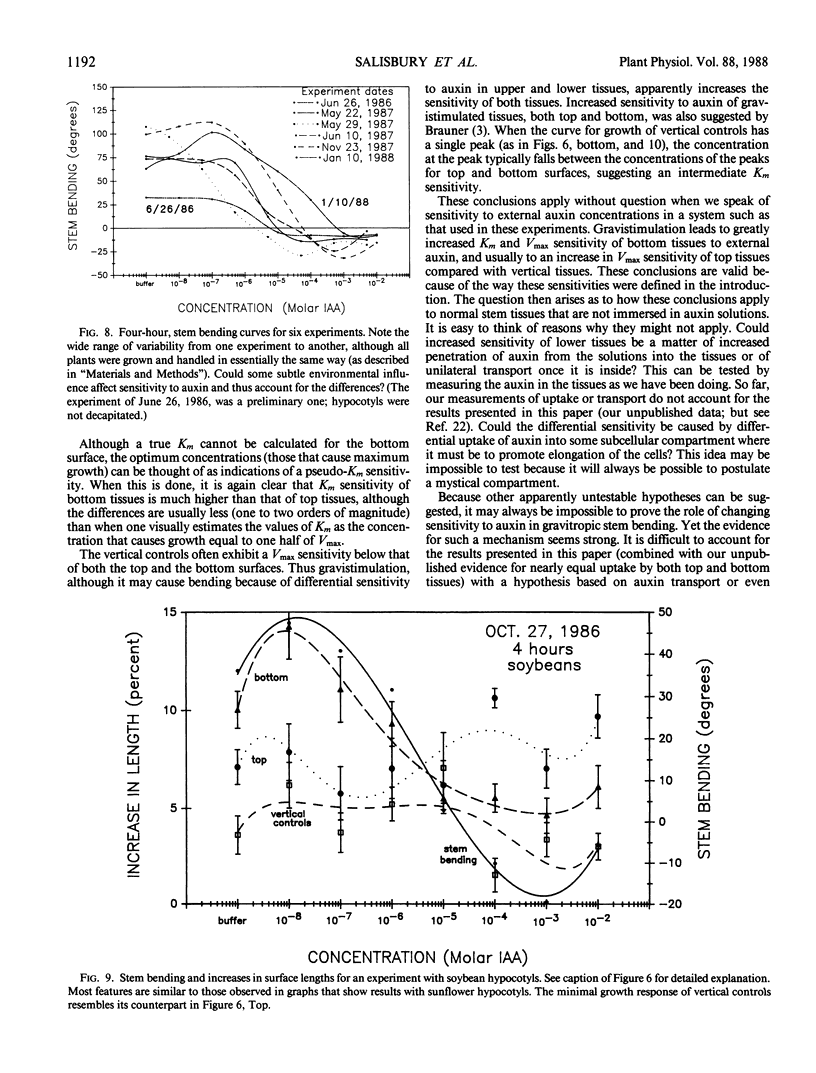
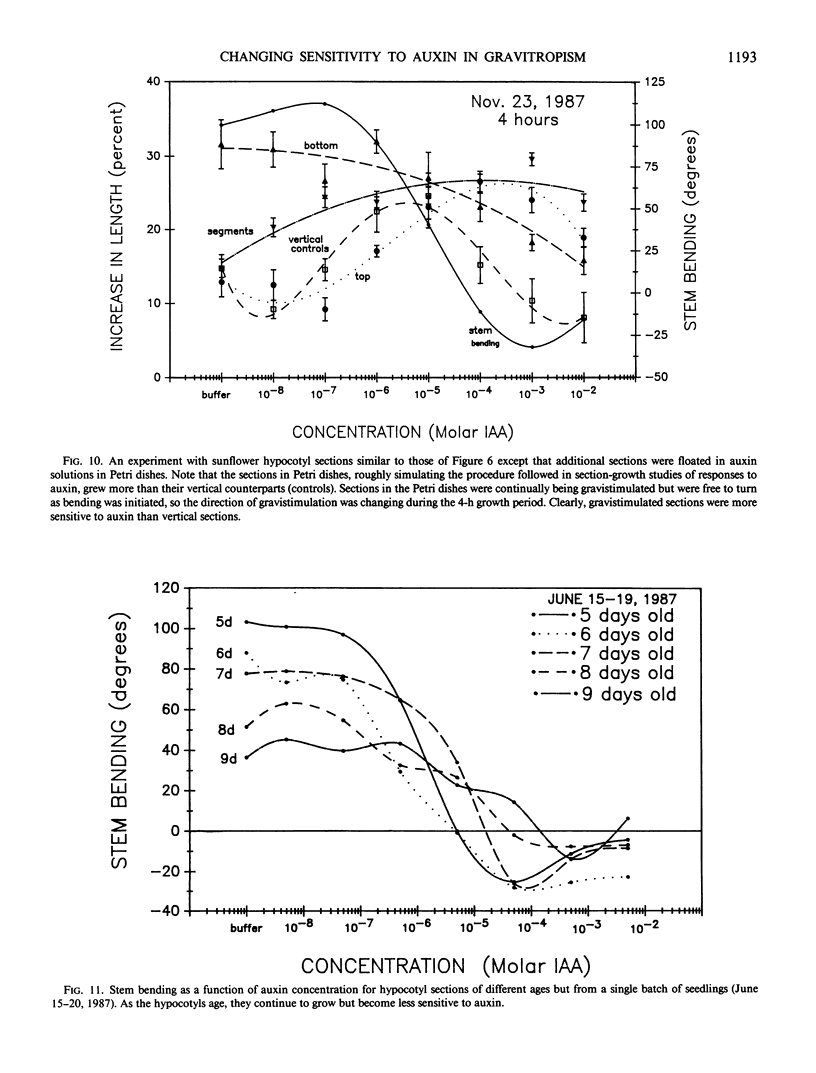
Abstract
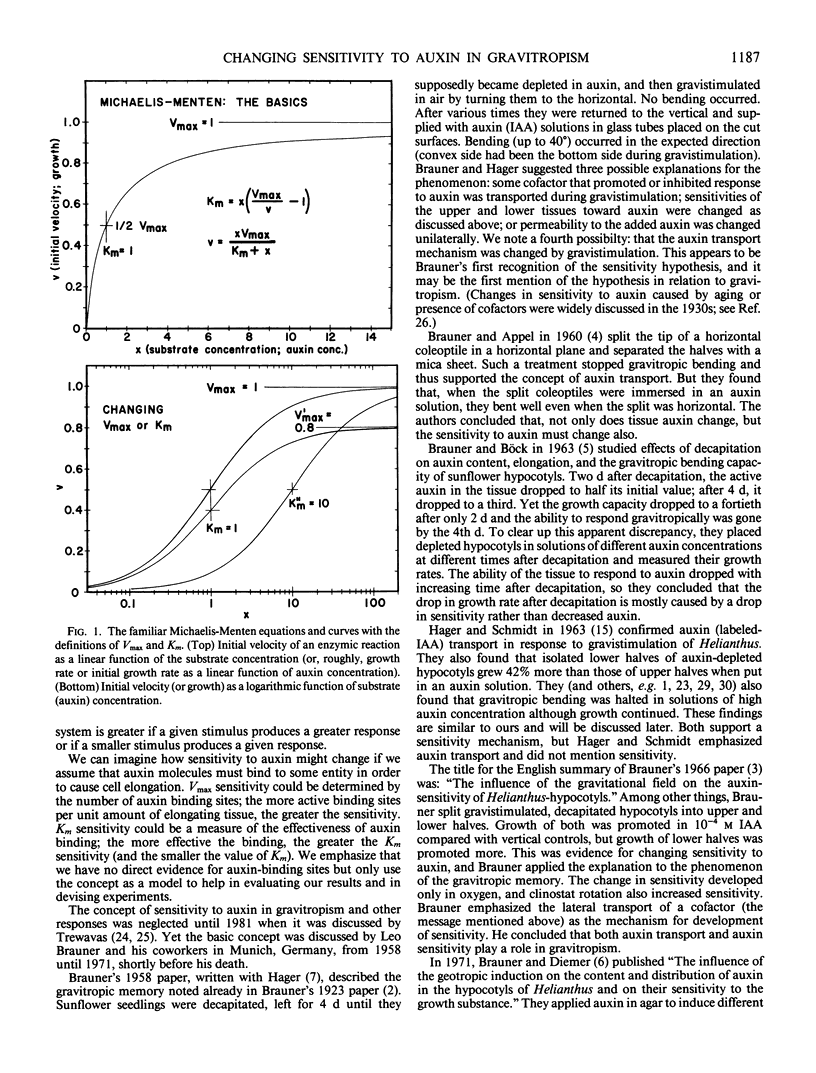
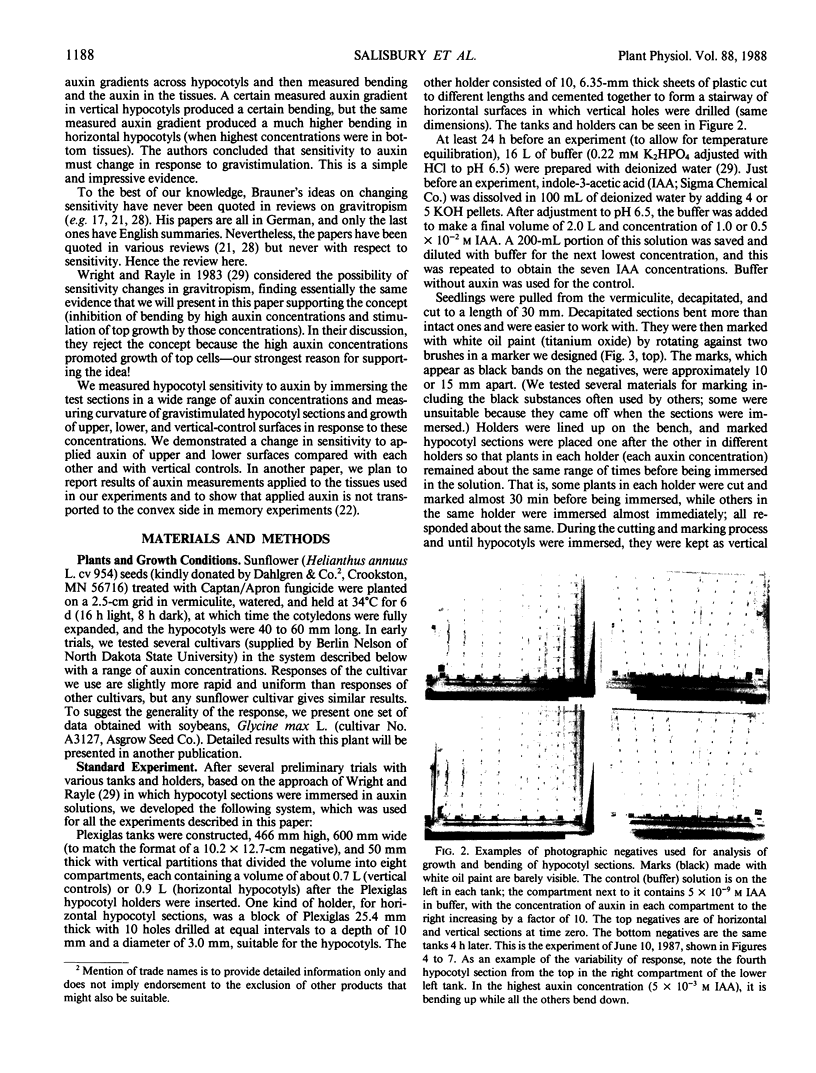
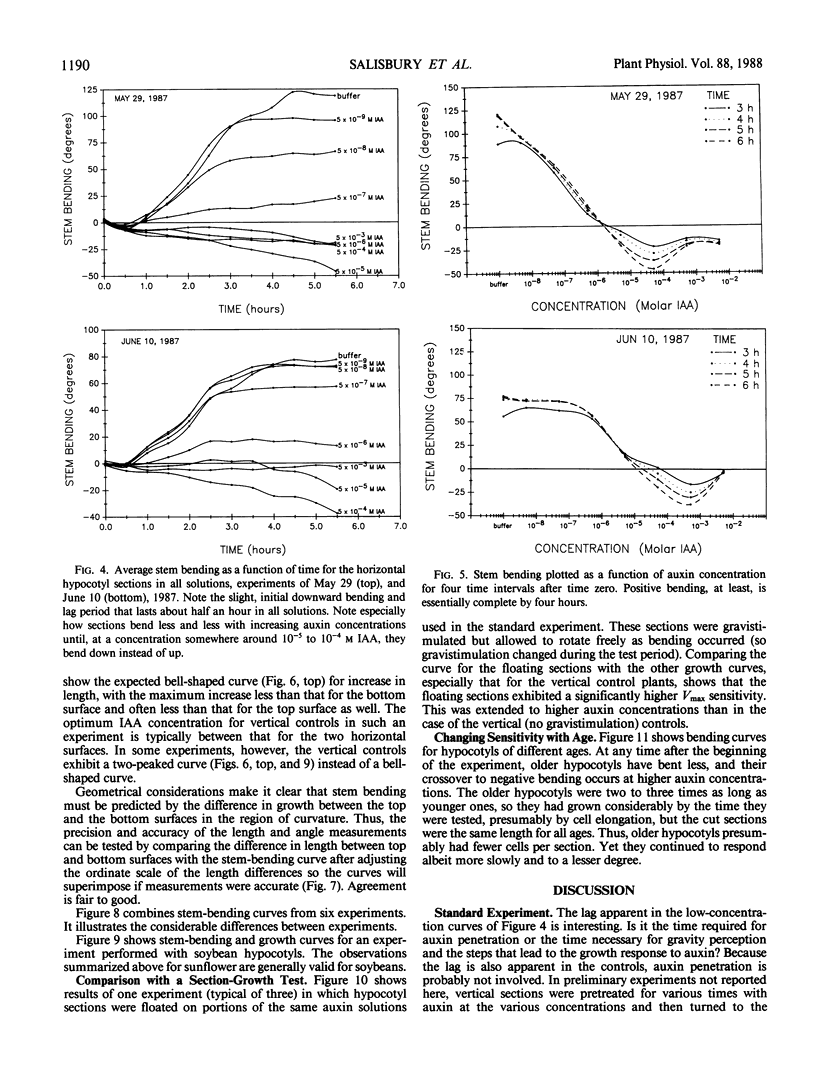
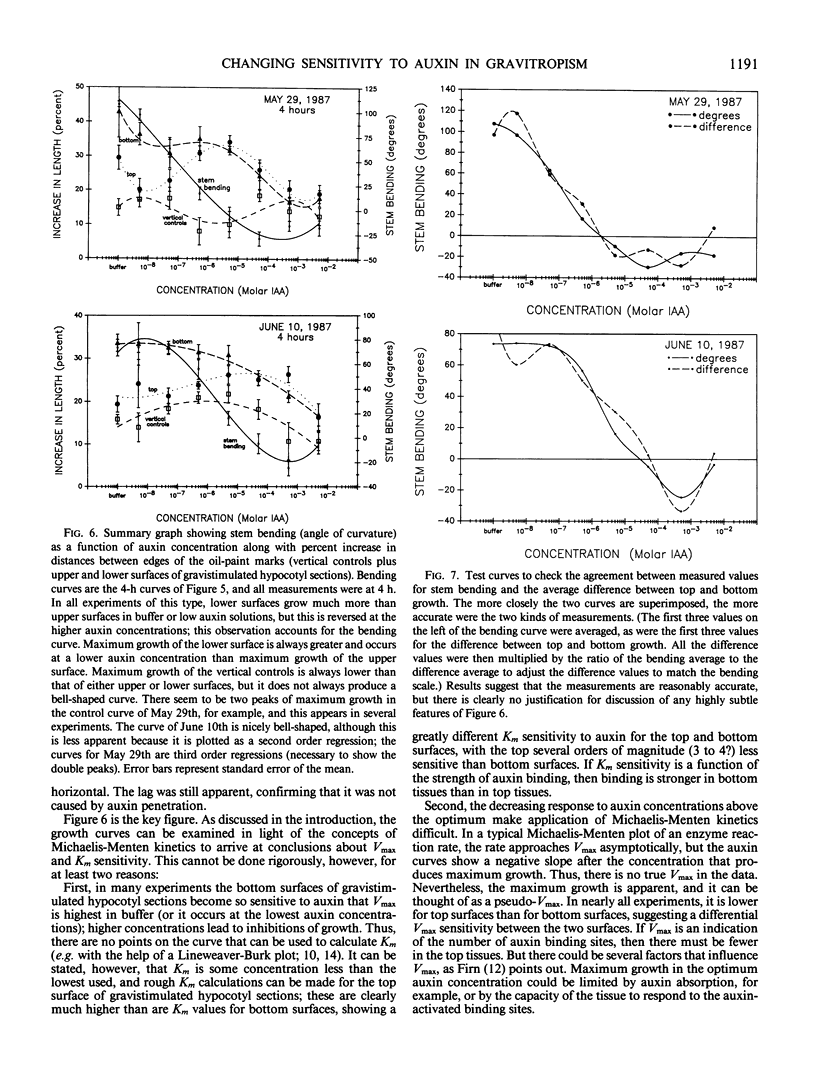
An alternative to the Cholodny-Went, auxin-transport hypothesis of gravitropic stem bending was proposed as early as 1958, suggesting that gravistimulation induces changes in sensitivity to auxin, accounting for differential growth and bending. To test the sensitivity hypothesis, we immersed marked, decapitated sunflower (Helianthus annuus L.) hypocotyl