoviridae family [81]. The members of this family include enveloped viruses with segmented single-strand negative-sense RNA fragments. This family of viruses is comprised of four genera: A, B, C, and Thogotovirus, among which, only types A and B are significant for human beings from a clinical viewpoint. While influenza B viruses nearly exclusively infect humans, influenza A viruses lead to infection in a great number of mammalian and avian hosts. Since influenza A viruses have led to pandemic outbreaks, they have received much more attention [82]. The three main parts of the influenza virus structure include the core, matrix, and envelope proteins. These biomolecules are hemagglutinin (HA), RNA polymerase (PA, PB1, and PB2), matrix protein 1 (M1), proton channel protein (M2), neuraminidase (NA), nonstructural protein 1 (NS1), nuclear export protein (NEP, NS2), and nucleoprotein (NP). The influenza virus has developed cellular systems that allow it to take advantage of human cell components in order to promote its proliferation while repressing host defenses. Recognizing these cellular processes helps the identification of anti-influenza drug targets [[82], [83], [84]]. Currently, one of the main therapeutic targets is the NA enzyme. NA, also known as sialidase, catalyzes the cleavage of sialic acid on the surface of the afflicted cell and releases progeny virions from these cells. It might also facilitate viral transmission through respiratory tract mucus, thereby increasing viral infectiousness. The capacity of the influenza virus to propagate to neighboring cells is greatly hindered when NA activity is impaired, causing viral progeny to congregate at the surface of the infected cell [85]. Despite the development of many viral NA inhibitors, such as rimantadine, oseltamivir, zanamivir, and amantadine, high levels of drug tolerance have been detected in influenza viruses [86]. Therefore, there is a great need to identify anti-influenza medications that are active against various influenza strains. In an anti-influenza assessment of natural compounds, several derivatives were reported to be implicated in mitigating the risk of virus strains with drug resistance or boosting the efficiency of antiviral drugs. NA is one of the main inhibitory targets of numerous natural and synthesized chalcones since suppressing its activity is crucial for limiting viral propagation.
Chalcone derivatives acquired from the acetone extract of Glycyrrhiza inflata were shown to exhibit potent inhibitory effects against NAs from several influenza virus subtypes, namely H1N1, novel H1N1 (WT), oseltamivir-resistant novel H1N1 (H274Y), and H9N2 expressed in 293T cells. Additionally, the presence of one chalcone derivative improved the efficiency of oseltamivir against H274Y NA [85]. In another study, a number of 2′-amino, 3′-amino, and 2′,4′-dihydroxy chalcone-based compounds were synthesized to act as inhibitors of the H1N1 influenza virus and NA enzyme using a structure-based drug design [87]. In an in silico study, it was shown that a synthetic chalcone derivative known as 2′-hydroxy-4-methoxychalcone, which was designed based on quercetin, was active against H5N1-NA in a non-competitive manner and could be used as an efficient treatment to circumvent the currently observed drug resistance [88]. In a different study, six chalcones with alkyl substituents were extracted from Angelica keiskei and their capacity to suppress NA hydrolysis was examined. It was indicated that 2-hydroxy-3-methyl-3-butenyl alkyl (HMB)-substituted chalcone had strong inhibitory effects (IC50: 12.3 μM) [89]. More research is presented in Table 3.
Table 3.
Chalcone derivatives investigated against the influenza virus.
| Compound “Chemical name(s)” | Virus subtype | Study model(s) | Results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| Echinantin “(E)-3-(4-hydroxy-2-methoxyphenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one” | H1N1, H9N2, H1N1 (WT), H1N1 (H274Y) |
In vitro | IC50 values of 5.80 ± 0.30, 5.70 ± 0.55, 2.49 ± 0.14, and 2.19 ± 0.06 μg/mL, respectively |
Inhibition of NAs | [85] |
| Isoliquiritigenin “(E)-1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one” | H1N1, H9N2, H1N1 (WT), H1N1 (H274Y) |
In vitro | IC50 values of 8.41 ± 0.39, 9.69 ± 0.37, 3.48 ± 0.19, and 3.42 ± 0.12 μg/mL, respectively |
Inhibition of NAs | [85] |
| “(E)-1-(2,4-dihydroxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one” | H1N1 | In vitro | IC50 of 2.23 μmol/L | Inhibition of NAs | [87] |
| “(E)-1-(2-hydroxy-4-methoxyphenyl)-3-phenylprop-2-en-1-one” | H5N1 | In silico | Binding to the 150-cavity of NA | Inhibition of NAs | [88] |
| 2-Hydroxy-3-methyl-3-butenyl alkyl | H1N1 | In vitro | IC50 of 12.3 μM | Inhibition of NAs | [89] |
IC50: Half maximal inhibitory concentration.
3.4. Chalcones and human rhinovirus (HRV)
The most frequent cause of upper respiratory system disorders in both adolescents and adults is HRV [90]. HRVs, which belong to the Picornaviridae family, contain more than 100 distinct viral strains. These viruses are the most significant etiological factors of the common cold. Although upper respiratory disorders caused by HRV are generally minor and self-limiting, the socioeconomic toll of missing work or school and the level of unnecessary antibiotic usage are considerable. Additionally, increasing evidence supports the association between HRV infection and more severe conditions. Infections caused by HRV are significant risk factors for acute otitis media and sinusitis and are one of the main reasons for asthma attacks in both adults and children. Moreover, in those with bronchitis, cystic fibrosis, and other preexisting respiratory conditions, HRV infections are also linked with lower respiratory system disorders [91]. Current antiviral therapies to combat this virus are aimed at decreasing cell sensitivity by blocking viral binding, attachment, replication, uncoating, and protein production. A number of studies have investigated the impact of chalcones on a variety of particular targets in the virus including the capsid pocket, binding sites, and viral proteases inside VP1, and these proteases were discovered to be the most efficient targets of chalcones with anti-rhinovirus activity. Ro 09–0410 (4′ ethoxy-2′-hydroxy-4,6′ dimethoxy-chalcone) was one of the first isolated chalcones which exclusively inactivates rhinovirus [92,93]. It is a synthetic chemical similar to a flavone that is found in a Chinese medicinal plant called Agastache follium (Agastache rugosa Kuntze). It displays in vitro antiviral effects against various rhinovirus strains and suppresses the function of the virus via adhering to the viral particles in suspension [92]. At doses of 4 μg/mL or less, this compound is non-toxic to cells [93]. However, it did not substantially lower the incidence of infection in human clinical studies [94,95]. Hence, researchers have attempted to design varying synthetic analogs similar to Ro 09–0410. Ninomiya et al. synthesized chalcone amides that are 4.5–10 times more effective against HRV in cell culture than the antirhinovirus drug Ro 09–0410. Ro 09–0696, Ro 09–0881, and Ro 09–0535 are amide analogs that blocked the replication of the virus at concentrations < 2–3 ng/mL and showed cytotoxic activity at doses ranging from 30 to 50 μg/mL [96] (Table 4).
Table 4.
Chalcone derivatives investigated against HRV.
| Compound(s) “Chemical name(s)” | Virus subtype | Study model(s) | Results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| Chalcone amides including: Ro 09–0535 “4-ethoxy-2-hydroxy-6-methoxy-N-[(4-methoxyphenyl)methyl]benzamide” Ro 09–0696 “2-hydroxy-6-methoxy-N-[(4-methoxyphenyl)methyl]-4-(3-methylbut-2-enoxy)benzamide” Ro 09–0881 “4-ethoxy-2-hydroxy-6-methoxy-N-[[4-(methylamino)phenyl]methyl]benzamide” |
HRV-2, | In vitro | IC50: 0.0040, 0.0014, and 0.052 μg/mL, respectively | Inhibition of the uncoating of rhinovirus by keeping the viral capsid protein stable | [96] |
| Ro 09–0410 “(E)-1-(4-ethoxy-2-hydroxy-6-methoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one” | HRV9 | In vitro | IC50: 0.03 μg/mL, | Inactivation of the capsid protein by binding to a specific site in the rhinovirus capsid | [92] |
HRV: Human rhinovirus, IC50: Half maximal inhibitory concentration.
3.5. Chalcones and herpes simplex virus (HSV)
HSV belongs to the Alphaherpesvirinae subfamily of the Herpesviridae family. It is a double-stranded DNA virus with a lytic and latent cycle, which infects various host cells. HSV is one of the most widely researched viruses since it was the first human herpes virus to be identified [97]. The antiviral effect of a group of chalcones was examined against a variety of viruses and it was reported that these derivatives showed favorable in vitro antiviral activity against HSP-1 and HSP-2. These compounds were designed by reacting 4-hydroxy-3-methylacetophenone with a suitable aldehyde while a base was present by using a typical Claisen-Schmidt condensation [98]. In another study, the anti-HSV potential of several chalcone derivatives extracted from the stem bark of Millettia leucantha KURZ (Leguminosae) was investigated. It was reported that dihydrochalcones exhibited mild antiviral effects on HSV-1 and HSV-2 [99]. A number of 3,5-bis(arylidene)-4-piperidones, as chalcone analogs, were designed and tested for their in vitro antiviral properties. A few of these molecules were shown to have anti-HSV-1 effects [100] (Table 5)
Table 5.
Chalcone derivatives investigated against HSV.
| Compound(s) “Chemical name(s)” | Virus subtype | Study model(s) | Results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| “5-(4-chlorophenyl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridyl methanone” | HSV-1 | In vitro | Minimum inhibitory concentration (MIC): >8 μg/mL (against HSV-1) |
Replication inhibitory activity | [98] |
| Chalcone derivatives isolated from Millettia leucantha KURZ “2′,4′,6′-Trimethoxy-3,4-methylenedioxydihydrochalcone, 3-(1,3-benzodioxol-5-yl)-1-(2,4-dimethoxyphenyl)-3-methoxypropan-1-one” | HSV-1 and HSV-2 | In vitro | IC50: 15.5 μg/mL (against HSV-1) and 17.0 μg/mL (against HSV-2) | N/A | [99] |
| 3,5-Bis(arylidene)-4-piperidones “(E)-1-Methyl-3,5-bis(2-thienylidene)-4-piperidone” | HSV-1 | In vitro | IC50: 0.66 μM | N/A | [100] |
HSV-1: herpes simplex virus type 1, HSV-2: herpes simplex virus type 2, IC50: Half maximal inhibitory concentration, MIC: Minimal inhibitory concentration.
3.6. Chalcones and dengue virus
Dengue virus is an arthropod-borne virus with four distinct serotypes (DEN-1, DEN-2, DEN-3, and DEN-4), which belongs to the Flavivirus genus of the Flaviviridae family. All these serotypes have a positive polarity single-stranded RNA, which encodes a polyprotein precursor including seven nonstructural proteins and three structural proteins (C, prM/M, and E) [101]. The cleavage of this polypeptide is catalyzed by the host protease and the viral NS2B/NS3 protease (protease complex) [102]. Dengue fever is regarded by the World Health Organization (WHO) as a serious worldwide health concern in tropical and subtropical regions [103]. Several investigations have been conducted in order to develop anti-dengue agents, such as antiviral inhibitors [[104], [105], [106], [107]] and vaccinations [[108], [109], [110]]. However, no efficient drugs have been discovered for the treatment of dengue fever as of yet. Clinically tested drugs, such as Balapiravir, are still ineffective at preventing viral replication [111]. In an investigation by Kiat et al. the effect of several chalcones extracted from fingerroot, Boesenbergia rotunda (L.) Mansf. Kulturpfl. (BR) on DEN-2 virus NS3 protease was examined. They reported that cardamonin showed non-competitive inhibitory activities against the NS3 protease of the DEN-2 virus. Two other cyclohexenyl chalcones obtained from fingerroot were panduratin A and 4-hydroxypanduratin A, which competitively inhibited the NS3 protease (Ki: 25 and 21 μM, respectively) [112] (Table 6).
Table 6.
Chalcone derivatives investigated against dengue virus.
| Compound(s) “Chemical name(s)” | Virus subtype | Study model(s) | Assay results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| Cardamonin “(E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop-2-en-1-one” | DEN-2 | In vitro | Ki: 377 μM | Non-competitive inhibition of NS2B/NS3 protease | [112] |
| 4-Hydroxypanduratin A “[(1R,2S,6R)-3-methyl-2-(3-methylbut-2-enyl)-6-phenylcyclohex-3-en-1-yl]-(2,4,6-trihydroxyphenyl)methanone” | DEN-2 | In vitro | Ki: 21 μM | Competitive inhibition of NS3 protease | [112] |
| Panduratin A “(2,6-dihydroxy-4-methoxyphenyl)-[(1R,2S,6R)-3-methyl-2-(3-methylbut-2-enyl)-6-phenylcyclohex-3-en-1-yl]methanone” | DEN-2 | In vitro | Ki: 25 μM | Competitive inhibition of NS3 protease | [112] |
Dengue virus (DENV), Ki: inhibitory constant.
3.7. Chalcones and human cytomegalovirus (HCMV)
Human herpesvirus 5, generally referred to as HCMV, is the prototype member of the Betaherpesvirinae family. It maintains latency and lives for the duration of the person's life, similar to all herpesviruses. Over 60% of individuals in industrialized regions and over 90% in many underdeveloped nations have specific IgG antibodies. Individuals from lower socioeconomic classes and those of non-Caucasian descent are more likely to develop this infection [113]. Xanthohumol, which is one of the main components of the hop extract, was revealed to have potent antiviral activity against HCMV [114]. Moreover, it has been indicated that HCMV causes serum- or density-arrested human lung (LU) cells to progress through the cell cycle, enabling it to propagate in cells that have completed their mitotic cycle, which serve as its in vivo cellular substrates. In addition, infection with HCMV increases p53 levels, which appears to contradict the detected cell cycle advancement. It was shown that the ubiquitination of p53 was suppressed by a chalcone derivative known as trans-4-iodo, 4-boranylchalcone [115]. Moreover, an aryl/heteroaryl-derived thienyl chalcone was proved to exert strong inhibitory effects against the D169 strain of HCMV (EC50: <0.05 μM) as compared with the standard treatment with ganciclovir (EC50: 0.12 μM) [116] (Table 7)
Table 7.
Chalcone derivatives investigated against human cytomegalovirus (HCMV).
| Compound(s) “Chemical name(s)” | Virus subtype | Study model(s) | Results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| Xanthohumol “(E)-1-[2,4-dihydroxy-6-methoxy-3-(3-methylbut-2-enyl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one” | HCMV | In vitro | IC50: 2.5 ± 0.56 (μg/mL) | Downregulation of CXCR4 chemokine receptors | [114] |
| “Trans-4-iodo-4′-boranyl-chalcone” | HCMV | In vitro | A concentration-dependent decrease in the abundance of p53 ladders | Inhibition of ubiquitination of p53 | [115] |
| “Thienylchalcone (1-phenyl-3-(2-thiophen-2-ylphenyl)prop-2-en-1-one)” derivatives | HCMV | In vitro | EC50: <0.05 μM | Strong growth inhibitory activity towards three major cancers (colon, breast, and leukemia) | [116] |
HCMV: Human cytomegalovirus, EC50: Half maximal effective concentration, IC50: Half maximal inhibitory concentration.
