Abstract
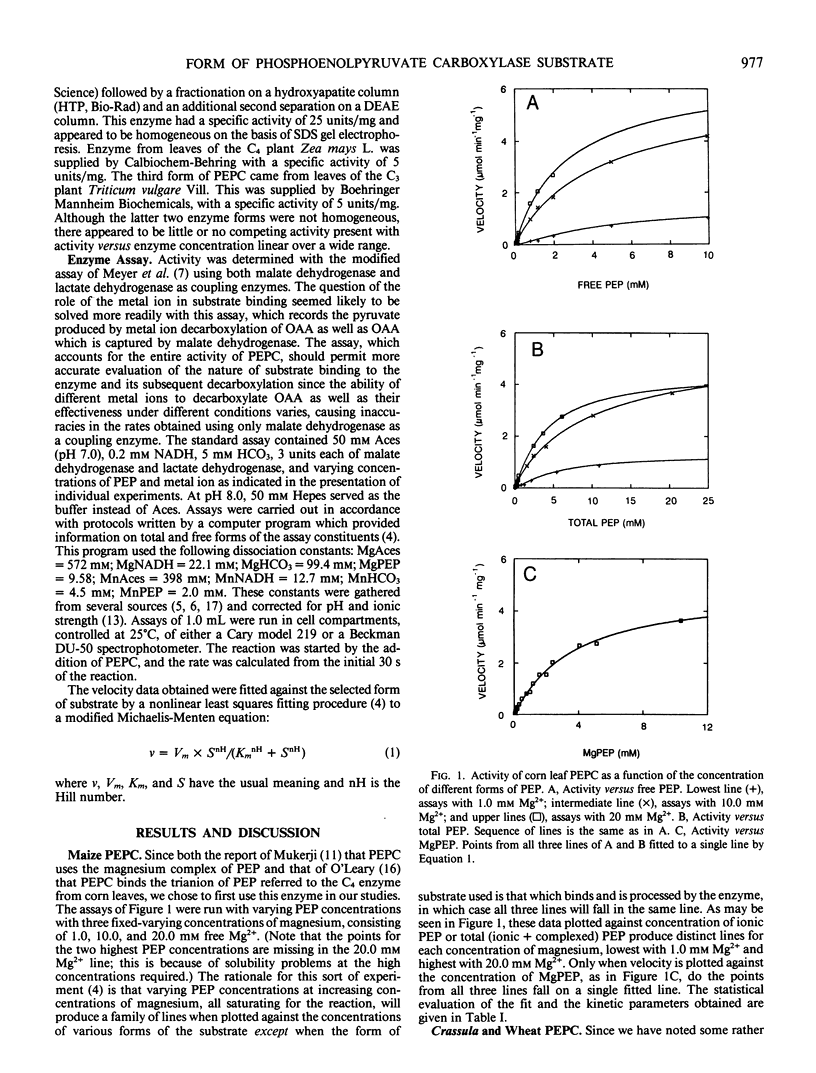
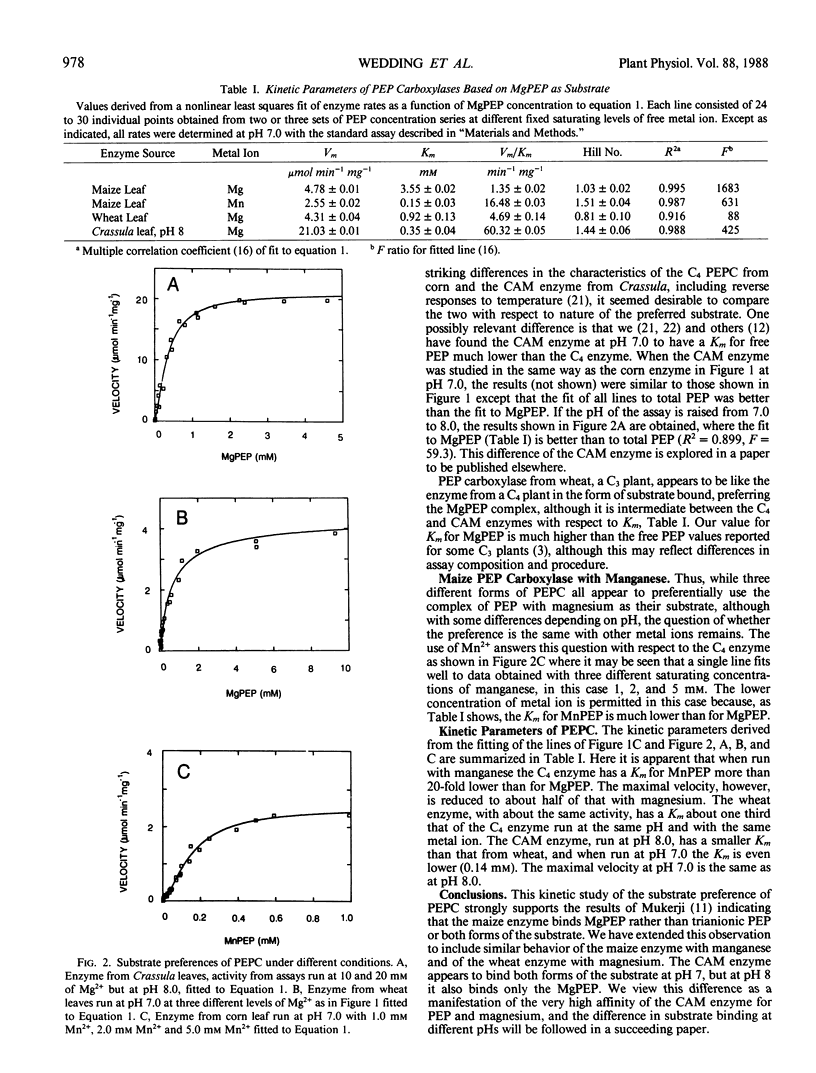
Phosphoenolpyruvate carboxylase isolated from maize (Zea mays L.) leaves was assayed with varying concentrations of free phosphoenolpyruvate at several fixed-varying concentrations of free magnesium higher than required to saturate the enzyme reaction. These assays produced velocity data which were found to form a family of individual lines when plotted against free phosphoenolpyruvate or against total phosphoenolpyruvate, but not when plotted against the concentration of the complex of phosphoenolpyruvate with magnesium. In this latter case, the points from all the fixed-varying concentrations fell on the same line, which can be fitted to a modified Michaelis-Menten equation with a multiple correlation coefficient R2 = 0.995. Similar results were obtained when the enzyme from the C4 plant maize was assayed with manganese rather than magnesium and when phosphoenolpyruvate carboxylase from leaves of the C3 plant wheat (Triticum vulgare Vill.) was assayed with magnesium. However, at pH 7.0 the enzyme from the Crassulacean acid metabolism plant Crassula argentea did not produce a satisfactory single line when plotted against the complex of metal ion and substrate, but did so when the assay pH was raised to 8.0. It is concluded that in general the preferred form of substrate for phosphoenolpyruvate carboxylase is the complex of phosphoenolpyruvate with the metal ion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., GREINER C. M. The enzymatic synthesis of oxalacetate from phosphoryl-enolpyruvate and carbon dioxide. J Biol Chem. 1953 Oct;204(2):781–786. [PubMed] [Google Scholar]
- Bauwe H., Chollet R. Kinetic properties of phosphoenolpyruvate carboxylase from c(3), c(4), and c(3)-c(4) intermediate species of flaveria (asteraceae). Plant Physiol. 1986 Nov;82(3):695–699. doi: 10.1104/pp.82.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canellas P. F., Wedding R. T. Substrate and metal ion interactions in the NAD+ malic enzyme from cauliflower. Arch Biochem Biophys. 1980 Jan;199(1):259–264. doi: 10.1016/0003-9861(80)90279-9. [DOI] [PubMed] [Google Scholar]
