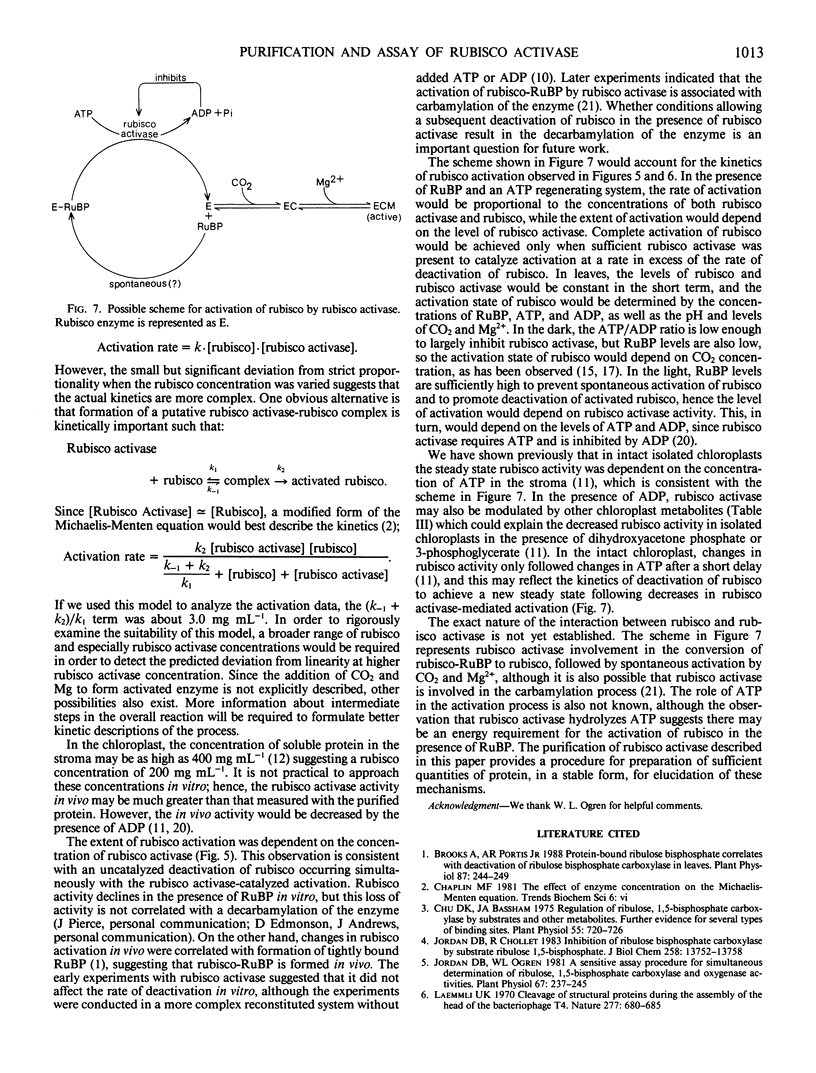
Abstract
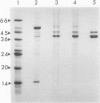
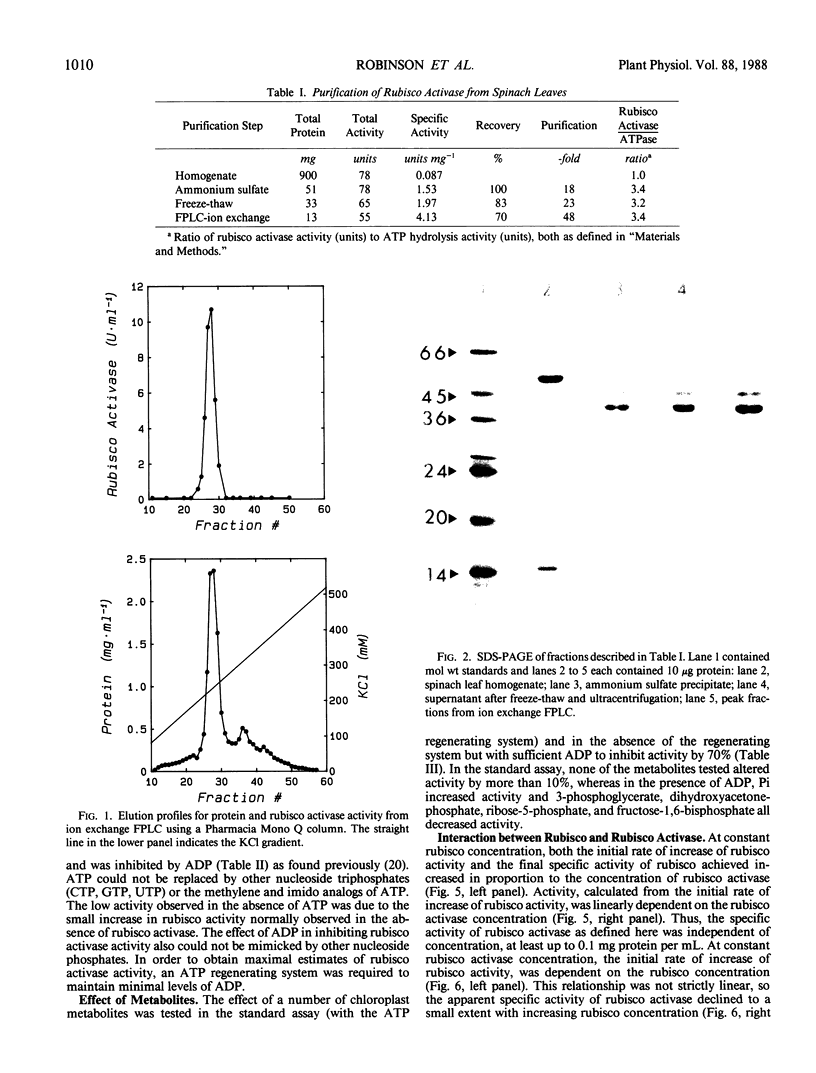
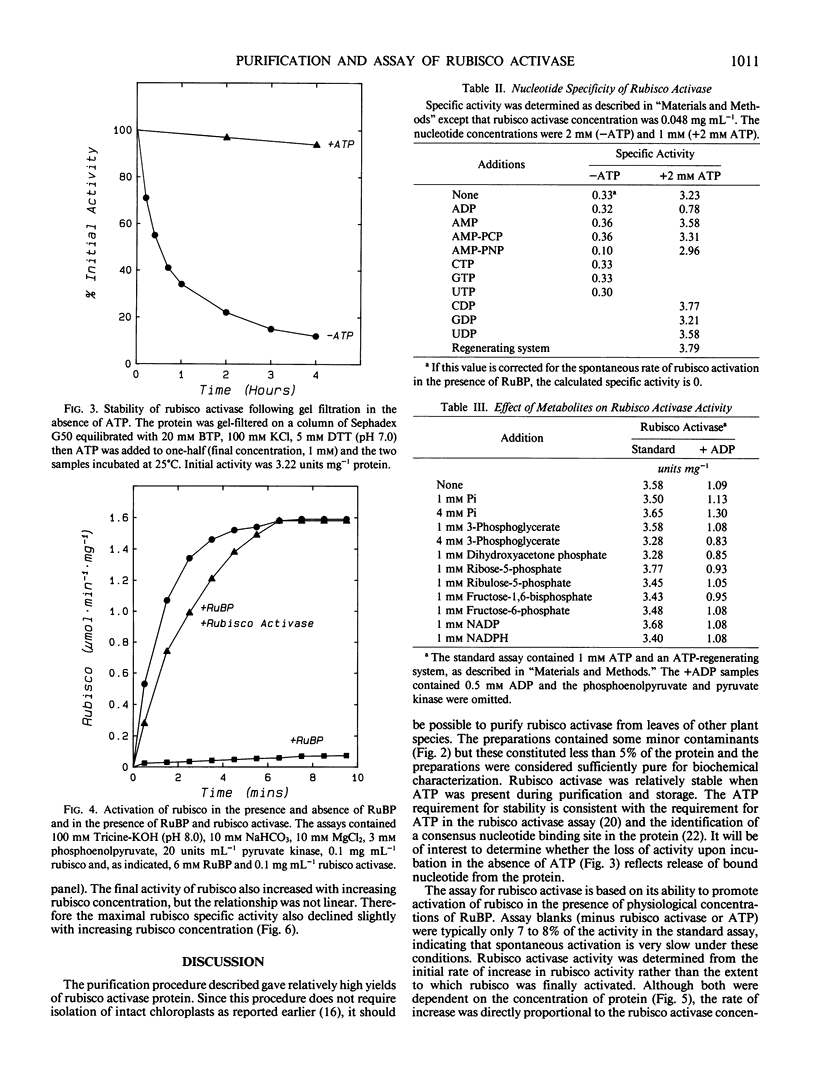
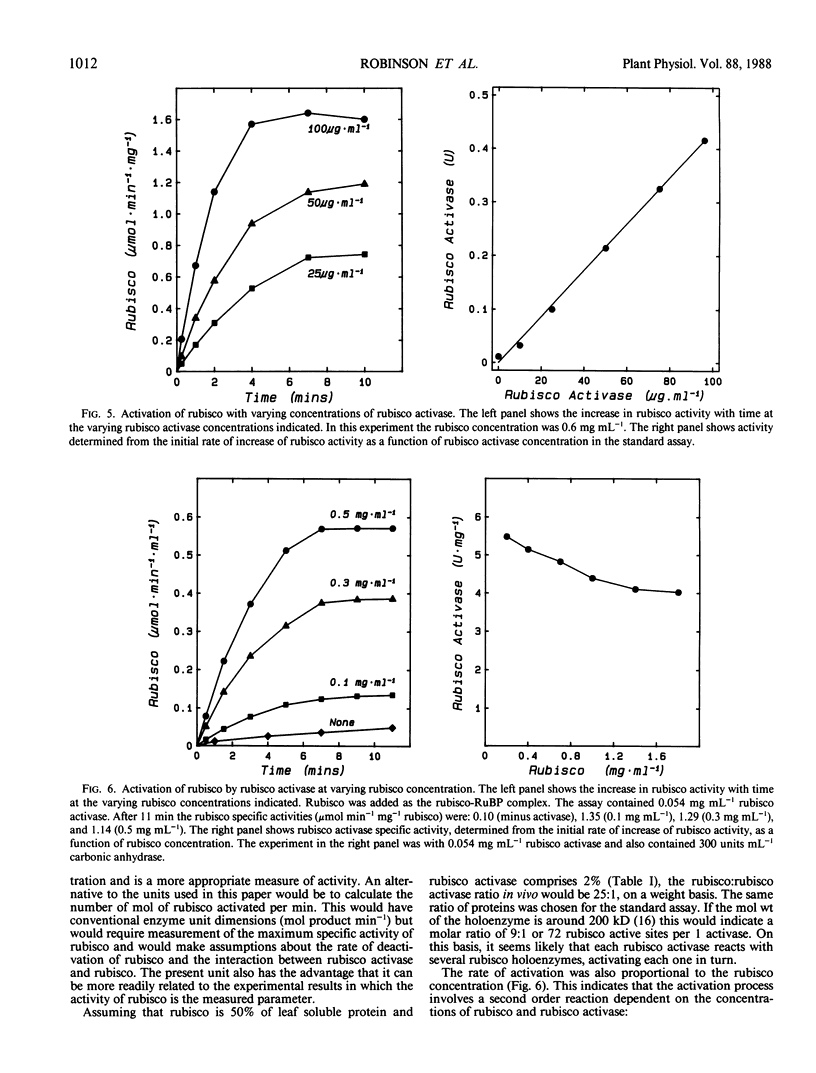
Ribulose 1,5-bisphosphate carboxylase/oxygenase (rubisco) activase protein was purified from spinach leaves by ammonium sulfate precipitation and ion exchange fast protein liquid chromatography. This resulted in 48-fold purification with 70% recovery of activity and yielded up to 18 milligrams of rubisco activase protein from 100 grams of leaves. Based on these figures, the protein comprised approximately 2% by weight of soluble protein in spinach (Spinacia oleracea L.) leaves. The preparations were at least 95% pure and were stable when frozen in liquid nitrogen. Addition of ATP during purification and storage was necessary to maintain activity. Assay of rubisco activase was based on its ability to promote activation of rubisco in the presence of ribulose-1,5-bisphosphate. There was an absolute requirement for ATP which could not be replaced by other nucleoside phosphates. The initial rate of increase of rubisco activity and the final rubisco specific activity achieved were both dependent on the concentration of rubisco activase. The initial rate was