3.8. Chalcones and hepatitis B virus (HBV)
HBV is a small DNA virus with retrovirus-like features belonging to the Hepadnaviridae family [117]. HBV replication is mediated by an RNA intermediary and it can incorporate the genetic material of the host. The distinct properties of the HBV replication cycle allow it to survive in infected tissue. HBV infection causes various hepatic disorders, from acute (including fulminant hepatic failure) to chronic hepatitis, cirrhosis, and hepatocellular cancer [118]. HBV is classified into eight genotypes, A through H, based on sequence comparison. Each of these genotypes has a unique geographical distribution. Electron microscopy has revealed three different types of viral remnants in infected sera. Two of these remnants are smaller components with a spherical shape, which are 20 nm in diameter, and filaments of different lengths, which are 22 nm in width. These particles are nonpathogenic because they are comprised of host-derived lipids and hepatitis B surface antigen (HBsAg) rather than viral nucleic acids [119]. In a study by Mathayan et al. two chalcone-based compounds, i.e., glabaarachalcone and isopongachromene, were extracted from an Indian medicinal herb called P. pinnata. The anti-HBV properties of these molecules were investigated via in silico screening, and it was indicated that these derivatives were potential ligands for the DNA polymerase of HBV [120].
3.9. Chalcones and hepatitis C virus (HCV)
HCV is a blood-borne infectious agent. HCV infection affects around 120–130 million individuals or 3% of the global populace. According to WHO, approximately 3–4 million new cases are reported each year [121]. HCV is regarded as a serious public health concern because the virus causes chronic hepatitis, which usually progresses to cirrhosis and hepatocellular cancer (HCC) [122]. From a clinical and economic standpoint, the discovery of alternative and/or complementary medications to treat HCV infection is still urgently required. Licochalcone A and isoliquiritigenin are two chalcone derivatives isolated from medicinal plants belonging to Glycyrrhiza species, which were shown to be active against HCV (IC50: 2.5 and 3.7 μg/mL, respectively). According to time-of-addition investigations, these compounds mainly function after the entry stage. Isoliquiritigenin was reported to impede the propagation of an HCV subgenomic RNA replicon in vitro [123]. The antiviral effects of xanthohumol, a prenylated chalcone isolated from hops, on HCV infection in Tupaia belangeri, which is an established HCV infection model, were investigated. The results revealed that xanthohumol can efficiently attenuate hepatic inflammation, steatosis, and fibrosis caused by HCV in Tupaias, mostly by inhibiting oxidative reactions, regulating cell death, and possibly suppressing the activation of hepatic stellate cells [124]. Table 8, Table 9 demonstrate the effects of chalcones on HBV and HCV, respectively.
Table 8.
Chalcone derivatives investigated against HBV.
| Compound(s) “Chemical name(s)” | Virus subtype | Study model(s) | Results | Inhibition mechanism | Ref. |
|---|---|---|---|---|---|
| Glabaarachalcone and Isopongachromene “2-(1,3-benzodioxol-5-yl)-6-methoxy-8,8-dimethylpyrano[2,3-h]chromen-4-one” | HBV | In vitro, In silico | Docking scores: 9.523 and −9.094 against HBV DNA polymerase, respectively | Binding to the DNA polymerase of HBV | [120] |
HBV: Hepatitis B virus.
Table 9.
Chalcone derivatives investigated against HCV.
| Compound(s) “Chemical name(s)” | Virus subtype | Study model(s) | Results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| Licochalcone A “(E)-3-[4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-yl)phenyl]-1-(4-hydroxyphenyl)prop-2-en-1-one” | HCV genotype 2a (J6/JFH1P47 | In vitro | IC50: 2.5 mg/mL |
Inhibition of the post-entry step | [123] |
| Isoliquiritigenin “(E)-1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one” | HCV genotype 2a (J6/JFH1P47 | In vitro | IC50: 3.7 mg/mL |
Inhibition of the post-entry step by preventing the HCV subgenomic RNA propagation | [123] |
| Xanthohumol “(E)-1-[2,4-dihydroxy-6-methoxy-3-(3-methylbut-2-enyl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one” | HCV genotype 1b | In vivo | Significantly reduced transforming growth factor β1 expression, hepatic steatosis score, aminotransferase levels, and histological activity index in liver tissue | Modulation of apoptosis, suppression of oxidative response, and regulation of MTP function | [124] |
HCV: hepatitis C virus, IC50: Half maximal inhibitory concentration, MTP: Microsomal triglyceride transfer protein.
4. Antibacterial activity
The antibacterial effects of natural chalcones have been extensively studied in the literature. In one study, 28 novel thiazole-based chalcones were prepared and their antibacterial activities against several bacterial species were investigated. All these derivatives exerted antibacterial effects against three resistant strains, including P. aeruginosa, methicillin-resistant S. aureus (MRSA), and E. coli. Moreover, docking studies were conducted and the results revealed that this antibacterial activity might be mediated by the inhibition of DNA gyrase, GyrB, and MurA [125]. Four synthetic chalcones, namely 3-(4-trifluoromethylphenyl)-1-(2-hydroxyphenyl)-2-propen-1-on (p-CF3), 3-(2,6-dimethoxyphenyl)-1-(2-hydroxyphenyl)-2-propen-1-on (2,6-OCH3), 3-(2-methoxyphenyl)-1-(2-hydroxyphenyl)-2-propen-1-on (o-OCH3), and 3-(4-fluoro-2-methylphenyl)-1-(2-hydroxyphenyl)-2-propen-1-on (p-F-o-CH3), were found to have antibacterial effects against two multiresistant bacterial strains including A. baumannii and P. aeruginosa, which were extracted from patients admitted to hospital (MICs = 100–175 μg/mL). Additionally, the effects of these chalcone derivatives on the expression of several virulence factors in A. baumannii (surface-related motion and twitching) and P. aeruginosa (pyocyanin synthesis, swimming and swarming motion), as well as their biofilm production, were investigated. Regarding the structures of these chalcones, it is commonly acknowledged that the α, β-unsaturated ketone group is necessary for antimicrobial activities. Furthermore, the authors concluded that the antimicrobial properties were supported by methoxy and halogen groups [126]. The treatment of staphylococcal infections using conventional medications, e.g., penicillin and cephalosporin, is no longer effective due to the advent and dissemination of multidrug-resistant strains [127]. Despite its efficacy against multidrug-resistant forms of Staphylococcus aureus, vancomycin has certain disadvantages, including toxicity and insufficient uptake [128]. The quest for novel drugs was prompted by the persistent development of bacterial resistance, which has become a critical issue in contemporary medicine. One of the most common pathways in multiresistant bacteria is resistance mediated by efflux pumps, which has prompted researchers to seek potential efflux pump inhibitors [129]. Therefore, developing new chemicals that are readily available is an effective approach to antimicrobial treatment.
Six 2′-hydroxychalcones were synthesized using the Claisen-Schmidt condensation reaction, and their effects were investigated alone and combined with several antibiotics against a number of multiresistant S. aureus strains including RN4220, K4414, 1199B, and K2068. In vitro and in silico studies indicated that these synthetic chalcones could suppress the NorA efflux pump [130], which is implicated in resistance to antiseptics, biocides, quaternary ammonium compounds, dyes, and fluoroquinolones (e.g., ciprofloxacin) [131]. These findings suggest that the addition of a furanic ring (A1), a chlorine atom, and a methoxy group at the C4 position (A2 and A4), as well as a second double bond (A5), and a fluorine atom at the C2 position (A6) had an impact on the synergistic effects shown by the prepared chalcones [130]. A number of azidosulfonamide-chalcones were synthesized and their antimicrobial effects were shown against several Gram-negative and Gram-positive bacteria, namely S. aureus, M. luteus, S. marcens, K. pneumonia, and E. coli [132]. It has been established that compounds with the sulfonamide group (sulfa drugs, –SO2NH2–) exhibit a wide range of biological effects, including anti-inflammatory, anticancer, and antimicrobial activities. Moreover, as previously mentioned, chalcones have been effectively employed as potential anti-infective compounds. The antibacterial effects of sulfonamides are related to their ability to target the dihydropteroate synthase enzyme (DHPS), which acts as a catalyst for the condensation of 6-(hydroxymethyl)-7,8-dihydropterin-pyrophosphate (DHPPP) and p-aminobenzoic acid (PABA) into dihydropteroate (DHPt). Therefore, the formation of dihydrofolic acid is prevented, which leads to the inhibition of the growth and proliferation of microorganisms [133]. In another study, the antimicrobial activity of five pyrazole-based adamantyl chalcones against Gram-negative and Gram-positive bacteria was shown with inhibition zone diameters (IZDs) between 8.3 and 15.3 [37]. The antibacterial effect of four synthesized chalcones including (E)-3-(4-fluorophenyl)-1-(2-hydroxyphenyl)prop-2en-1-one, (E)-1-(2-hydroxyphenyl)-3-(4-ethoxyphenyl)prop-2en-1-one, (E)-3-(4-(dimethylamino)phenyl)-1-(2-hydroxyphenyl)prop-2-en1-one, and (E)-1-(2-hydroxyphenyl)-3-(thiophen-2-yl)prop-2-en-1-one was shown against Gram-negative E. coli ATCC 25922, Gram-positive S. aureus ATCC 25923, and the S. aureus 11999-B strain overexpressing the norA gene that encodes the NorA efflux pump. Moreover, it was reported that these compounds could reduce the resistance of the SA1199B strain to norfloxacin, which was possibly mediated by the inhibition of the NorA reflux [134]. In addition, the antibacterial effects of norfloxacin against the SA1199B strain were shown to be improved by four chalcone derivatives, namely 4′-hydroxy-3-4-dimethoxy-chalcone, 3′-hydroxy-3-acetate, 4-methoxy-chalcone, 3′,4′-dihydroxy, 3,4,4′-trimethoxy-chalcone, and 3,4-dimethoxy-chalcone, which were extracted from Arrabidaea brachypoda (Bignoniaceae) flowers. According to molecular docking studies, these chalcones could bind to the hydrophobic pockets of MepA and NorA at the same binding sites as norfloxacin, suggesting that they could compete for these binding sites with this antibiotic. It was concluded that combining chalcone derivatives with norfloxacin could be an effective strategy to combat multidrug-resistant S. aureus over-producing MepA or NorA [135]. Several thiazolyl chalcones were prepared based on the Claisen–Schmidt condensation reaction of 5-acetyl-4-methyl-2-(3-pyridyl) thiazole with different heterocyclic aldehydes in alkali media. These chalcone derivatives were evaluated for antibacterial activity with good results. Among these derivatives, 2-thienylchalcone exhibited the highest activity against P. aeruginosa [136]. Several synthetic chalcones containing trifluoromethyl and trifluoromethoxy substituents showed varying degrees of antibacterial activity against Gram-positive (S. aureus and B. subtilis) and Gram-negative (E. coli and B. subtilis) bacteria [137]. Five fluorinated chalcones were prepared using the required aromatic aldehydes via a revised Claisen-Schmidt condensation of 4-fluoro-2-hydroxyacetophenone. The antibacterial effects of these compounds were investigated against Gram-negative (S. typhi, E. coli, C. ulcerans, P. aeruginosa, and P. mirabilis) and Gram-positive (Vancomycin-resistant enterococci, S. aureus, methicillin-resistant S. aureus, S. pyrogenes, and S. faecalis) bacteria utilizing the agar diffusion approach. It was reported that these chalcones showed broad-spectrum effects against eight of the bacteria, with the most potent molecules showing zones of inhibition between 23 and 28 mm and MICs ranging from 25 to 50 μg/mL [138]. Other studies are listed in Table 10.
Table 10.
The antibacterial activity of chalcones.
| Compound(s) “Chemical name(s)” | Bacterial species | Study model(s) | Results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| Thiazole-based chalcones “(E)-3-(2-chloro-6-fluorophenylo-1-(2-(ethylamino)- 4–2.1.4.methylthiazol-5-yl)prop-2-en-1-on” | Pseudomonas aeruginosa, MRSA, and Escherichia coli | In vitro, In silico |
MIC: 0.65, 1, 1.3 μmol/mL × 10−2 against E. coli, P. aeruginosa, and MRSA, respectively | Inhibition of DNA gyrase, GyrB, and MurA | [125] |
| “3-(4-Trifluoromethylphenyl)-1-(2-hydroxyphenyl)-2-propen-1-on” “3-(2,6-dimethoxyphenyl)-1-(2-hydroxyphenyl)-2-propen-1-on” “3-(2-methoxyphenyl)-1-(2-hydroxyphenyl)-2-propen-1-on” “3-(4-fluoro-2-methylphenyl)-1-(2-hydroxyphenyl)-2-propen-1-on” |
P. aeruginosa and Acinetobacter baumannii | In vitro | MICs: 100–175 μg/mL | Supported by methoxy and halogen groups | [126] |
| 2′-Hydroxychalcones “(E)-3-(2-hydroxyphenyl)-1-phenylprop-2-en-1-one” | MRSA strains including RN4220, K4414, 1199B, and K2068 | In vitro, In silico |
Binding energies: 6.4, −7.4, −7.0, −7.2, −7.5, and −7.2 kcal/mol |
Inhibition of the NorA efflux pump | [130] |
| Azidosulfonamide chalcones “N-[4-[3-(4-Bromophenyl)acryloyl]phenyl]-4-azidobenzene- sulfonamide” | S. aureus, Micrococcus luteus, Serratia marcescens, Klebsiella pneumoniae, and E. coli | In vitro, In silico |
IZDs: 33 mm and MIC: 1.5 μg/cm3 against S. aureus; IZD: 29 mm and MIC: 1.5 μg/cm3 against M. luteus (most active compound) |
Possible inhibition of the microbial DHPS enzyme | [132] |
| Pyrazole-based adamantyl chalcones “2-(3-((1S,3S)-adamatan-1-yl)-1-(2,4-dinitrophenyl)-4,5-dihydro-1H-pyrazol-5-yl)pyridine” |
P. aeruginosa, E. coli, S. aureus, K. pneumonia, Salmonella typhimurium, and Bacillus subtilis | In vitro | IZDs: 15.3 mm against K. pneumonia (most active compound) | N/A | [37] |
| “(E)-3-(4-fluorophenyl)-1-(2-hydroxyphenyl)prop-2en-1-one” “(E)-1-(2-hydroxyphenyl)-3-(4-ethoxyphenyl)prop-2en-1-one” “(E)-3-(4-(dimethylamino)phenyl)-1-(2-hydroxyphenyl)prop-2-en1-one” “(E)-1-(2-hydroxyphenyl)-3-(thiophen-2-yl)prop-2-en-1-one” |
E. coli ATCC 25922, S. aureus ATCC 25923, and the S. aureus 11999-B strain overexpressing the norA gene | In vitro, In silico |
Significantly reduced the MICs of norfloxacin from 64 to 8 μg/mL | Reduced the resistance to norfloxacin by inhibiting the NorA reflux | [134] |
| 4′-Hydroxy-3-4-dimethoxy-chalcone, 3′-hydroxy-3-acetate, 4-methoxy-chalcone, 3′,4′-dihydroxy, 3,4,4′-trimethoxy-chalcone, and 3,4-dimethoxy-chalcone | SA1199B strain | In vitro | Strengthened the effects of norfloxacin and EtBr against the SA1199-B (norA) strain | Competes with norfloxacin for similar MepA and NorA binding sites | [135] |
| Thiazolyl chalcones “3-(5-[4,5-Dihydro-5-(1H-pyrrol-2-yl)-1H-pyrazol3-yl]-4-methylthiazol-2-yl)pyridine” “4-(4,5-Dihydro-3-[4-methyl-2-(pyridin-3-yl)thiazol-5-yl]-1H-pyrazol-5-yl)pyridine” |
B. subtilis, Shigella flexneri | In vitro | MIC: 0.48 μg/Ml (most active compound) | Results indicate that the effect of pyrazolines derivatives on Gram negative bacterial activity is attributed to its NH group which can act as a hydrogen donor to the target receptor | [136] |
| Trifluoromethyl- and trifluoromethoxy-substituted chalcones “(E)-3-(1”H-indol-3″-yl)-1-[40 -(trifluoromethoxy)phenyl]prop-2-en-1-one” “(E)-3-(1”H-indol-3″-yl)-1-[40 -(trifluoromethyl)phenyl]prop-2-en-1-one” |
S. aureus, B. subtilis, E. coli, and, Proteus vulgaris | In vitro | MIC: 24 μM against B. subtilis and E. coli, MIC: 25 μM against E. coli and P. vulgaris, respectively | N/A | [137] |
| Fluorinated chalcones “(E)-3-(2′-ethoxyphenyl)-1-(4-Fluoro-2-hydroxyphenyl) prop-2-en-1-one” “(E)-3-(3′,4′-diethoxyphenyl)-1-(4-fuoro-2-hydroxyl-ph enyl)prop-2-en-1-one” “(E)-3-(3′,5′-Bis[trifuoromethyl]phenyl)-1-(4-Fluoro- 2-hydroxylphenyl)prop-2-en-1-one” |
MRSA, S. aureus, E. coli, S. pyrogenes, Vancomycin-resistant enterococci, Enterococcus faecalis, Salmonella typhi, Proteus mirabilis, Pseudomonas aeruginosa, Corynebacterium ulcerans | In vitro | IZD: 28 mm against E. coli; 28 mm against MRSA; 28 mm against S. pyrogenes | The electron-releasing group on the B-ring and the fluorine atom on the A-ring enable easy penetration into the negatively charged bacterial wall. | [138] |
IZD: Inhibition zone diameter, MRSA: Methicillin-resistant Staphylococcus aureus, MIC: Minimal inhibitory concentration.