sections in buffered auxin solutions over a wide concentration range (0, 10−8 to 10−2 molar IAA), photographed them at half-hour intervals, analyzed the negatives with a digitizer/computer, and evaluated surface-length changes in terms of Michaelis-Menten enzyme kinetics. Bending decreases with increasing auxin concentration; above about 10−4 molar IAA the hypocotyls bend down; increasing auxin inhibits elongation growth of lower surfaces (which is high at zero or relatively low auxin levels) but promotes upper-surface growth (which is low at low auxin levels). Thus, lower surfaces have a greater Km sensitivity to applied auxin than upper surfaces. At optimum auxin levels (maximum growth), growth of bottom surfaces exceeds that of top surfaces, so bottom tissues have a greater Vmax sensitivity. Vmax sensitivity of vertical controls is slightly lower than it is for either horizontal surface; Km sensitivity is intermediate. Clearly, gravistimulation leads to significant changes in tissue sensitivity to applied auxin. Perhaps these changes are also important in normal gravitropism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. G., Kaufman P. B. Altered growth response to exogenous auxin and gibberellic acid by gravistimulation in pulvini of Avena sativa. Plant Physiol. 1988;87:130–133. doi: 10.1104/pp.87.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. J., McRae D. H., Bonner J. Auxin-Induced Growth Inhibition a Natural Consequence of Two-Point Attachment. Proc Natl Acad Sci U S A. 1952 Dec;38(12):1014–1022. doi: 10.1073/pnas.38.12.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idso S. B. Three Phases of Plant Response to Atmospheric CO(2) Enrichment. Plant Physiol. 1988 May;87(1):5–7. doi: 10.1104/pp.87.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald I. R., Hart J. W. New light on the cholodny-went theory. Plant Physiol. 1987 Jul;84(3):568–570. doi: 10.1104/pp.84.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meudt W. J. Investigations on the Mechanism of the Brassinosteroid Response: VI. Effect of Brassinolide on Gravitropism of Bean Hypocotyls. Plant Physiol. 1987 Jan;83(1):195–198. doi: 10.1104/pp.83.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L. Z., Rayle D. L. Evidence for a Relationship between H Excretion and Auxin in Shoot Gravitropism. Plant Physiol. 1983 May;72(1):99–104. doi: 10.1104/pp.72.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]




