Abstract
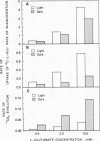
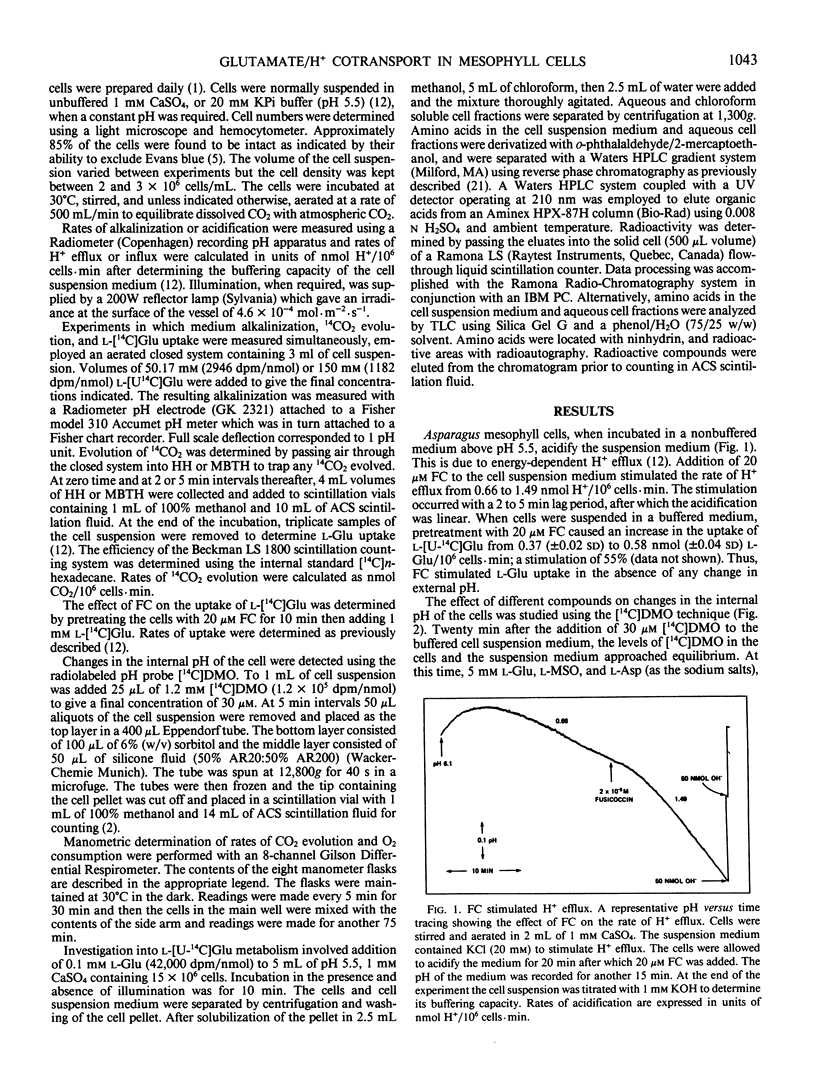
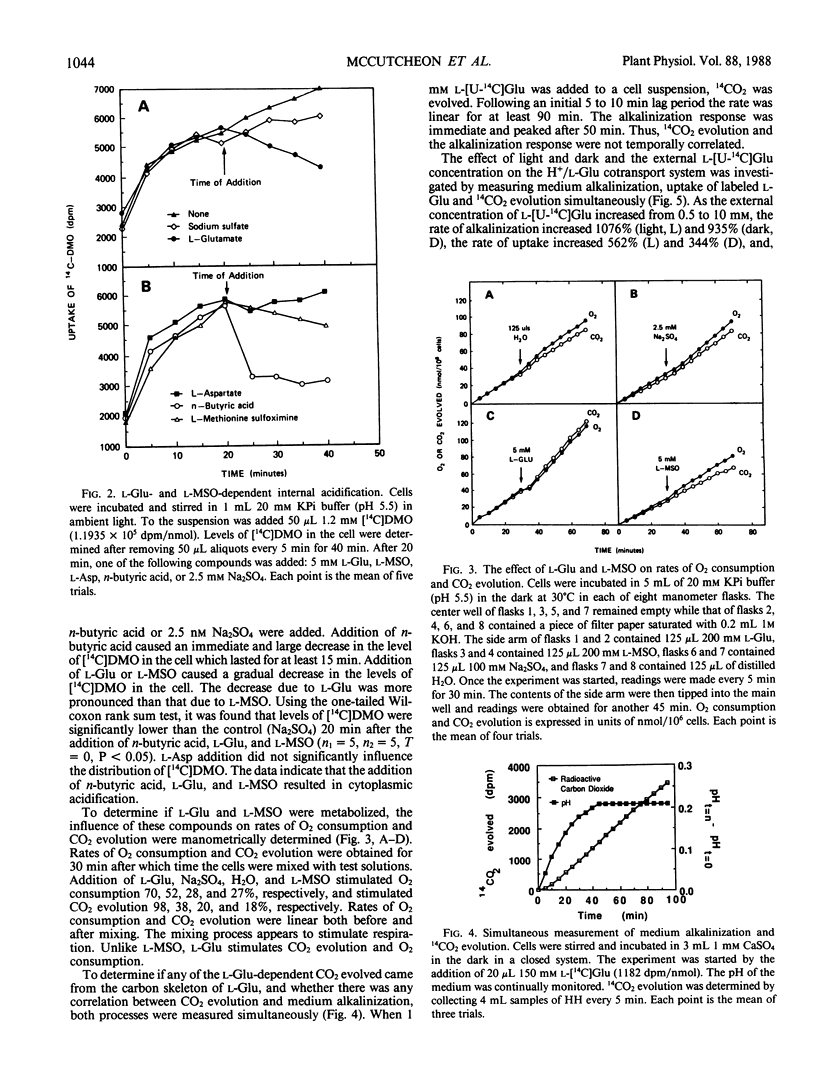
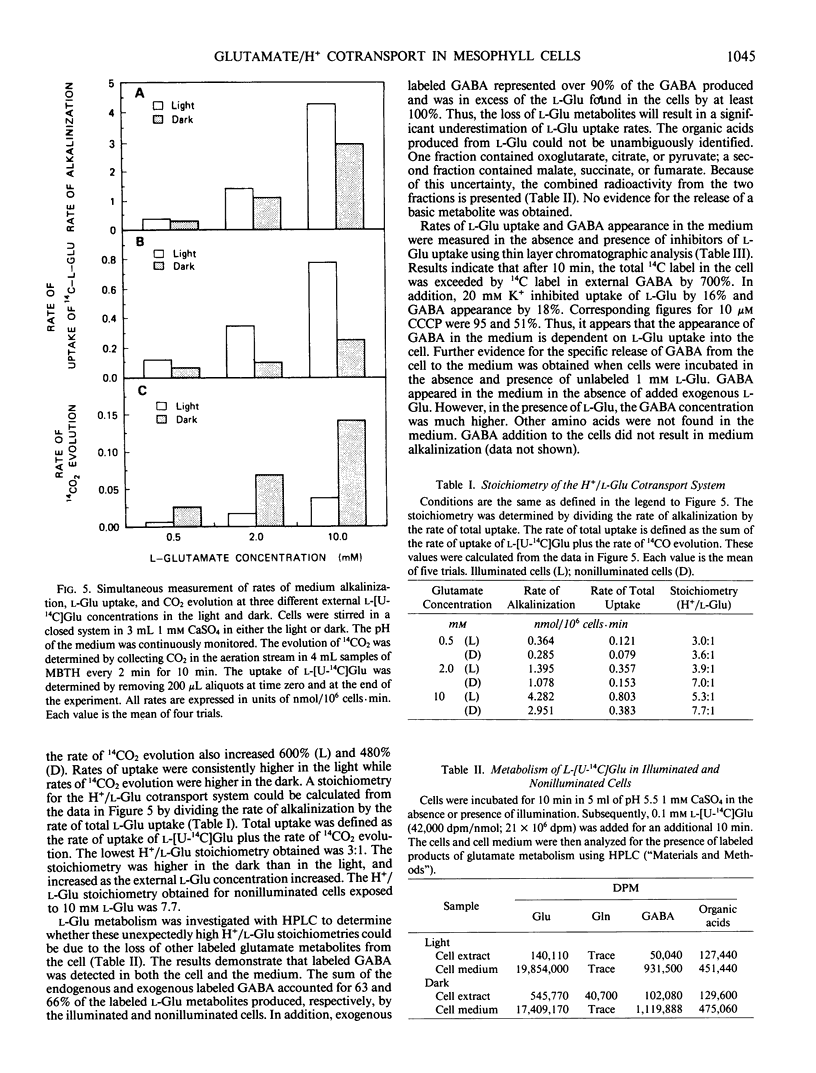
Mechanically isolated Asparagus sprengeri Regel mesophyll cells cause alkalinization of the suspension medium on the addition of l-glutamate or its analog l-methionine-d,l-sulfoximine. Using a radiolabeled pH probe, it was found that both compounds caused internal acidification whereas l-aspartate did not. Fusicoccin stimulated H+ efflux from the cells by 111% and the uptake of l-[U-14C]glutamate by 55%. Manometric experiments demonstrated that, unlike l-methionine-d,l-sulfoximine, l-glutamate stimulated CO2 evolution from nonilluminated cells. Simultaneous measurements of medium alkalinization and 14CO2 evolution upon the addition of labeled l-glutamate showed that alkalinization was immediate and reached a maximum value after 45 minutes whereas 14CO2 evolution exhibited a lag before its appearance and continued in a linear manner for at least 100 minutes. Rates of alkalinization and uptake of l-[U-14C]glutamate were higher in the light while rates of 