The antibacterial mechanisms of chalcones have been previously reported. These mechanisms include the inhibition of fatty acid synthesis, efflux pumps, DNA replication (DNA gyrase), filamentous temperature-sensitive mutant Z (FtsZ), virulence factors, and protein tyrosine phosphatase, resulting in the inhibition of bacterial division and eventually bactericidal effects. A schematic representation is adapted from a review study conducted by Dhaliwal et al. (Fig. 5).
Fig. 5.
Antibacterial mechanisms of chalcone and its derivatives. Reproduced with permission from Ref. [139].
5. Antifungal activity
The prevalence of infectious diseases caused by fungi appears to be globally on the rise. This may partly be due to the use of immune-suppressing treatments, namely hematopoietic stem cell transplants, and medications such as tissue necrosis factor antagonists, which increase the number of individuals who are predisposed to otherwise uncommon fungal diseases [140]. This prompted researchers to seek novel molecular targets for new antifungal agents. In recent years, numerous antifungal medications have been created; however, it is still challenging to treat certain patients [141]. The primary cause of this issue is intrinsic or acquired antifungal resistance. In addition, several recently created antifungal drugs lead to adverse effects. Consequently, there is an ongoing need for novel antifungal agents that can selectively target the fungi without impairing the host's biochemical processes [140,142].
In an effort to discover effective antifungal drugs, a number of chalcones and new dihydrochromane-chalcone derivatives were developed and characterized using spectroscopic techniques. The activity of these molecules was assessed in vitro against the phytopathogens B. cinerea and M. fructicola, which influence a variety of commercially important plants. Several of the synthetic derivatives were shown to be active against B. cinerea and M. fructicola. In order to investigate the mode of action of these compounds, structure-activity models were developed and studied. The results revealed that in the case of B. cinerea, this inhibitory effect was mediated by the atomic charge on C5 and the hydrogen bonding acceptor and donor. However, the inhibitory activity against M. fructicola was mediated by the dipole moment and the carbonyl carbon and the atomic charge on C1′ [143]. In another study, eight chalcones were designed using 2-hydroxy-3,4,6-trimethoxyacetophenone extracted from Croton anisodontus, and their antifungal activity was examined in vitro. The chalcone derivatives (E)-1-(2-hydroxy-3,4,6- trimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one and (E)-3-(furan-2-yl)-1-(2-hydroxy3,4,6-trimethoxyphenyl)prop-2-en-1-one were shown to be active against C. albicans LABMIC 0107 (MIC: 0.31 mg/mL) and C. albicans LABMIC 0105 (MIC: 0.62 mg/mL), respectively. Moreover, the combination of amphotericin B and the chalcone derivative (E)-1-(2-hydroxy-3,4,6-trimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one was reported to exhibit synergistic effects against C. albicans LABMIC 0105 (IFIC = 0.124) [144]. A different study reported the fungicidal impact of 2-hydroxychalcone encapsulated in a nanoemulsion on Paracoccidioides brasiliensis and Paracoccidioides lutzii, which are the causative agents of paracoccidioidomycosis [145]. Azulene-containing chalcones were developed by Bala via the Claisen–Schmidt condensation reaction and showed strong inhibitory effects on Candida parapsilosis (MIC: 0.156–0.312 mg/mL). The chalcone derivative with azulene groups on either side of the 2-propene-1-one bond was the most active compound and showed good antifungal activity [146]. Synthesized polyoxygenated chalcones were shown to be active against the clinically significant yeasts C. neoformans (ATCC 32264) and C. albicans (ATCC 10231) [147]. 2,3-Dihydroxy-chalcone (DHC) demonstrated inhibitory properties against clinical isolates of C. albicans that produce phospholipase (MIC: 16.24–130.16 μM and MFC: 260.32–1041.31 μM) [148]. Ten chalcone derivatives synthesized from 3′-methoxy-4′-hydroxyacetophenone and p-aminoacetophenone were tested against four Trichophyton rubrum strains, namely CEMM 0201, CEMM 0202, LABMIC 0203, and LABMIC 0204, and showed good antifungal activity (MIC = 0.015–1.25 μg/mL) [149]. Synthetic pyrrole chalcone derivatives were examined for their in vitro fungicidal effects on Aspergillus niger and showed acceptable outcomes (zones of inhibition between 10 and 24 mm) [150]. In another study, several difluoro phenyl pyrazole chalcone-based compounds were prepared and their activities against human pathogenic fungi were evaluated in vitro. It was indicated that a number of these compounds displayed promising inhibitory effects (MICs of 25 and 50 μg/mL) [151]. In a different study, ferrocenyl chalcone and organic chalcone were used as intermediates to synthesize sulfones and bis-sulfones and their fungicidal activities were investigated. Among these compounds, the ferrocenyl chalcone-based dimethyl-substituted derivative displayed the strongest antifungal properties [152]. Five chalcones were prepared from 2-hydroxyacetophenone with substituted benzaldehydes, including (E)-3-(2′-ethoxyphenyl), (E)-3-(4′-diethylaminophenyl), (E)-3-(2′,3′-dihydrobenzofuran-5-yl), (E)-3-(3′,4′-diethoxyphenyl), and (E)-3-(3′,5′-bis[trifluoromethyl]phenyl)-1-(2-hydroxyphenyl) prop-2-en-1-one. The inhibitory properties of these molecules were examined against several candida species utilizing the agar diffusion technique. All the derivatives were active against the candida species to varying degrees while those with the 2′-ethoxyphenyl and 4′-(diethylamino)phenyl moieties were the most effective against the fungi with MICs ranging from 25 to 50 μg/mL [138] (Table 11).
Table 11.
The antifungal activity of chalcones.
| Compound(s) “Chemical name(s)” | Fungal species | Study model(s) | Results | Inhibition mechanism | Refs. | |
|---|---|---|---|---|---|---|
| Dihydrochromane–chalcone derivatives “(2E)-1,3-diphenylprop-2-en-1-one” |
Botrytis cinerea and Monilinia fructicola | In vitro | IC50 against B. cinerea: 43.9 and against M. fructicola: 48.5 μg/mL | Mycelial growth inhibition (for B. cinerea) modulated by the atomic charge on C5 and the hydrogen bonding acceptor and donor. Modification of the inhibitory properties (for M. fructicola) via dipole moment and the carbonyl carbon and the atomic charge on C1′. | [143] | |
| Chalcones synthesized from natural acetophenone “(E)-3-(furan-2-yl)-1-(2-hydroxy3,4,6-trimethoxyphenyl)prop-2-en-1-one” “(E)-1-(2-hydroxy-3,4,6- trimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one” |
Candida albicans LABMIC 0105 and C. albicans LABMIC 0107 | In vitro | MIC: 0.62 and 0.31 mg/mL, respectively | Similar to the mode of action of amphotericin B. | [144] | |
| 2-Hydroxychalcone “(E)-3-(2-hydroxyphenyl)-1-phenylprop-2-en-1-one” | Paracoccidioides brasiliensis and Paracoccidioides lutzii | In vitro | MIC: 0.06 and 0.12 μg/mL, respectively | Reaction between the internal stage of the formulation and lipid membrane molecules such as ergosterol, destabilizing fungi integrity, and the disruption of the fungal membrane leading to its death. | [145] | |
| Azulene-containing chalcone “(E)-1,3-Di(azulen-1-yl)prop-2-en-1-one” | Candida parapsilosis | In vitro | MIC: 0.156 mg/mL | N/A | [146] | |
| Polyoxygenated chalcones “1-Phenyl-3-(4-hydroxy-3-methoxyphenyl)-prop-2-en-1-one” “1-(2,5-Dimethoxyphenyl)-3-phenyl-prop-2-en-1-one” |
C. albicans, Cryptococcus neoformans | In vitro | IC50: 50 and 125 μg/mL against C. albicans; 15.6 and 7.8 μg/mL against C. neoformans, respectively | Anticryptococcal activity mediated by the methylation of the 3-OH of the B ring and the substitution pattern of the A ring. | [147] | |
| “(E) 2,3-dyhydroxy-chalcone” | C. albicans isolates 1-9 | In vitro | MIC: 16.24 for isolate 1 and MFC: 32.54 μM for isolate 1 | N/A | [148] | |
| “(2E)-1-(4′-aminophenyl)-3-(phenyl)-prop-2- en-1-one” | Trichophyton rubrum (LAMBIC 0208) | In vitro | MIC: 0.07 and MFC: 0.015–1.25 μg/mL | Inhibition activity mediated by the phenyl group and electron-withdrawing groups such as fluorine and chlorine, the existence of a heterocyclic ring, hydroxy and methoxy groups on ring A, and the nitro group on ring B. | [149] | |
| Ferrocenyl chalcone-based dimethyl-substituted derivative “1-Ferrocenyl-3-(3,4dimethylphenyl)-3- (phenylsulfonyl)propan-1-one” | Aspergillus niger, C. albicans, Aspergillus fumigatus, Candida tropicalis, Cryptococcus neoformans, and C. parapsilosis | In vitro | MIC: 3.9, 7.8, 10.5, 3.9, 7.81, and 15.5 μg/mL, respectively | N/A | [152] | |
| “(E)-3-(4′-diethylaminophenyl)” “(E)-3-(2′-ethoxyphenyl)” “(E)-3-(3′,4′-diethoxyphenyl)” “(E)-3-(2′,3′-dihydrobenzofuran-5-yl)” “(E)-3-(3′,5′-bis[trifluoromethyl]phenyl)-1-(2-hydroxyphenyl) prop-2-en-1-one” |
C. albicans, C. tropicalis, Candida stellatoidea, Candida pseudotropicalis and Candida krusei | In vitro | MIC:25–50 μg/mL | Improvement of inhibitory activity by the 4′-(diethylamino) substituents, which is a potent electron-donating group. | [138] | |
MIC: Minimum Inhibitory, MFC: Fungicidal Concentrations, IC50: Half maximal inhibitory concentration.
6. Antiparasitic activity
Parasitic disorders, namely leishmaniasis and malaria, continue to be a serious worldwide health problem. In addition to being fatal, they have a substantially damaging effect on the quality of life of millions of individuals around the world [153]. Many of the currently accessible medications have certain drawbacks, including toxicity, disputable efficiency, and prolonged treatment periods, which lead to resistance. Therefore, researchers have focused on developing new therapeutic agents that are safe and effective [154]. Due to the lack of a vaccine and the challenges associated with establishing a vector control system, existing antiparasitic management strategies have centered on chemotherapy procedures. The demand for novel pharmacological approaches for managing parasitic disorders stems from a variety of issues, such as the scarcity of medications available, their decreased efficacy owing to microbial resistance, prolonged therapies, and substantial toxicity [155]. As a result, continuous research into biologically active chemicals to combat these pathogens remains critical. A number of natural compounds have been previously introduced in the search for antiparasitic drugs. Chalcone‐type molecules, in particular, were active against Leishmania species, P. falciparum and T. cruzi [153].