directly proportional to the rubisco activase concentration and was used as the basis of activity. The rate of activation of rubisco was also dependent on the rubisco concentration, suggesting that the activation process is a second order reaction dependent on the concentrations of both rubisco and rubisco activase. It is suggested that deactivation of rubisco occurs simultaneously with rubisco activase-mediated activation, and that rubisco activation state represents a dynamic equilibrium between these two processes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks A., Portis A. R. Protein-bound ribulose bisphosphate correlates with deactivation of ribulose bisphosphate carboxylase in leaves. Plant Physiol. 1988 May;87(1):244–249. doi: 10.1104/pp.87.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. K., Bassham J. A. Regulation of ribulose 1,5-diphosphate carboxylase by substrates and other metabolites: further evidence for several types of binding sites. Plant Physiol. 1975 Apr;55(4):720–726. doi: 10.1104/pp.55.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. B., Chollet R. Inhibition of ribulose bisphosphate carboxylase by substrate ribulose 1,5-bisphosphate. J Biol Chem. 1983 Nov 25;258(22):13752–13758. [PubMed] [Google Scholar]
- Jordan D. B., Ogren W. L. A Sensitive Assay Procedure for Simultaneous Determination of Ribulose-1,5-bisphosphate Carboxylase and Oxygenase Activities. Plant Physiol. 1981 Feb;67(2):237–245. doi: 10.1104/pp.67.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Miziorko H. M. Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+. Biochemistry. 1980 Nov 11;19(23):5321–5328. doi: 10.1021/bi00564a027. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Salvucci M. E., Ogren W. L. Activation of Ribulosebisphosphate Carboxylase/Oxygenase at Physiological CO(2) and Ribulosebisphosphate Concentrations by Rubisco Activase. Plant Physiol. 1986 Dec;82(4):967–971. doi: 10.1104/pp.82.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988 Jan;86(1):293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Portis A. R., Jr, Ogren W. L. Purification of ribulose-1, 5-bisphosphate carboxylase/oxygenase with high specific activity by fast protein liquid chromatography. Anal Biochem. 1986 Feb 15;153(1):97–101. doi: 10.1016/0003-2697(86)90066-7. [DOI] [PubMed] [Google Scholar]
- Salvucci M. E., Portis A. R., Ogren W. L. Light and CO(2) Response of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Activation in Arabidopsis Leaves. Plant Physiol. 1986 Mar;80(3):655–659. doi: 10.1104/pp.80.