14CO2 evolution were higher in the dark. The major labeled product of glutamate decarboxylation, γ-aminobutyric acid, was found in the cells and the suspension medium. Its addition to the cell suspension did not result in medium alkalinization and evidence indicates that it is lost from the cell to the medium. The data suggest that the origin of medium alkalinization is co-transport not metabolism, and that the loss of labeled CO2 and γ-aminobutyric acid from the cell result in an overestimation of the stoichiometry of the H+/l-glutamate uptake process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jordan B. R., Givan C. V. Effects of Light and Inhibitors on Glutamate Metabolism in Leaf Discs of Vicia faba L: Sources of ATP for Glutamine Synthesis and Photoregulation of Tricarboxylic Acid Cycle Metabolism. Plant Physiol. 1979 Dec;64(6):1043–1047. doi: 10.1104/pp.64.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Newman I. A., Etherton B. A Quantitative Simulation Model for H-Amino Acid Cotransport To Interpret the Effects of Amino Acids on Membrane Potential and Extracellular pH. Plant Physiol. 1984 Nov;76(3):806–813. doi: 10.1104/pp.76.3.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Energetics of sucrose transport into protoplasts from developing soybean cotyledons. Plant Physiol. 1985 May;78(1):41–45. doi: 10.1104/pp.78.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon S. L., Bown A. W. Evidence for a specific glutamate/h cotransport in isolated mesophyll cells. Plant Physiol. 1987 Mar;83(3):691–697. doi: 10.1104/pp.83.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio R. A., Rowe W. B., Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969 Mar;8(3):1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Walker K. A., Givan C. V., Keys A. J. Glutamic Acid metabolism and the photorespiratory nitrogen cycle in wheat leaves: metabolic consequences of elevated ammonia concentrations and of blocking ammonia assimilation. Plant Physiol. 1984 May;75(1):60–66. doi: 10.1104/pp.75.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. E., Komor E. Mechanism of amino Acid uptake by sugarcane suspension cells. Plant Physiol. 1984 Dec;76(4):865–870. doi: 10.1104/pp.76.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]