Two chalcones, namely 2′,5′-dimethoxy-4′,6′-dihydroxychalcone and 2′,4′-dimethoxy-6′-hydroxychalcone were isolated from Polygonum salicifolium, which is found in Iraq. These compounds exhibited antitrypanosomal and antileishmanial properties, with 2′,4′-dimethoxy-6′-hydroxychalcone showing the strongest activity against T. congolense, Trypanosoma brucei brucei, and Leishmania mexicana (EC50 of 2.5, 0.5, and 5.2 μg/mL, respectively) with no toxicity toward a human cell line [156]. The antileishmanial effects of 25 synthetic prenylated chalcones were investigated and these compounds were reported to display good activity against Leishmania mexicana. Eleven derivatives showed a metabolic inhibition of at least 50% and three of the most potent ones had IC50 values less than 10 μM. According to docking studies, prenylated chalcones may affect fumarate reductase activity by binding with high affinity to two binding sites that are essential for the target [157]. In another work, six chalcone derivatives were designed and developed using a drug-food-homologous chalcone skeleton, and nearly half of the compounds demonstrated effective anti-Toxoplasma activity in vitro. Among these, four compounds displayed strong activity against T. gondii and minimal cytotoxicity in vitro. Moreover, the proliferation of Toxoplasma tachyzoites was inhibited by three of these derivatives in vivo [158]. Three types of chalcones were designed with substituents H, OH, or NH2 at the C‐2′ position in ring A and substituents with electron‐releasing and electron‐withdrawing characteristics, such as OCH3, NO2, and halogens (Cl, F), in ring B. The antiparasitic effects of these compounds against Trypanosoma cruzi, Leishmania braziliensis, and Plasmodium falciparum were evaluated, and they exhibited structure‐dependent activity against L. braziliensis. Only one derivative displayed strong effects against T. cruzi, and none of the molecules showed considerable antiplasmodial activity. The antiparasitic activity might be mediated by the hydrogen bonds at C‐2′ with carbonyl and the electron‐donating substituents in ring B [153]. A set of chalcone-thiosemicarbazones were prepared and their effects were examined against axenic amastigotes, promastigotes, and intracellular amastigotes of Leishmania amazonensis. The IC50 value of the most effective derivative against L. amazonensis promastigotes was reported to be 5.22 ± 0.75 μM, whereas the IC50 value of pentamidine, as the positive control, was 4.90 ± 0.60 μM. Several spectroscopic approaches combined with molecular docking were used for the early pharmacokinetic assessment of the most effective chalcone derivative via its interaction with human serum albumin (HSA). The results demonstrated that chalcone-thiosemicarbazone compounds were novel prototypes, which could be used for developing anti-leishmaniasis drugs [159]. One study indicated a strong interaction between several chalcone derivatives, including (E)-1-(4-aminophenyl)-3-(4-methoxyphenyl)-prop-2-en-1-one, (E)-1-(4-aminophenyl)-3-phenylprop-2-en-1-one, (E)-1-(4-aminophenyl)-3-(4ethoxyphenyl)-prop-2-en-1-one, and the Leishmania major receptor using in silico assays [160]. In a different study, 12 chalcone and benzimidazolyl-arylpropenone hybrids were developed and tested against promastigotes of Leishmania donovani, which leads to visceral leishmaniasis, the most severe form of leishmaniasis. Among these compounds, seven derivatives exhibited stronger activity against L. donovani compared with pentamidine (the reference drug) with IC50 values between 0.5 and 1.8 μM [161]. The in-silico inhibitory effects of a series of chalcone-quinoline conjugates against cruzipain, which is a key cysteine protease in T. cruzi, were investigated via molecular docking. It was reported that two of the most active compounds, i.e., (E)-1-(3,4-dimethoxyphenyl)-3-(4-((9-(quinolin-8-yloxy)nonyl)oxy)phenyl)prop-2- en-1-one (1e) and (E)-3-(furan-2-yl)-1-(4-((5-(quinolin-8-yloxy) pentyl)oxy)phenyl)prop-2-en-1-one (2c), exhibited the highest scores in docking studies (7.3 and 7.4 kcal/mol, respectively). These findings indicated that the chalcone-quinoline derivatives exert their antiparasitic activity through the inhibition of cruzipain [162]. The addition of chloride substituents to 2-hydroxy-3,4,6-trimethoxyphenylchalcones was shown to decrease the toxicity of the host cell against these compounds without impairing their inhibitory effects on the Trypanosoma cruzi Y strain forms [163]. The antiparasitic effect of chalcones and three of their amino analogs was evaluated against Trichomonas vaginalis, the causative agent of a non-viral illness known as Trichomoniasis, which is transmitted through sexual contact. Among these derivatives, 3′-aminochalcone had the strongest activity and displayed elevated cytotoxicity toward human vaginal cells. After the treatment of trophozoites of T. vaginalis with this compound, no considerable accumulation of reactive oxygen species (ROS) was observed; however, after co-incubation, it significantly increased ROS accumulation in human neutrophils [164]. The ultra-structural alterations as a result of the activity of chalcones and their derivatives in various Plasmodium falciparum organelles were investigated in vitro in order to clarify the mode of action of the parasite. RBCs infected with CQ-resistant (RKL-9) and CQ-sensitive (MRC-2) Plasmodium isolates were subjected to treatment with three chalcones and standard medications (artemisinin and CQ) for 24 h. The RBCs were then collected, rinsed, fixed, embedded, and stained to examine the ultra-structural alterations pre- and post-intervention using a transmission electron microscope. The results revealed that all of the membranes of the parasite, including the membranes of the nucleus, food vacuole, and mitochondria, were significantly disrupted. Additionally, there was a notable decrease in the number of ribosomes in the trophozoites and the development of schizonts was prevented, which indicates possible modes of action by which chalcones affect the malaria parasite [165]. The anti-chagasic properties of the synthesized chalcone CPN2F were investigated using in vitro experiments and in silico structural classification, ADMET analyses, and molecular docking. The in vitro assays revealed that the protozoan metabolism of host cells (LLC-MK2) had decreased. The molecular docking simulations assessed this decrease via the synergistic impact related to benznidazole against key therapeutic targets (cruzipain, trypanothione reductase, and T. cruzi glyceraldehyde-3-phosphate dehydrogenase (TcGAPDH)) in the development cycle of Trypanosoma cruzi. The in silico results demonstrated an association between pharmacokinetic features, e.g., renal uptake and release, which suggests that CPN2F has the potential to be used as an antichagasic medication with minimal organic toxicity [166]. Chalcone-based compounds with hydroxy and methoxy substitution on the aromatic ring A have been shown to exhibit potential inhibitory effects against leishmania and trypanosome strains [167,168]. Furthermore, heterocyclic components in the B ring have been used to develop antimalarial compounds [169]. In another study, the structure-activity relationships (SAR) of lophirone E, a natural chalcone with a benzofuran B-ring, and its analogs were evaluated. Accordingly, an efficient synthesis technique was suggested for the development of lophirone E as well as the synthesis of a number of chalcone derivatives with various substituents in the A and heterocyclic B rings. The resultant molecules were screened for their impact against the promastigotes of Leishmania infantum (IC50 values of the most active compounds were reported to be 15.3, 27.2, and 15.9 μM) [170]. The antileishmanial effects of 12 benzimidazolylchalcones against promastigote strains of L. donovani were investigated using molecular docking, quantitative structure-activity relationship (QSAR) analyses, and ADME prediction models. Binding free energies were calculated using MM-GBSA to determine the affinity of the ligands for the proteins. Moreover, the three most active derivatives were docked with Leishmania phosphodiesterase B1 (PDB ID: 2JK6). Docking results indicated that the most active compound formed hydrogen bonds with SER 464, pi-cation contact with LYS 61, and hydrophobic interactions with LEU 62, TYR 64, and LEU72 of the active site of L. donovani phosphodiesterase B1. According to the ADME results, all three compounds have favorable pharmacokinetic characteristics, suggesting that benzimidazolylchalcones can be used as potential antileishmanial drugs [171] (Table 12).
Table 12.
The antiparasitic activity of chalcones.
| Compound(s) “Chemical name(s)” | Parasite species | Study model(s) | Results | Inhibition mechanism | Refs. |
|---|---|---|---|---|---|
| “(E)-1-(2-hydroxy-4-methoxyphenyl)-3-(2-methoxyphenyl)prop-2-en-1-one” | Trypanosoma brucei brucei, Trypanosoma congolense, and Leishmania Mexicana | In vitro | EC50: 0.5, 2.5, and 5.2 μg/mL, respectively | N/A | [156] |
| “(E)-1-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy) phenyl)-3-(3-nitrophenyl)prop-2-en-1-one” “(E)-1-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy) phenyl)-3-(3-(trifuoromethyl)phenyl)prop-2-en-1-one” “(E)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy) phenyl)-1-(2-(trifuoromethyl)phenyl)prop-2-en-1-one” |
L. mexicana | In vitro, In silico |
IC50 < 10 μM | Possibly modulates the activity of fumarate reductase by binding to two crucial binding sites for the target with good affinity. | [157] |
| “(E)-3-(2-bromophenyl)-1-(4-(isopropylamino)phenyl)prop2-en-1-one” “(E)-3-(2-bromophenyl)-1-(4-(ethylamino)phenyl)prop-2-en1-one” |
Toxoplasma gondii | In vitro, In vivo |
Significant anti-toxoplasma activity and reduction of biochemical variables as well as liver and spleen indices | Anti-Toxoplasma effects are enhanced by the Michael receptor found in chalcones' molecular skeleton. | [158] |
| Chalcone derivatives with substituents in the A and B rings “1‐(2‐Aminophenyl)‐3‐(3,4,5‐trimethoxyphenyl)prop‐2‐en‐1‐one” “1‐(2‐Aminophenyl)‐3‐(3,5‐dimethoxyphenyl)prop‐2‐en‐1‐one” “1‐(2‐Hydroxyphenyl)‐3‐(3,4,5‐trimethoxyphenyl)prop‐2‐en‐1‐one” |
Leishmania braziliensis, Trypanosoma cruzi, Plasmodium falciparum | In vitro | EC50: 5.7 against L. braziliensis; EC50 against T. cruzi: 8.1 μM; EC50 against P. falciparum: 59.2 μM | The antiparasitic properties were affected by the hydrogen bonds at C-2′ with carbonyl and the electron-donating substituents in ring B. | [153] |
| Chalcone-thiosemicarbazones “(1E,2E)-3-(phenyl)-1-phenylprop-2-en-1-one thiosemicarbazone” “(1E,2E)-3-(40 -chlorophenyl)-1-phenylprop-2-en-1-one thiosemicarbazone” |
Leishmania amazonensis | In vitro | IC50 against intracellular amastigotes: 3.40 μM, IC50 against Promastigotes: 5.22 μM | Anti-leishmanial properties were improved by moieties with electronic withdrawing effects. | [159] |
| “(E)-1-(4-aminophenyl)-3-phenylprop-2-en-1-one (C1)” “(E)-1-(4-aminophenyl)-3-(4-methoxyphenyl)-prop-2-en-1-one (C4)” “(E)-1-(4-aminophenyl)-3-(4ethoxyphenyl)-prop-2-en-1-one (C9)” |
Leishmania major | In silico | Strong interaction of C9 ligand with the Leishmania major receptor, particularly for the Tyr 217, His 219, and Phe 88 residues | N/A | [160] |
| Benzimidazolyl-chalcones “1-(5-chloro-1H-benzo[d]imidazole-2-yl)-3-(2- chlorophenyl)prop-2-en-1-one” | Leishmania donovani | In vitro | IC50: 0.47 μM (most active compound) | The antileishmanial activity could be affected by the C-5 group of benzimidazole, an electron-withdrawing group in the arylpropenone functional group, or the presence of a heterocycle or hydroxyl group in chalcones. | [161] |
| Chalcone-quinoline conjugates “(E)-3-(furan-2-yl)-1-(4-((5-(quinolin-8-yloxy) pentyl)oxy)phenyl)prop-2-en-1-one” | T. cruzi | In silico | Binding energy: 7.2 kcal/mol (most active compound) | Inhibition of cruzipain | [162] |
| Chloride substituted 2-hydroxy-3,4,6-trimethoxyphenylchalcones “(E)-3-(3,4-dimethoxyphenyl)-1-(2-hydroxy-6-methoxyphenyl)prop-2-en-1-one” | T. cruzi | In vitro, in silico | Reduction of the toxicity of chlorine-substituted molecules on host cells; reduction of intracellular amastigotes and infected cells. | Inhibitory effects are associated with a rise in cytoplasmic ROS, mitochondrial malfunction, necrotic events, as well as the activity of TcTR and TcCr enzymes. | [163] |
| Chalcone and its amino analogs | T. vaginalis | In vitro, In vivo | IC50: 29 μM; no toxicity against Galleria mellonella larvae. | ROS accumulation in human neutrophils induced by trophozoites treated with aminochalcone 3. | [164] |
| (E)-1-(2,5-Dimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one, (E)-(3,4,5-Trimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one, (E)-1-(3,4,5-Trimethoxyphenyl)-3-(3,4-dimethoxyphenyl)prop-2-en-1-one | P. falciparum | In vitro | Disruption of all parasite membranes, significant drop in ribosome content of trophozoites, and the cessation of schizont development. | The ultrastructural changes suggest multiple mechanisms of action for the activity of chalcone derivatives | [165] |
| Synthetic chalcone CPN2F “(2E)-3-(2-fluorophenyl)-1-(2-hydroxy- 3,4,6-trimethoxyphenyl)prop-2-en-1-one” |
T. cruzi | In vitro, In silico |
A decrease in the metabolism of protozoa in host cells | N/A | [166] |
| Lophirone E analogs “(E)-1-(2,4-Dihydroxyphenyl)-3-(2-(4-hydroxyphenyl)benzofuran-5-yl)prop-2-en-1-one” “(E)-1-(2,4-Dihydroxyphenyl)-3-(2-(4-hydroxyphenyl)-1-methyl-1H-indol-5-yl)prop-2-en-1-one” “(E)-3-(1-Benzyl-2-(4-hydroxyphenyl)-1H-indol-5-yl)-1-(2,4-dihydroxyphenyl)prop-2-en-1-one” |
Leishmania infantum | In vitro | IC50: 15.3, 27.2, and 15.9 μM, | A free OH at C2′ is essential for the activity of the compound. | [170] |
| Benzimidazolylchalcones | L. donovani | In silico, In vitro |
Binding energies (most active compounds): 6.50–6.24 kcal/mol; IC50 (most active compounds): 0.47, 0.50, and 0.53 μM. | electrophilic substituents such as halogens (Cl), nitro, or hydroxyl on the benzimidazolyl-chalcone raises the negativity of the electron affinity of the molecules, boosting their antileishmanial activities. | [171] |
EC50: Half maximal effective concentration, IC50: Half maximal inhibitory concentration.
7. Quantitative structure–activity relationships (QSAR) of chalcone derivatives
A structure-activity relationship (SAR) is a relation between a specific chemical substructure and its capacity to produce a particular biological effect. In a quantitative structure-activity relationship (QSAR), a mathematical model was used for establishing a quantitative link between the structural features of a chemical complex and its biological activity. This means that the relation between the structures and activity of molecules can be explained in a quantitative manner. With advancements in computer technology, it is now possible to estimate the molecular properties of compounds without synthesizing them. This allows the use of predictive computational models (in silico) to anticipate the biological properties of virtual compounds, aiding in the selection of target molecules for synthesis. By employing computational approaches to predict the activity of potential drug candidates before their synthesis, resources can be saved, and the drug discovery process can be accelerated. These computational models consider various chemical descriptors such as hydrophobicity, inductive, electronic, polar features, and steric impacts, which can be specified through empirical or calculated methods [172].