- Meyer C. R., Rustin P., Wedding R. T. A simple and accurate spectrophotometric assay for phosphoenolpyruvate carboxylase activity. Plant Physiol. 1988 Feb;86(2):325–328. doi: 10.1104/pp.86.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. S., Mildvan A. S., Chang H. C., Easterday R. L., Maruyama H., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. IV. The binding of manganese and substrates by phosphoenolpyruvate carbosy-kinase and phosphoenolypyruvate carboxylase. J Biol Chem. 1968 Nov 25;243(22):6030–6040. [PubMed] [Google Scholar]
- Miziorko H. M., Nowak T., Mildvan A. S. Spinach leaf phosphoenolpyruvate carboxylase: purification, properties, and kinetic studies. Arch Biochem Biophys. 1974 Jul;163(1):378–389. doi: 10.1016/0003-9861(74)90489-5. [DOI] [PubMed] [Google Scholar]
- Mukerji S. K. Corn leaf phosphoenolpyruvate carboxylases. The effect of divalent cations on activity. Arch Biochem Biophys. 1977 Jul;182(1):352–359. doi: 10.1016/0003-9861(77)90316-2. [DOI] [PubMed] [Google Scholar]
- Nguyen T. T., Ngam-Ek A., Jenkins J., Grover S. D. Metal Ion Interactions with Phosphoenolpyruvate Carboxylase from Crassula argentea and Zea mays. Plant Physiol. 1988 Jan;86(1):104–107. doi: 10.1104/pp.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary M. H., Rife J. E., Slater J. D. Kinetic and isotope effect studies of maize phosphoenolpyruvate carboxylase. Biochemistry. 1981 Dec 8;20(25):7308–7314. doi: 10.1021/bi00528a040. [DOI] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K. Role of Magnesium in the Binding of Substrate and Effectors to Phosphoenolpyruvate Carboxylase from a CAM Plant. Plant Physiol. 1988 Jun;87(2):443–446. doi: 10.1104/pp.87.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Diurnal regulation of phosphoenolpyruvate carboxylase from crassula. Plant Physiol. 1985 Mar;77(3):667–675. doi: 10.1104/pp.77.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Temperature Effects on Phosphoenolpyruvate Carboxylase from a CAM and a C(4) Plant : A Comparative Study. Plant Physiol. 1987 Oct;85(2):497–501. doi: 10.1104/pp.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]