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Werneke J. M., Ogren W. L., Portis A. R. Purification and species distribution of rubisco activase. Plant Physiol. 1987 Jul;84(3):930–936. doi: 10.1104/pp.84.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. R., Portis A. R., Ogren W. L. A Mutant of Arabidopsis thaliana Which Lacks Activation of RuBP Carboxylase In Vivo. Plant Physiol. 1982 Aug;70(2):381–387. doi: 10.1104/pp.70.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Streusand V. J., Portis A. R. Rubisco Activase Mediates ATP-Dependent Activation of Ribulose Bisphosphate Carboxylase. Plant Physiol. 1987 Sep;85(1):152–154. doi: 10.1104/pp.85.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke J. M., Chatfield J. M., Ogren W. L. Catalysis of Ribulosebisphosphate Carboxylase/Oxygenase Activation by the Product of a Rubisco Activase cDNA Clone Expressed in Escherichia coli. Plant Physiol. 1988 Aug;87(4):917–920. doi: 10.1104/pp.87.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke J. M., Zielinski R. E., Ogren W. L. Structure and expression of spinach leaf cDNA encoding ribulosebisphosphate carboxylase/oxygenase activase. Proc Natl Acad Sci U S A. 1988 Feb;85(3):787–791. doi: 10.1073/pnas.85.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]