Several studies in the literature have focused on the design of new chalcone derivatives based on structure-activity relationship (SAR) principles. For instance, introducing methoxy or methyl groups to the phenyl ring has been shown to result in losing antibacterial action. SAR analysis has indicated the positive correlation between the antibacterial action and the alkyl chain length, with medium-length alkyl chains (n = 7), demonstrating higher activity compared to longer chains. Nevertheless, the antibacterial activity of cationic molecules can be different considerably due to differences in the hydrophobicity of the alkyl chain. Additionally, it was noted that the antibacterial activity reduces by increasing the spacer length. This decline in activity can be attributed to the affinity of long hydrophobic chains to aggregate, affecting the overall effectiveness of the compounds [173]. In the study conducted by Bheru et al. novel variations of chalcone structures were created by incorporating pyrazine and aromatic para-substituted aldehydes. These modified chalcones were evaluated for their potential biological activities, specifically their anticancer, antimicrobial, and antioxidant effects. The QSAR analysis results indicated that the substitution at the para position on the phenyl ring and alkyl substitution on the pyrazine rings played a crucial role in determining the activity levels. Different substituents like –Cl, –F, –NO2, - Br, –N(CH3)2, and –OCH2Ph were introduced at the para position on the phenyl ring, while methyl and ethyl groups were added at the 3-position on the pyrazine ring to introduce structural diversity. The biological assays demonstrated that electron-withdrawing groups on the phenyl ring generally exhibited better antibacterial activity [174]. The antibacterial activity of the 2-((5E)-5-(4-((E)-3-(2,4-dichlorophenyl)-3-oxoprop-1-enyl)benzylidene)-4-oxo-2 thioxothiazolidin-3-yl)acetic acid hybrid compound was assessed using SAR analysis. It was found to exhibit potent antibacterial activity against S. aureus, with a MIC value of 2 μg/mL. Although it exhibited higher activity compared to standard drug norfloxacin, it was less active compared to oxacillin. This suggested that introducing robust electron-withdrawing chloro groups at the 2 and 4-positions of the phenyl ring enhanced the compound's antibacterial action. Conversely, the inclusion of electron-donating groups (p-CH3, 2,4-(CH3)2, m-OCH3, p-OCH3, m-OCH2OCH3, p-OCH2OCH3) on the phenyl ring resulted in a decrease in antibacterial activity [175]. According to the SAR, it was observed that the presence of a bromine atom at a specific position on the benzene ring significantly improves the antibacterial activity. Conversely, substituting the bromine atom with a chlorine or a methoxy group at the 4-position of the phenyl ring leads to a decrease in antibacterial action. It could be due to the stronger hydrophobic effect exhibited by the bromine group compared to the chlorine and methoxy groups at the 4-position. Overall, the contribution order of the R group in terms of antibacterial activity was determined to be bromine (Br) > chlorine (Cl) > methoxy (OMe) [176]. In another research study, a total of 48 chalcones were developed and evaluated for their antifungal action against Fusarium proliferatum NCIM1105, Aspergillus flavus NCIM 594, Candida tropicalis NCIM 3556, and Aspergillus niger NCIM 596 in vitro. The compounds that exhibited significant activity against all four fungi generally possessed electron-withdrawing substitutions in the para-position of ring B. QSAR analysis revealed that the antifungal activity was associated with ADME (absorption, distribution, metabolism, and excretion), electrophilicity, as well as topological and spatial descriptors. The QSAR predictions were highly reliable, with a 99% confidence level, with the exception of two cases [177]. Furthermore, a set of 48 chalcone analogs was developed and tested for their antibacterial action against Bacillus subtilis NCIM 2718, Staphylococcus aureus NCIM 5021, Phaseolus vulgaris NCIM 2813, Salmonella typhi 2501, Escherichia coli NCIM 2931, and Enterobacter aerogenes NCIM 5139 using a micro-dilution broth assay. QSARs were established for these compounds. The polarizability, size, hydrophilic nature, and electron-donating/withdrawing properties of molecules were identified as key factors influencing the activities against both Gram-negative and Gram-positive bacteria. Among the microorganisms evaluated, S. aureus exhibited the highest hydrophobicity, while S. typhi showed the least hydrophobicity [178]. In the study conducted by Xiuhai Gan et al. a series of 28 chalcone derivatives containing a purine (sulfur) ether moiety were developed and tested for their antiviral action against tobacco mosaic virus (TMV). The initial SARs indicated that the compounds with diethyl malonate showed improved inactivity against TMV [179].
8. Conclusions and future outlooks
A wide variety of compounds with suitable antiviral, antibacterial, antifungal, and anti-parasitic activities have been synthesized using chalcones. However, very limited clinical trial studies have been conducted on the antiviral and antimicrobial potential of chalcones and their derivatives. Although clinical trials on chalcone derivatives have shown promising results with acceptable plasma levels and negligible toxicity, additional assessments should be focused on biosafety issues and clinical translation studies, as well as scale-up production and optimization of synthesis conditions. Studies on the structure of chalcones have revealed that the α, β-unsaturated ketone group, as well as methoxy and halogen groups, can play significant roles in their antimicrobial properties. However, more comprehensive in vitro and in vivo studies are required to understand the mechanisms behind the antiviral and antimicrobial effects of both synthetic and natural chalcones. Biofilm reduction mechanisms and antibacterial effects against antibiotic-resistant strains are of great importance. Chalcone structures have shown the ability to specifically target viral enzymes and can be employed to combat pathogenic microorganisms through the inhibition of GyrB, MurA, and protein NorA, among others. One of the major challenges in the management and treatment of bacterial and viral infections is drug resistance due to possible phenotype alterations, mutations, and genetic modifications, leading to a higher risk of disease transmission and an increased mortality. Chalcones and their derivatives can be considered potentially efficient antiviral and antimicrobial agents used for designing structures/molecules against new viral strains or multidrug-resistant microorganisms. Despite the advances in greener and more sustainable techniques for synthesizing chalcones, in-depth investigations are warranted for the optimization and standardization of these techniques as well as their up-scaling/industrial applications.
Ethics declarations
This study was approved by the ethics committee (IR.KMU.REC.1401.321) of Kerman University of Medical Sciences, Kerman, Iran.
Data availability statement
Data will be made available on request.
Funding
This work was supported by Kerman University of Medical Sciences, Kerman, Iran (grant No. 401000576).
CRediT authorship contribution statement
Mohammad Hadi Nematollahi: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. Mehrnaz Mehrabani: Investigation, Validation, Writing – review & editing. Yaser Hozhabri: Investigation, Methodology, Writing – original draft. Maryamossadat Mirtajaddini: Investigation, Methodology, Writing – original draft. Siavash Iravani: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Mohammad Hadi Nematollahi, Email: mh.nematollahi@yahoo.com, m.nematollahi@kmu.ac.ir.
Siavash Iravani, Email: siavashira@gmail.com.
References
- 1.Rajizadeh M.A., Nematollahi M.H., Jafari E., Bejeshk M.A., Mehrabani M., Razeghinia M.S., Najafipour H. Niosome nanocarrier enhances the ameliorating effects of myrtenol in the lungs of rats with experimental asthma. OpenNano. 2023 [Google Scholar]
- 2.Sarhadynejad Z., Sharififar F., Pardakhty A., Nematollahi M.-H., Sattaie-Mokhtari S., Mandegary A. Pharmacological safety evaluation of a traditional herbal medicine “Zereshk-e-Saghir” and assessment of its hepatoprotective effects on carbon tetrachloride induced hepatic damage in rats. J. Ethnopharmacol. 2016;190:387–395. doi: 10.1016/j.jep.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 3.Amiri K., Nasibi S., Mehrabani M., Nematollahi M.H., Harandi M.F. In vitro evaluation on the scolicidal effect of Myrtus communis L. and Tripleurospermum disciforme L. methanolic extracts. Exp. Parasitol. 2019;199:111–115. doi: 10.1016/j.exppara.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Raeiszadeh M., Pardakhty A., Sharififar F., Mehrabani M., Mehrabani M. Phytoniosome: a novel drug delivery for myrtle extract. Iran. J. Pharm. Res. (IJPR): IJPR. 2018;17(3):804. [PMC free article] [PubMed] [Google Scholar]
- 5.Demeke C.A., Woldeyohanins A.E., Kifle Z.D. Herbal medicine use for the management of COVID-19: a review article. Metabolism Open. 2021;12 doi: 10.1016/j.metop.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oloumi M.M., Vosough D., Derakhshanfar A., Nematollahi M.H. The healing potential of Plantago lanceolata ointment on collagenase-induced tendinitis in burros (Equus asinus) J. Equine Vet. Sci. 2011;31(8):470–474. [Google Scholar]
- 7.Poursalehi H.R., Fekri M.S., Far F.S., Mandegari A., Izadi A., Mahmoodi R., Nematollahi H., Porgholamhosein F., Ghorani V., Fekri M.S. Early and late preventive effect of Nigella sativa on the bleomycin-induced pulmonary fibrosis in rats: an experimental study. Avicenna Journal of Phytomedicine. 2018;8(3):263. [PMC free article] [PubMed] [Google Scholar]
- 8.Ebrahimpour N., Mehrabani M., Iranpour M., Kordestani Z., Mehrabani M., Nematollahi M.H., Asadipour A., Raeiszadeh M., Mehrbani M. The efficacy of a traditional medicine preparation on second-degree burn wounds in rats. J. Ethnopharmacol. 2020;252 doi: 10.1016/j.jep.2020.112570. [DOI] [PubMed] [Google Scholar]
- 9.Harandi H., Falahati-Pour S.K., Mahmoodi M., Faramarz S., Maleki H., Nasab F.B., Shiri H., Fooladi S., Nematollahi M.H. Nanoliposomal formulation of pistachio hull extract: preparation, characterization and anti-cancer evaluation through Bax/Bcl2 modulation. Mol. Biol. Rep. 2022:1–9. doi: 10.1007/s11033-021-07083-5. [DOI] [PubMed] [Google Scholar]
- 10.Sargazi M.L., Juybari K.B., Tarzi M.E., Amirkhosravi A., Nematollahi M.H., Mirzamohammdi S., Mehrbani M., Mehrabani M., Mehrabani M. Naringenin attenuates cell viability and migration of C6 glioblastoma cell line: a possible role of hedgehog signaling pathway. Mol. Biol. Rep. 2021;48(9):6413–6421. doi: 10.1007/s11033-021-06641-1. [DOI] [PubMed] [Google Scholar]
- 11.Mutha R.E., Tatiya A.U., Surana S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Future journal of pharmaceutical sciences. 2021;7:1–13. doi: 10.1186/s43094-020-00161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasim H.A., Nahar L., Jasim M.A., Moore S.A., Ritchie K.J., Sarker S.D. Chalcones: synthetic chemistry follows where nature leads. Biomolecules. 2021;11(8):1203. doi: 10.3390/biom11081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuang C., Zhang W., Sheng C., Zhang W., Xing C., Miao Z. Chalcone: a privileged structure in medicinal chemistry. Chemical reviews. 2017;117(12):7762–7810. doi: 10.1021/acs.chemrev.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valavanidis A., Vlachogianni T. Plant polyphenols: recent advances in epidemiological research and other studies on cancer prevention. Stud. Nat. Prod. Chem. 2013;39:269–295. [Google Scholar]
- 15.Opletalova V. Chalcones and their heterocyclic analogs as potential therapeutic agents in bacterial diseases. Ces. Slov. Farm.: Casopis Ceske Farmaceuticke Spolecnosti a Slovenske Farmaceuticke Spolecnosti. 2000;49(6):278–284. [PubMed] [Google Scholar]
- 16.Liu M., Wilairat P., Go M.-L. Antimalarial alkoxylated and hydroxylated chalones: structure− activity relationship analysis. J. Med. Chem. 2001;44(25):4443–4452. doi: 10.1021/jm0101747. [DOI] [PubMed] [Google Scholar]
- 17.Narender T., Khaliq T., Goyal N., Gupta S. Synthesis of chromenochalcones and evaluation of their in vitro antileishmanial activity. Bioorg. Med. Chem. 2005;13(23):6543–6550. doi: 10.1016/j.bmc.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.H., Nan J.-X., Zhao Y.Z., Woo S.W., Park E.-J., Kang T.-H., Seo G.S., Kim Y.-C., Sohn D.H. The chalcone butein from Rhus verniciflua shows antifibrogenic activity. Planta Med. 2003;69(11):990–994. doi: 10.1055/s-2003-45143. [DOI] [PubMed] [Google Scholar]
- 19.Konieczny M.T., Konieczny W., Sabisz M., Skladanowski A., Wakieć R., Augustynowicz-Kopeć E., Zwolska Z. Synthesis of isomeric, oxathiolone fused chalcones, and comparison of their activity toward various microorganisms and human cancer cells line. Chemical and pharmaceutical Bulletin. 2007;55(5):817–820. doi: 10.1248/cpb.55.817. [DOI] [PubMed] [Google Scholar]
- 20.Jin F., Jin X.Y., Jin Y.L., Sohn D.W., Kim S.-A., Sohn D.H., Kim Y.C., Kim H.S. Structural requirements of 2′, 4′, 6′-tris (methoxymethoxy) chalcone derivatives for anti-inflammatory activity: the importance of a 2′-hydroxy moiety. Arch Pharm. Res. (Seoul) 2007;30(11):1359–1367. doi: 10.1007/BF02977357. [DOI] [PubMed] [Google Scholar]
- 21.Barfod L., Kemp K., Hansen M., Kharazmi A. Chalcones from Chinese liquorice inhibit proliferation of T cells and production of cytokines. Int. Immunopharm. 2002;2(4):545–555. doi: 10.1016/s1567-5769(01)00202-8. [DOI] [PubMed] [Google Scholar]
- 22.Letafat B., Shakeri R., Emami S., Noushini S., Mohammadhosseini N., Shirkavand N., Kabudanian Ardestani S., Safavi M., Samadizadeh M., Letafat A., Sha Ee A., Foroumadi A. Synthesis and in vitro cytotoxic activity of novel chalcone-like agents. Iran J Basic Med Sci. 2013;16(11):1155–1162. [PMC free article] [PubMed] [Google Scholar]
- 23.Viana G.S., Bandeira M.A., Matos F.J. Analgesic and antiinflammatory effects of chalcones isolated from Myracrodruon urundeuva allemão. Phytomedicine. 2003;10(2–3):189–195. doi: 10.1078/094471103321659924. [DOI] [PubMed] [Google Scholar]
- 24.Shaik A., Bhandare R.R., Palleapati K., Nissankararao S. Antimicrobial, Antioxidant, and Anticancer Activities of Some Novel Isoxazole Ring Containing Chalcone and Dihydropyrazole Derivatives. 2020;25(5) doi: 10.3390/molecules25051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansari F.L., Umbreen S., Hussain L., Makhmoor T., Nawaz S.A., Lodhi M.A., Khan S.N., Shaheen F., Choudhary M.I. Syntheses and biological activities of chalcone and 1, 5‐benzothiazepine derivatives: promising new free‐radical scavengers, and esterase, urease, and α‐glucosidase inhibitors. Chem. Biodivers. 2005;2(4):487–496. doi: 10.1002/cbdv.200590029. [DOI] [PubMed] [Google Scholar]
- 26.Araico A., Terencio M., Alcaraz M., Dominguez J., Leon C., Ferrandiz M. Phenylsulphonyl urenyl chalcone derivatives as dual inhibitors of cyclo-oxygenase-2 and 5-lipoxygenase. Life Sci. 2006;78(25):2911–2918. doi: 10.1016/j.lfs.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Nyandoro S., Nkunya M., Josepha C., Odalo J., Sattler I. New glucopyranosylglyceryl-N-octenyl adipate and bioactivity of retro and condensed chalcones from Toussaintia orientalis. Tanzan. J. Sci. 2012;38(3):108–126. [Google Scholar]
- 28.Shibata S. Anti-tumorigenic chalcones. Stem Cell. 1994;12(1):44–52. doi: 10.1002/stem.5530120109. [DOI] [PubMed] [Google Scholar]
- 29.De Vincenzo R., Scambia G., Benedetti Panici P., Ranelletti F.O., Bonanno G., Ercoli A., Delle Monache F., Ferrari F., Piantelli M., Mancuso S. Effect of synthetic and naturally occurring chalcones on ovarian cancer cell growth: structure-activity relationships. Anti Cancer Drug Des. 1995;10(6):481–490. [PubMed] [Google Scholar]
- 30.Rahaman S., Prasad Y.R., Bhuvaneswari K., Kumar P. Synthesis and antihistaminic activity of novel pyrazoline derivatives. Int. J. ChemTech Res. 2010;2:16–20. [Google Scholar]
- 31.Tomar V., Bhattacharjee G., Kumar A. Synthesis and antimicrobial evaluation of new chalcones containing piperazine or 2, 5-dichlorothiophene moiety. Bioorg. Med. Chem. Lett. 2007;17(19):5321–5324. doi: 10.1016/j.bmcl.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Bandgar B.P., Gawande S.S. Synthesis and biological screening of a combinatorial library of β-chlorovinyl chalcones as anticancer, anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. 2010;18(5):2060–2065. doi: 10.1016/j.bmc.2009.12.077. [DOI] [PubMed] [Google Scholar]
- 33.Filosa R., Peduto A., De Caprariis P., Saturnino C., Festa M., Petrella A., Pau A., Pinna G.A., La Colla P., Busonera B. Synthesis and antiproliferative properties of N3/8-disubstituted 3, 8-diazabicyclo [3.2. 1] octane analogues of 3, 8-bis [2-(3, 4, 5-trimethoxyphenyl) pyridin-4-yl] methyl-piperazine. Eur. J. Med. Chem. 2007;42(3):293–306. doi: 10.1016/j.ejmech.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Mai C.W., Yaeghoobi M., Abd-Rahman N., Kang Y.B., Pichika M.R. Chalcones with electron-withdrawing and electron-donating substituents: anticancer activity against TRAIL resistant cancer cells, structure-activity relationship analysis and regulation of apoptotic proteins. Eur. J. Med. Chem. 2014;77:378–387. doi: 10.1016/j.ejmech.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Mahapatra D.K., Bharti S.K., Asati V. Chalcone scaffolds as anti-infective agents: structural and molecular target perspectives. Eur. J. Med. Chem. 2015;101:496–524. doi: 10.1016/j.ejmech.2015.06.052. [DOI] [PubMed] [Google Scholar]
- 36.Irwin K.K., Renzette N., Kowalik T.F., Jensen J.D. Antiviral drug resistance as an adaptive process. Virus evolution. 2016;2(1) doi: 10.1093/ve/vew014. [Internet] 2016/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Saheb R., Makharza S., Al-Battah F., Abu-El-Halawa R., Kaimari T., Abu Abed O.S. Synthesis of new pyrazolone and pyrazole-based adamantyl chalcones and antimicrobial activity. Biosci. Rep. 2020;40(9) doi: 10.1042/BSR20201950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhingra S., Rahman N.A.A., Peile E., Rahman M., Sartelli M., Hassali M.A., Islam T., Islam S., Haque M. Microbial resistance movements: an overview of global public health threats posed by antimicrobial resistance, and how best to counter. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.535668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marotta L., Rossi S., Ibba R., Brogi S., Calderone V., Butini S., Campiani G., Gemma S. The green chemistry of chalcones: valuable sources of privileged core structures for drug discovery. Front. Chem. 2022;10 doi: 10.3389/fchem.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieira L.C.C., Paixão M.W., Corrêa A.G. Green synthesis of novel chalcone and coumarin derivatives via Suzuki coupling reaction. Tetrahedron Lett. 2012;53:2715–2718. [Google Scholar]
- 41.Palleros D.R. Solvent-free synthesis of chalcones. J Chem Educ. 2004;81:1345. [Google Scholar]
- 42.Borade R.M., Somvanshi S.B., Kale S.B., Pawar R.P., Jadhav K. Spinel zinc ferrite nanoparticles: an active nanocatalyst for microwave irradiated solvent free synthesis of chalcones. Mater. Res. Express. 2020;7 [Google Scholar]
- 43.Khan S.A., Asiri A.M., Al-Ghamdi N.S.M., Asad M., Zayed M.E.M., Elroby S.A.K., Aqlan F.M., Wani M.Y., Sharma K. Microwave assisted synthesis of chalcone and its polycyclic heterocyclic analogues as promising antibacterial agents: in vitro, in silico and DFT studies. J. Mol. Struct. 2019;1190:77–85. [Google Scholar]
- 44.Ritter M., Martins R.M., Rosa S.A., Malavolta J.L., Lund R.G., Flores A.F.C., Pereira C.M.P. Green synthesis of chalcones and microbiological evaluation. J. Braz. Chem. Soc. 2015;26 [Google Scholar]
- 45.Gu Y., Jérôme F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010;12:1127–1138. [Google Scholar]
- 46.Achutha D.K., Vagish C.B., Renuka N., Lokeshwari D.M., Kariyappa A.K. Green synthesis of novel pyrazoline carbothioamides: a potent antimicrobial and antioxidant agents. Chemical Data Collections. 2020;28 [Google Scholar]
- 47.Yin H., Shi X., Wang H., Liu G., Ma L. VB1 promoted green synthesis of chalcones and its neuroprotection. Potency Evaluation Processes. 2019;7:236. [Google Scholar]
- 48.Mohammed Musthafa T.N., Snigdha K., Asiri A.M., Sobahi T.R., Asad M. Green synthesis of chromonyl chalcone and pyrazoline as potential antimicrobial agents – DFT, molecular docking and antimicrobial studies. J. Mol. Struct. 2023;1271 [Google Scholar]
- 49.Kumar A., Rout L., Achary L.S.K., Mohanty S.K., Nayak P.S., Barik B., Dash P. Solvent free synthesis of chalcones over graphene oxide-supported MnO2 catalysts synthesized via combustion route. Mater. Chem. Phys. 2021;259 [Google Scholar]
- 50.Tanpure S., Ghanwat V., Shinde B., Tanpure K., Lawande S. The eggshell waste transformed green and efficient synthesis of K-Ca(OH)2 catalyst for room temperature synthesis of chalcones. Polycycl. Aromat. Comp. 2022;42:1322–1340. [Google Scholar]
- 51.Pal M., Berhanu G., Desalegn C., Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12(3) doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chafekar A., Fielding B.C. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10(2):93. doi: 10.3390/v10020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar S., Nyodu R., Maurya V.K., Saxena S.K. Springer; 2020. Morphology, Genome Organization, Replication, and Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Coronavirus Disease 2019 (COVID-19) pp. 23–31. [Google Scholar]
- 55.Gyebi G.A., Ogunro O.B., Adegunloye A.P., Ogunyemi O.M., Afolabi S.O. Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CLpro): an in silico screening of alkaloids and terpenoids from African medicinal plants. J. Biomol. Struct. Dyn. 2021;39(9):3396–3408. doi: 10.1080/07391102.2020.1764868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batovska D.I., Todorova I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010;5(1):1–29. doi: 10.2174/157488410790410579. [DOI] [PubMed] [Google Scholar]
- 58.Mathpal S., Joshi T., Sharma P., Pande V., Chandra S. 2022. Assessment of Activity of Chalcone Compounds as Inhibitors of 3-Chymotrypsin like Protease (3CLPro) of SARS-CoV-2: in Silico Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lam T.-P., Nguyen D.-N., Mai T.T., Tran T.-D., Le M.-T., Huynh P.N.H., Nguyen D.-T., Tran V.-H., Trinh D.-T.T., Truong P. Exploration of chalcones as 3-chymotrypsin-like protease (3CLpro) inhibitors of SARS-CoV-2 using computational approaches. Struct. Chem. 2022;33(5):1707–1725. doi: 10.1007/s11224-022-02000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alsafi M.A., Hughes D.L., Said M.A. First COVID-19 molecular docking with a chalcone-based compound: synthesis, single-crystal structure and Hirshfeld surface analysis study. Acta Crystallogr., Sect. C: Struct. Chem. 2020;76(12):1043–1050. doi: 10.1107/S2053229620014217. [DOI] [PubMed] [Google Scholar]
- 61.Duran N., Polat M.F., Aktas D.A., Alagoz M.A., Ay E., Cimen F., Tek E., Anil B., Burmaoglu S., Algul O. New chalcone derivatives as effective against SARS‐CoV‐2 agent. Int. J. Clin. Pract. 2021;75(12) doi: 10.1111/ijcp.14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J.-Y., Ko J.-A., Kim D.W., Kim Y.M., Kwon H.-J., Jeong H.J., Kim C.Y., Park K.H., Lee W.S., Ryu Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzym. Inhib. Med. Chem. 2016;31(1):23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- 63.Jo S., Kim H., Kim S., Shin D.H., Kim M.S. Characteristics of flavonoids as potent MERS‐CoV 3C‐like protease inhibitors. Chem. Biol. Drug Des. 2019;94(6):2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanjanasirirat P., Suksatu A., Manopwisedjaroen S., Munyoo B., Tuchinda P., Jearawuttanakul K., Seemakhan S., Charoensutthivarakul S., Wongtrakoongate P., Rangkasenee N. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci. Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-77003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nyamweya S., Hegedus A., Jaye A., Rowland‐Jones S., Flanagan K.L., Macallan D.C. Comparing HIV‐1 and HIV‐2 infection: lessons for viral immunopathogenesis. Rev. Med. Virol. 2013;23(4):221–240. doi: 10.1002/rmv.1739. [DOI] [PubMed] [Google Scholar]
- 66.Sierra S., Walter H. Targets for inhibition of HIV replication: entry, enzyme action, release and maturation. Intervirology. 2012;55(2):84–97. doi: 10.1159/000331995. [DOI] [PubMed] [Google Scholar]
- 67.Gulick R.M., Flexner C. Long-acting HIV drugs for treatment and prevention. Annu. Rev. Med. 2019;70:137–150. doi: 10.1146/annurev-med-041217-013717. [DOI] [PubMed] [Google Scholar]
- 68.Mathaiyan M., Suresh A., Balamurugan R. Binding property of HIV p24 and Reverse transcriptase by chalcones from Pongamia pinnata seeds. Bioinformation. 2018;14(6):279. doi: 10.6026/97320630014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hameed A., Abdullah M.I., Ahmed E., Sharif A., Irfan A., Masood S. Anti-HIV cytotoxicity enzyme inhibition and molecular docking studies of quinoline based chalcones as potential non-nucleoside reverse transcriptase inhibitors (NNRT) Bioorg. Chem. 2016;65:175–182. doi: 10.1016/j.bioorg.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Pham P.A., Flexner C.W. In: Pharmacology and Therapeutics. Waldman S.A., Terzic A., Egan L.J., Elghozi J.-L., Jahangir A., Kane G.C., et al., editors. W.B. Saunders; Philadelphia: 2009. Chapter 84 - HIV infections and AIDS; pp. 1187–1199. [Google Scholar]
- 71.Ali S.A., Teow S.Y., Omar T.C., Khoo A.S., Choon T.S., Yusoff N.M. A cell internalizing antibody targeting capsid protein (p24) inhibits the replication of HIV-1 in T cells lines and PBMCs: a proof of concept study. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0145986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Craigie R. The molecular biology of HIV integrase. Future Virol. 2012;7(7):679–686. doi: 10.2217/FVL.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monserrat J.-P., Al-Safi R.I., Tiwari K.N., Quentin L., Chabot G.G., Vessières A., Jaouen G., Neamati N., Hillard E.A. Ferrocenyl chalcone difluoridoborates inhibit HIV-1 integrase and display low activity towards cancer and endothelial cells. Bioorg. Med. Chem. Lett. 2011;21(20):6195–6197. doi: 10.1016/j.bmcl.2011.07.078. [DOI] [PubMed] [Google Scholar]
- 74.Chiu T.K., Davies D.R. Structure and function of HIV-1 integrase. Current topics in medicinal chemistry. 2004;4(9):965–977. doi: 10.2174/1568026043388547. [DOI] [PubMed] [Google Scholar]
- 75.Wu J., Ao M-t, Shao R., Wang H-r, Yu D., Fang M-j, Gao X., Wu Z., Zhou Q., Xue Y-h. A chalcone derivative reactivates latent HIV-1 transcription through activating P-TEFb and promoting Tat-SEC interaction on viral promoter. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-10728-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grau-Exposito J., Luque-Ballesteros L., Navarro J., Curran A., Burgos J., Ribera E., Torrella A., Planas B., Badia R., Martin-Castillo M. Latency reversal agents affect differently the latent reservoir present in distinct CD4+ T subpopulations. PLoS Pathog. 2019;15(8) doi: 10.1371/journal.ppat.1007991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meher B.R., Vaishnavi M., Mattaparthi V.S.K., Patel S., Kaushik S. In: Encyclopedia of Bioinformatics and Computational Biology. Ranganathan S., Gribskov M., Nakai K., Schönbach C., editors. Academic Press; Oxford: 2019. Mutation effects on 3D-structural reorganization using HIV-1 protease as a case study; pp. 706–721. [Google Scholar]
- 78.Panthong A., Tassaneeyakul W., Kanjanapothi D., Tantiwachwuttikul P., Reutrakul V. Anti-inflammatory activity of 5,7-dimethoxyflavone. Planta Med. 1989;55(2):133–136. doi: 10.1055/s-2006-961905. [DOI] [PubMed] [Google Scholar]
- 79.Cheenpracha S., Karalai C., Ponglimanont C., Subhadhirasakul S., Tewtrakul S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg. Med. Chem. 2006;14(6):1710–1714. doi: 10.1016/j.bmc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 80.Tewtrakul S., Subhadhirasakul S., Puripattanavong J., Panphadung T. HIV-1 protease inhibitory substances from the rhizomes of Boesenbergia pandurata Holtt. Songklanakarin J. Sci. Technol. 2003;25(4):504–508. [Google Scholar]
- 81.Asha K., Kumar B. Emerging influenza D virus threat: what we know so far. J. Clin. Med. 2019;8(2):192. doi: 10.3390/jcm8020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lofgren E., Fefferman N.H., Naumov Y.N., Gorski J., Naumova E.N. Influenza seasonality: underlying causes and modeling theories. J. Virol. 2007;81(11):5429–5436. doi: 10.1128/JVI.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arbeitskreis Blut UBBK Influenza Virus. Transfusion medicine and hemotherapy : offizielles. Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2009;36(1):32–39. doi: 10.1159/000197314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shie J.-J., Fang J.-M. Development of effective anti-influenza drugs: congeners and conjugates – a review. J. Biomed. Sci. 2019;26(1):84. doi: 10.1186/s12929-019-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dao T.T., Nguyen P.H., Lee H.S., Kim E., Park J., Lim S.I., Oh W.K. Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg. Med. Chem. Lett. 2011;21(1):294–298. doi: 10.1016/j.bmcl.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 86.McKimm-Breschkin J.L. Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Respir Viruses. 2013;7(Suppl 1):25–36. doi: 10.1111/irv.12047. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malbari K., Gonsalves H., Chintakrindi A., Gohil D., Joshi M., Kothari S., Srivastava S., Chowdhary A., Kanyalkar M. In search of effective H1N1 neuraminidase inhibitor by molecular docking, antiviral evaluation and membrane interaction studies using NMR. Acta Virol. 2018;62(2):179–190. doi: 10.4149/av_2018_209. [DOI] [PubMed] [Google Scholar]
- 88.Chintakrindi A S., Af Martis E., Gohil D J., T Kothari S., S Chowdhary A., C Coutinho E., Kanyalkar M A. A computational model for docking of noncompetitive neuraminidase inhibitors and probing their binding interactions with neuraminidase of influenza virus H5N1. Curr. Comput. Aided Drug Des. 2016;12(4):272–281. doi: 10.2174/1573409912666160713111242. [DOI] [PubMed] [Google Scholar]
- 89.Park J.-Y., Jeong H.J., Kim Y.M., Park S.-J., Rho M.-C., Park K.H., Ryu Y.B., Lee W.S. Characteristic of alkylated chalcones from Angelica keiskei on influenza virus neuraminidase inhibition. Bioorg. Med. Chem. Lett. 2011;21(18):5602–5604. doi: 10.1016/j.bmcl.2011.06.130. [DOI] [PubMed] [Google Scholar]
- 90.Hayden F.G. Rhinovirus and the lower respiratory tract. Rev. Med. Virol. 2004;14(1):17–31. doi: 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Passioti M., Maggina P., Megremis S., Papadopoulos N.G. The common cold: potential for future prevention or cure. Curr. Allergy Asthma Rep. 2014;14(2):1–11. doi: 10.1007/s11882-013-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishitsuka H., Ninomiya Y., Ohsawa C., Fujiu M., Suhara Y. Direct and specific inactivation of rhinovirus by chalcone Ro 09-0410. Antimicrobial agents and chemotherapy. 1982;22(4):617–621. doi: 10.1128/aac.22.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmad A., Dowsett A., Tyrrell D. Studies of rhinovirus resistant to an antiviral chalcone. Antivir. Res. 1987;8(1):27–39. doi: 10.1016/0166-3542(87)90085-4. [DOI] [PubMed] [Google Scholar]
- 94.Al-Nakib W., Higgins P.G., Barrow I., Tyrrell D.A., Lenox-Smith I., Ishitsuka H. Intranasal chalcone, Ro 09-0410, as prophylaxis against rhinovirus infection in human volunteers. J. Antimicrob. Chemother. 1987;20(6):887–892. doi: 10.1093/jac/20.6.887. [DOI] [PubMed] [Google Scholar]
- 95.Phillpotts R.J., Higgins P.G., Willman J.S., Tyrrell D.A., Lenox-Smith I. Evaluation of the antirhinovirus chalcone Ro 09-0415 given orally to volunteers. J. Antimicrob. Chemother. 1984;14(4):403–409. doi: 10.1093/jac/14.4.403. [DOI] [PubMed] [Google Scholar]
- 96.Ninomiya Y., Shimma N., Ishitsuka H. Comparative studies on the antirhinovirus activity and the mode of action of the rhinovirus capsid binding agents, chalcone amides. Antivir. Res. 1990;13(2):61–74. doi: 10.1016/0166-3542(90)90022-y. [DOI] [PubMed] [Google Scholar]
- 97.Cliffe A., Chang L., Colgrove R., Knipe D.M. Elsevier; 2014. Herpes Simplex Virus. Reference Module in Biomedical Sciences. [Google Scholar]
- 98.Ali M.A., Shaharyar M., Clercq E.D. Synthesis of 5-(4-hydroxy-3-methylphenyl)-5-(substituted phenyl)-4, 5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone derivatives with anti-viral activity. J. Enzym. Inhib. Med. Chem. 2007;22(6):702–708. doi: 10.1080/14756360701265832. [DOI] [PubMed] [Google Scholar]
- 99.Phrutivorapongkul A., Lipipun V., Ruangrungsi N., Kirtikara K., Nishikawa K., Maruyama S., Watanabe T., Ishikawa T. Studies on the chemical constituents of stem bark of Millettia leucantha: isolation of new chalcones with cytotoxic, anti-herpes simplex virus and anti-inflammatory activities. Chemical and Pharmaceutical bulletin. 2003;51(2):187–190. doi: 10.1248/cpb.51.187. [DOI] [PubMed] [Google Scholar]
- 100.El-Subbagh H.I., Abu-Zaid S.M., Mahran M.A., Badria F.A., Al-Obaid A.M. Synthesis and biological evaluation of certain α, β-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J. Med. Chem. 2000;43(15):2915–2921. doi: 10.1021/jm000038m. [DOI] [PubMed] [Google Scholar]
- 101.Junjhon J., Edwards T.J., Utaipat U., Bowman V.D., Holdaway H.A., Zhang W., Keelapang P., Puttikhunt C., Perera R., Chipman P.R. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. J. Virol. 2010;84(16):8353–8358. doi: 10.1128/JVI.00696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leung J.Y., Pijlman G.P., Kondratieva N., Hyde J., Mackenzie J.M., Khromykh A.A. Role of nonstructural protein NS2A in flavivirus assembly. J. Virol. 2008;82(10):4731–4741. doi: 10.1128/JVI.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hasan S., Jamdar S.F., Alalowi M., Al Ageel Al Beaiji S.M. Dengue virus: a global human threat: review of literature. J. Int. Soc. Prev. Community Dent. 2016;6(1):1–6. doi: 10.4103/2231-0762.175416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hrobowski Y.M., Garry R.F., Michael S.F. Peptide inhibitors of dengue virus and West Nile virus infectivity. Virol. J. 2005;2(1):49. doi: 10.1186/1743-422X-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lescar J., Luo D., Xu T., Sampath A., Lim S.P., Canard B., Vasudevan S.G. Towards the design of antiviral inhibitors against flaviviruses: the case for the multifunctional NS3 protein from Dengue virus as a target. Antivir. Res. 2008;80(2):94–101. doi: 10.1016/j.antiviral.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 106.Frimayanti N., Chee C.F., Zain S.M., Rahman N.A. Design of new competitive dengue ns2b/ns3 protease inhibitors—a computational approach. Int. J. Mol. Sci. 2011;12(2):1089–1100. doi: 10.3390/ijms12021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frecer V., Miertus S. Design, structure-based focusing and in silico screening of combinatorial library of peptidomimetic inhibitors of Dengue virus NS2B-NS3 protease. J. Comput. Aided Mol. Des. 2010;24(3):195–212. doi: 10.1007/s10822-010-9326-8. [DOI] [PubMed] [Google Scholar]
- 108.Edelman R. Dengue vaccines approach the finish line. Clin. Infect. Dis. 2007;45(Suppl 1):S56–S60. doi: 10.1086/518148. [DOI] [PubMed] [Google Scholar]
- 109.Durbin A.P., Karron R.A., Sun W., Vaughn D.W., Reynolds M.J., Perreault J.R., Thumar B., Men R., Lai C.J., Elkins W.R., Chanock R.M., Murphy B.R., Whitehead S.S. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3'-untranslated region. Am. J. Trop. Med. Hyg. 2001;65(5):405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 110.Durbin A.P., Whitehead S.S., McArthur J., Perreault J.R., Blaney J.E., Jr., Thumar B., Murphy B.R., Karron R.A. rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J. Infect. Dis. 2005;191(5):710–718. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 111.Kurniawan I., Rosalinda M., Ikhsan N. Implementation of ensemble methods on QSAR Study of NS3 inhibitor activity as anti-dengue agent. SAR QSAR Environ. Res. 2020;31(6):477–492. doi: 10.1080/1062936X.2020.1773534. [DOI] [PubMed] [Google Scholar]
- 112.Kiat T.S., Pippen R., Yusof R., Ibrahim H., Khalid N., Rahman N.A. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L.), towards dengue-2 virus NS3 protease. Bioorg. Med. Chem. Lett. 2006;16(12):3337–3340. doi: 10.1016/j.bmcl.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 113.Griffiths P., Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021;19(12):759–773. doi: 10.1038/s41579-021-00582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buckwold V.E., Wilson R.J., Nalca A., Beer B.B., Voss T.G., Turpin J.A., Buckheit R.W., 3rd, Wei J., Wenzel-Mathers M., Walton E.M., Smith R.J., Pallansch M., Ward P., Wells J., Chuvala L., Sloane S., Paulman R., Russell J., Hartman T., Ptak R. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antivir. Res. 2004;61(1):57–62. doi: 10.1016/s0166-3542(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 115.Chen Z., Knutson E., Wang S., Martinez L.A., Albrecht T. Stabilization of p53 in human cytomegalovirus-initiated cells is associated with sequestration of HDM2 and decreased p53 ubiquitination. J. Biol. Chem. 2007;282(40):29284–29295. doi: 10.1074/jbc.M705349200. [DOI] [PubMed] [Google Scholar]
- 116.Patil V., Patil S.A., Patil R., Bugarin A., Beaman K., Patil S.A. Exploration of (hetero) aryl derived thienylchalcones for antiviral and anticancer activities. Med. Chem. 2019;15(2):150–161. doi: 10.2174/1573406414666180524074648. [DOI] [PubMed] [Google Scholar]
- 117.Tong S., Li J., Wen Y.-M. Elsevier; 2019. Hepatitis B Virus: Molecular Biology☆. Reference Module in Biomedical Sciences. [Google Scholar]
- 118.Hollinger F., Liang T. Lippincott Williams & Wilkins; Philadelphia: 2001. Hepatitis B Virus. Fields Virology; pp. 2971–3036. [Google Scholar]
- 119.Liang T.J. Hepatitis B: the virus and disease. Hepatology. 2009;49(5 Suppl):S13–S21. doi: 10.1002/hep.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mathayan M., Jayaraman S., Kulanthaivel L., Suresh A. Inhibition studies of HBV DNA polymerase using seed extracts of Pongamia pinnata. Bioinformation. 2019;15(7):506–512. doi: 10.6026/97320630015506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Groeger J., Flaxman A., Wiersma S.T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 122.Morozov V.A., Lagaye S. Hepatitis C virus: morphogenesis, infection and therapy. World J. Hepatol. 2018;10(2):186–212. doi: 10.4254/wjh.v10.i2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adianti M., Aoki C., Komoto M., Deng L., Shoji I., Wahyuni T.S., Lusida M.I., Soetjipto Fuchino H., Kawahara N., Hotta H. Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species. Microbiol. Immunol. 2014;58(3):180–187. doi: 10.1111/1348-0421.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang M., Li N., Li F., Zhu Q., Liu X., Han Q., Wang Y., Chen Y., Zeng X., Lv Y., Zhang P., Yang C., Liu Z. Xanthohumol, a main prenylated chalcone from hops, reduces liver damage and modulates oxidative reaction and apoptosis in hepatitis C virus infected Tupaia belangeri. Int. Immunopharm. 2013;16(4):466–474. doi: 10.1016/j.intimp.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 125.Tratrat C., Haroun M., Xenikakis I., Liaras K., Tsolaki E., Eleftheriou P., Petrou A., Aldhubiab B., Attimarad M., Venugopala K.N. Design, synthesis, evaluation of antimicrobial activity and docking studies of new thiazole-based chalcones. Curr. Top. Med. Chem. 2019;19(5):356–375. doi: 10.2174/1568026619666190129121933. [DOI] [PubMed] [Google Scholar]
- 126.Ušjak D., Ivković B., Božić D.D., Bošković L., Milenković M. Antimicrobial activity of novel chalcones and modulation of virulence factors in hospital strains of Acinetobacter baumannii and Pseudomonas aeruginosa. Microb. Pathog. 2019;131:186–196. doi: 10.1016/j.micpath.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 127.Cai S., Qiao X., Feng L., Shi N., Wang H., Yang H., Guo Z., Wang M., Chen Y., Wang Y. Python cathelicidin CATHPb1 protects against multidrug-resistant staphylococcal infections by antimicrobial-immunomodulatory duality. J. Med. Chem. 2018;61(5):2075–2086. doi: 10.1021/acs.jmedchem.8b00036. [DOI] [PubMed] [Google Scholar]
- 128.Dinu V., Lu Y., Weston N., Lithgo R., Coupe H., Channell G., Adams G.G., Torcello Gómez A., Sabater C., Mackie A. The antibiotic vancomycin induces complexation and aggregation of gastrointestinal and submaxillary mucins. Sci. Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-57776-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nishino K., Yamasaki S., Nakashima R., Zwama M., Hayashi-Nishino M. Function and inhibitory mechanisms of multidrug efflux pumps. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.737288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.da Cunha Xavier J., de Almeida-Neto F.W., Rocha J.E., Freitas T.S., Freitas P.R., de Araujo A.C., da Silva P.T., Nogueira C.E., Bandeira P.N., Marinho M.M. Spectroscopic analysis by NMR, FT-Raman, ATR-FTIR, and UV-Vis, evaluation of antimicrobial activity, and in silico studies of chalcones derived from 2-hydroxyacetophenone. J. Mol. Struct. 2021;1241 [Google Scholar]
- 131.Zimmermann S., Klinger-Strobel M., Bohnert J.A., Wendler S., Rödel J., Pletz M.W., Löffler B., Tuchscherr L. Clinically approved drugs inhibit the Staphylococcus aureus multidrug NorA efflux pump and reduce biofilm formation. Front. Microbiol. 2019;10:2762. doi: 10.3389/fmicb.2019.02762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mustafa M., Mostafa Y.A. A facile synthesis, drug-likeness, and in silico molecular docking of certain new azidosulfonamide–chalcones and their in vitro antimicrobial activity. Monatshefte für Chemie-Chemical Monthly. 2020;151(3):417–427. [Google Scholar]
- 133.Ovung A., Bhattacharyya J. Sulfonamide drugs: structure, antibacterial property, toxicity, and biophysical interactions. Biophysical Reviews. 2021;13(2):259–272. doi: 10.1007/s12551-021-00795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leal A.L.A.B., da Silva P.T., da Rocha M.N., Marinho E.M., Marinho E.S., Marinho M.M., Bandeira P.N., Nogueira C.E.S., Barreto H.M., Teixeira A.M.R. Potentiating activity of Norfloxacin by synthetic chalcones against NorA overproducing Staphylococcus aureus. Microb. Pathog. 2021;155 doi: 10.1016/j.micpath.2021.104894. [DOI] [PubMed] [Google Scholar]
- 135.Rezende-Júnior L.M., Andrade LMdS., Leal A.L.A.B., Mesquita ABdS., Santos AlpdAd, Neto JdSL., Siqueira-Júnior J.P., Nogueira C.E.S., Kaatz G.W., Coutinho H.D.M. Chalcones isolated from Arrabidaea brachypoda flowers as inhibitors of NorA and MepA multidrug efflux pumps of Staphylococcus aureus. Antibiotics. 2020;9(6):351. doi: 10.3390/antibiotics9060351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bondock S., Nasr T. Synthesis and antimicrobial activity of new 4-Methyl-2-(3-pyridyl) thiazolyl chalcones and pyrazolines. Russ. J. Gen. Chem. 2021;91(3):488–494. [Google Scholar]
- 137.Lagu S.B., Yejella R.P., Bhandare R.R., Shaik A.B. Design, synthesis, and antibacterial and antifungal activities of novel trifluoromethyl and trifluoromethoxy substituted chalcone derivatives. Pharmaceuticals. 2020;13(11):375. doi: 10.3390/ph13110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Amole K.L., Bello I.A., Oyewale A.O. Synthesis, characterization and antibacterial activities of new fluorinated chalcones. Chemistry Africa. 2019;2(1):47–55. [Google Scholar]
- 139.Dhaliwal J.S., Moshawih S., Goh K.W., Loy M.J., Hossain M.S., Hermansyah A., Kotra V., Kifli N., Goh H.P., Dhaliwal S.K.S. Pharmacotherapeutics applications and chemistry of chalcone derivatives. Molecules. 2022;27(20):7062. doi: 10.3390/molecules27207062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lockhart S.R., Guarner J. Emerging and reemerging fungal infections. Semin. Diagn. Pathol. 2019;36(3):177–181. doi: 10.1053/j.semdp.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Van Daele R., Spriet I., Wauters J., Maertens J., Mercier T., Van Hecke S., Brüggemann R. Antifungal drugs: what brings the future? Med. Mycol. 2019;57(Supplement_3):S328–S343. doi: 10.1093/mmy/myz012. [DOI] [PubMed] [Google Scholar]
- 142.Nicola A.M., Albuquerque P., Paes H.C., Fernandes L., Costa F.F., Kioshima E.S., Abadio A.K.R., Bocca A.L., Felipe M.S. Antifungal drugs: new insights in research & development. Pharmacol. Ther. 2019;195:21–38. doi: 10.1016/j.pharmthera.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 143.Mellado M., Espinoza L., Madrid A., Mella J., Chávez-Weisser E., Diaz K., Cuellar M. Design, synthesis, antifungal activity, and structure–activity relationship studies of chalcones and hybrid dihydrochromane–chalcones. Mol. Divers. 2020;24(3):603–615. doi: 10.1007/s11030-019-09967-y. [DOI] [PubMed] [Google Scholar]
- 144.Silva PTd, Lopes L.M.A., Xavier J., Carvalho M., Moraes M., Pessoa C., Barros-Nepomuceno F., Bandeira P.N., Targino C., Teixeira A. Cytotoxic and antifungal activity of chalcones synthesized from natural acetophenone isolated from Croton anisodontus. Revista Virtual de Química. 2020;12(3) [Google Scholar]
- 145.Medina-Alarcón K.P., L Singulani Jd, Dutra L.A., S Pitangui Nd, Pereira-da-Silva M.A., Dos Santos M.B., Ayusso G.M., Regasini L.O., Soares C.P., Chorilli M. Antifungal activity of 2′-hydroxychalcone loaded in nanoemulsion against Paracoccidioides spp. Future Microbiol. 2020;15(1):21–33. doi: 10.2217/fmb-2019-0095. [DOI] [PubMed] [Google Scholar]
- 146.Bala D., Jinga L.-I., Popa M., Hanganu A., Voicescu M., Bleotu C., Tarko L., Nica S. Design, synthesis, and biological evaluation of new azulene-containing chalcones. Materials. 2022;15(5):1629. doi: 10.3390/ma15051629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vásquez-Martínez Y.A., Osorio M.E., San Martín D.A., Carvajal M.A., Vergara A.P., Sanchez E., Raimondi M., Zacchino S.A., Mascayano C., Torrent C. Antimicrobial, anti-inflammatory and antioxidant activities of polyoxygenated chalcones. J. Braz. Chem. Soc. 2019;30:286–304. [Google Scholar]
- 148.Sardi JdCO. Antifungal activity and pharmacokinetic prediction of chalcone on phospholipase-producing C. Albicans isolates. EC Pharmacology and Toxicology. 2020;8:29–37. [Google Scholar]
- 149.Bandeira P.N., Fontenele ROdS., Costa P.S., Santos HSd, Lemos TLGd. In vitro antifungal activity against trychophyton rubrum of p-aminochalcones and 3'-methoxy-4'-hydroxy chalcone. Revista Virtual de Quimica. 2020;12(3):703–711. [Google Scholar]
- 150.Rawat P., Singh R., Ranjan A., Gautam A., Trivedi S., Kumar M. Study of antimicrobial and antioxidant activities of pyrrole-chalcones. J. Mol. Struct. 2021;1228 [Google Scholar]
- 151.Jadhav S., Peerzade N., Hublikar M., Varpe B., Kulkarni A., Bhosale R. Synthesis and pharmacological screening of difluorophenyl pyrazole chalcone conjugates as antifungal, anti-inflammatory, and antioxidant agents. Russ. J. Bioorg. Chem. 2020;46(6):1128–1135. [Google Scholar]
- 152.Ahmed N., Konduru N.K., Owais M. Design, synthesis and antimicrobial activities of novel ferrocenyl and organic chalcone based sulfones and bis-sulfones. Arab. J. Chem. 2019;12(8):1879–1894. [Google Scholar]
- 153.González L.A., Upegui Y.A., Rivas L., Echeverri F., Escobar G., Robledo S.M., Quiñones W. Effect of substituents in the A and B rings of chalcones on antiparasite activity. Arch. Pharmazie. 2020;353(12) doi: 10.1002/ardp.202000157. [DOI] [PubMed] [Google Scholar]
- 154.Nweze J.A., Mbaoji F.N., Li Y.-M., Yang L.-Y., Huang S.-S., Chigor V.N., Eze E.A., Pan L.-X., Zhang T., Yang D.-F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: a review of recent articles. Infectious Diseases of Poverty. 2021;10(1):1–19. doi: 10.1186/s40249-021-00796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Britten N.S., Butler J.A. Ruthenium metallotherapeutics: novel approaches to combatting parasitic infections. Curr. Med. Chem. 2022;29(31):5159–5178. doi: 10.2174/0929867329666220401105444. [DOI] [PubMed] [Google Scholar]
- 156.Zheoat A.M., Alenezi S., Elmahallawy E.K., Ungogo M.A., Alghamdi A.H., Watson D.G., Igoli J.O., Gray A.I., de Koning H.P., Ferro V.A. Antitrypanosomal and antileishmanial activity of chalcones and flavanones from Polygonum salicifolium. Pathogens. 2021;10(2):175. doi: 10.3390/pathogens10020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hernández-Rivera J.L., Espinoza-Hicks J.C., Chacón-Vargas K.F., Carrillo-Campos J., Sánchez-Torres L.E., Camacho-Dávila A.A. Synthesis, characterization and evaluation of prenylated chalcones ethers as promising antileishmanial compounds. Mol. Divers. 2022:1–20. doi: 10.1007/s11030-022-10542-1. [DOI] [PubMed] [Google Scholar]
- 158.Jiang L., Liu B., Hou S., Su T., Fan Q., Alyafeai E., Tang Y., Wu M., Liu X., Li J. Discovery and evaluation of chalcone derivatives as novel potential anti-Toxoplasma gondii agents. Eur. J. Med. Chem. 2022;234 doi: 10.1016/j.ejmech.2022.114244. [DOI] [PubMed] [Google Scholar]
- 159.Mendes E.P., Goulart C.M., Chaves O.A., Faiões VdS., Canto-Carvalho M.M., Machado G.C., Torres-Santos E.C., Echevarria A. Evaluation of novel chalcone-thiosemicarbazones derivatives as potential anti-Leishmania amazonensis agents and its HSA binding studies. Biomolecules. 2019;9(11):643. doi: 10.3390/biom9110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bezerra L.L., Almeida-Neto F.W.Q., Marinho M.M., Santos Oliveira L., Teixeira A.M.R., Bandeira P.N., Dos Santos H.S., Lima-Neto Pd, Marinho E.S. Synthesis of aminochalcones and in silico evaluation of their antiparasitic potential against Leishmania. J. Biomol. Struct. Dyn. 2022:1–8. doi: 10.1080/07391102.2022.2103030. [DOI] [PubMed] [Google Scholar]
- 161.U Jp N'Guessan D., Ac Kablan L., Kacou A., Bories C., Coulibaly S., Sissouma D., M Loiseau P., Ouattara M. 2021. Synthesis and Biological Profiles of Some Benzimidazolyl-Chalcones as Anti-leishmanial and Trypanocidal Agents. [Google Scholar]
- 162.Yepes A.F., Quintero‐Saumeth J., Cardona‐G W. Chalcone‐quinoline conjugates as potential T. Cruzi cruzipain inhibitors: docking studies, molecular dynamics and evaluation of drug‐likeness. ChemistrySelect. 2020;5(23):7104–7112. [Google Scholar]
- 163.Magalhães E.P., Gomes N.D.B., de Freitas T.A., Silva B.P., Ribeiro L.R., Ameida-Neto F.W.Q., Marinho M.M., de Lima-Neto P., Marinho E.S., Dos Santos H.S. Chloride substitution on 2-hydroxy-3, 4, 6-trimethoxyphenylchalcones improves in vitro selectivity on Trypanosoma cruzi strain Y. Chem. Biol. Interact. 2022;361 doi: 10.1016/j.cbi.2022.109920. [DOI] [PubMed] [Google Scholar]
- 164.Trein M.R., Rodrigues e Oliveira L., Rigo G.V., Garcia M.A.R., Petro-Silveira B., da Silva Trentin D., Macedo A.J., Regasini L.O., Tasca T. Anti-Trichomonas vaginalis activity of chalcone and amino-analogues. Parasitol. Res. 2019;118(2):607–615. doi: 10.1007/s00436-018-6164-4. [DOI] [PubMed] [Google Scholar]
- 165.Sinha S., Radotra B., Medhi B., Batovska D.I., Markova N., Sehgal R. Ultrastructural alterations in Plasmodium falciparum induced by chalcone derivatives. BMC Res. Notes. 2020;13(1):1–6. doi: 10.1186/s13104-020-05132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cavalcante C.H.L., Almeida-Neto FWdQ., da Rocha M.N., Bandeira P.N., de Menezes R.R.P.P.B., Paula Magalhães E., Sampaio T.L., Marinho E.S., Marinho M.M., Maria Costa Martins A. Antichagasic evaluation, molecular docking and ADMET properties of the chalcone (2 E)-3-(2-fluorophenyl)-1-(2-hydroxy-3, 4, 6-trimethoxyphenyl) prop-2-en-1-one against Trypanosoma cruzi. J. Biomol. Struct. Dyn. 2022:1–17. doi: 10.1080/07391102.2022.2123394. [DOI] [PubMed] [Google Scholar]
- 167.Garcia A.R., Oliveira D.M., Jesus J.B., Souza A.M., Sodero A.C.R., Vermelho A.B., Leal I.C., Souza R.O.M., Miranda L.S., Pinheiro A.S. Identification of chalcone derivatives as inhibitors of Leishmania infantum arginase and promising antileishmanial agents. Front. Chem. 2021;8 doi: 10.3389/fchem.2020.624678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ortalli M., Ilari A., Colotti G., De Ionna I., Battista T., Bisi A., Gobbi S., Rampa A., Di Martino R.M., Gentilomi G.A. Identification of chalcone-based antileishmanial agents targeting trypanothione reductase. Eur. J. Med. Chem. 2018;152:527–541. doi: 10.1016/j.ejmech.2018.04.057. [DOI] [PubMed] [Google Scholar]
- 169.Jyoti Gaur R., Kumar Y., Cheema H.S., Kapkoti D.S., Darokar M.P., Khan F., Bhakuni R.S. Synthesis, molecular modelling studies of indolyl chalcone derivatives and their antimalarial activity evaluation. Nat. Prod. Res. 2021;35(19):3261–3268. doi: 10.1080/14786419.2019.1696788. [DOI] [PubMed] [Google Scholar]
- 170.Pozzetti L., Ibba R., Rossi S., Taglialatela-Scafati O., Taramelli D., Basilico N., D'Alessandro S., Parapini S., Butini S., Campiani G. Total synthesis of the natural chalcone lophirone E, synthetic studies toward benzofuran and indole-based analogues, and investigation of anti-leishmanial activity. Molecules. 2022;27(2):463. doi: 10.3390/molecules27020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.N'guessan D.U.J.-P., Alzain A.A., Adouko E.M., Sékou D., Coulibaly S., Ouattara M. Molecular modeling studies of benzimidazolyl-chalcones as antileishmanial agents using qsar, docking, ADME and molecular dynamics studies. Journal of Applied Pharmaceutical Sciences and Research. 2022;4(3):18–28. [Google Scholar]
- 172.Cherkasov A., Muratov E.N., Fourches D., Varnek A., Baskin, Cronin M., Dearden J., Gramatica P., Martin Y.C., Todeschini R. QSAR modeling: where have you been? Where are you going to? J. Med. Chem. 2014;57(12):4977–5010. doi: 10.1021/jm4004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu M., Wu P., Shen F., Ji J., Rakesh K. Chalcone derivatives and their antibacterial activities: current development. Bioorg. Chem. 2019;91 doi: 10.1016/j.bioorg.2019.103133. [DOI] [PubMed] [Google Scholar]
- 174.Kitawat B.S., Singh M., Kale R.K. Solvent free synthesis, characterization, anticancer, antibacterial, antifungal, antioxidant and SAR studies of novel (E)-3-aryl-1-(3-alkyl-2-pyrazinyl)-2-propenone. New J. Chem. 2013;37(8):2541–2550. [Google Scholar]
- 175.Chen Z.-H., Zheng C.-J., Sun L.-P., Piao H.-R. Synthesis of new chalcone derivatives containing a rhodanine-3-acetic acid moiety with potential anti-bacterial activity. Eur. J. Med. Chem. 2010;45(12):5739–5743. doi: 10.1016/j.ejmech.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 176.Abdullah M.I., Mahmood A., Madni M., Masood S., Kashif M. Synthesis, characterization, theoretical, anti-bacterial and molecular docking studies of quinoline based chalcones as a DNA gyrase inhibitor. Bioorg. Chem. 2014;54:31–37. doi: 10.1016/j.bioorg.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 177.Sivakumar P.M., Muthu Kumar T., Doble M. Antifungal activity, mechanism and QSAR studies on chalcones. Chem. Biol. Drug Des. 2009;74(1):68–79. doi: 10.1111/j.1747-0285.2009.00828.x. [DOI] [PubMed] [Google Scholar]
- 178.Sivakumar P.M., Priya S., Doble M. Synthesis, biological evaluation, mechanism of action and quantitative structure–activity relationship studies of chalcones as antibacterial agents. Chem. Biol. Drug Des. 2009;73(4):403–415. doi: 10.1111/j.1747-0285.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 179.Fu Y., Liu D., Zeng H., Ren X., Song B., Hu D., Gan X. New chalcone derivatives: synthesis, antiviral activity and mechanism of action. RSC advances. 2020;10(41):24483–24490. doi: 10.1039/d0ra03684f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.