Abstract
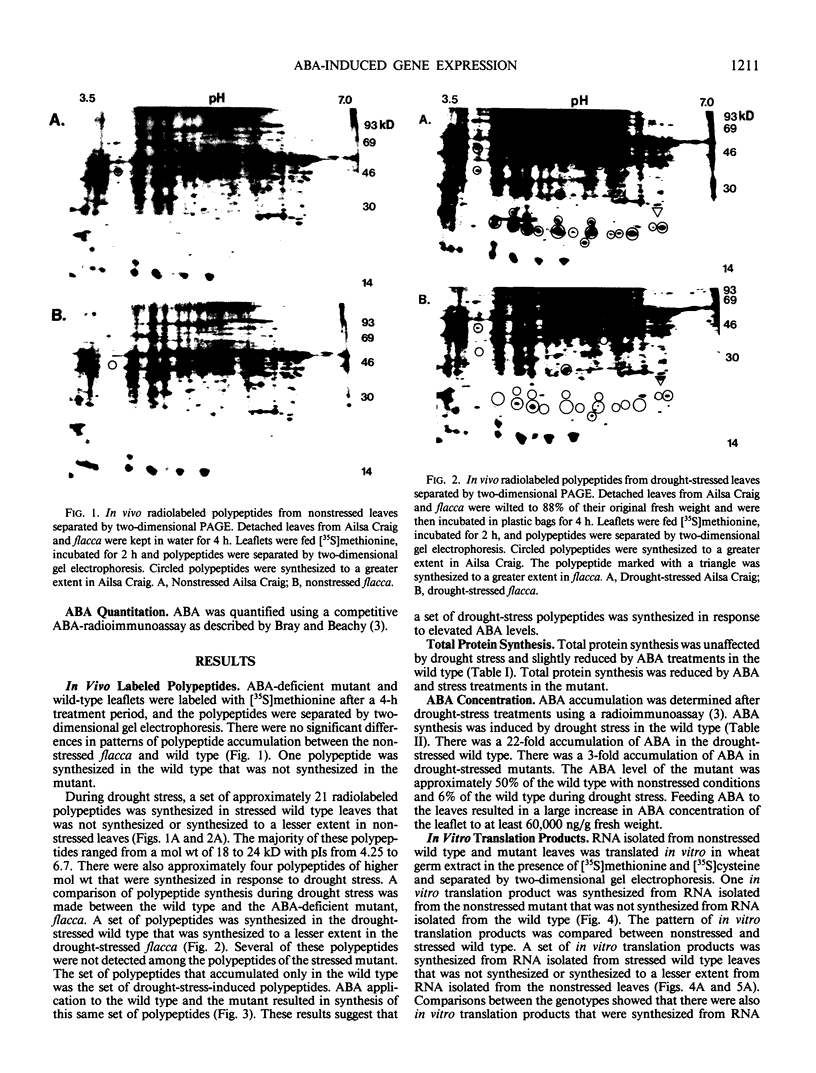
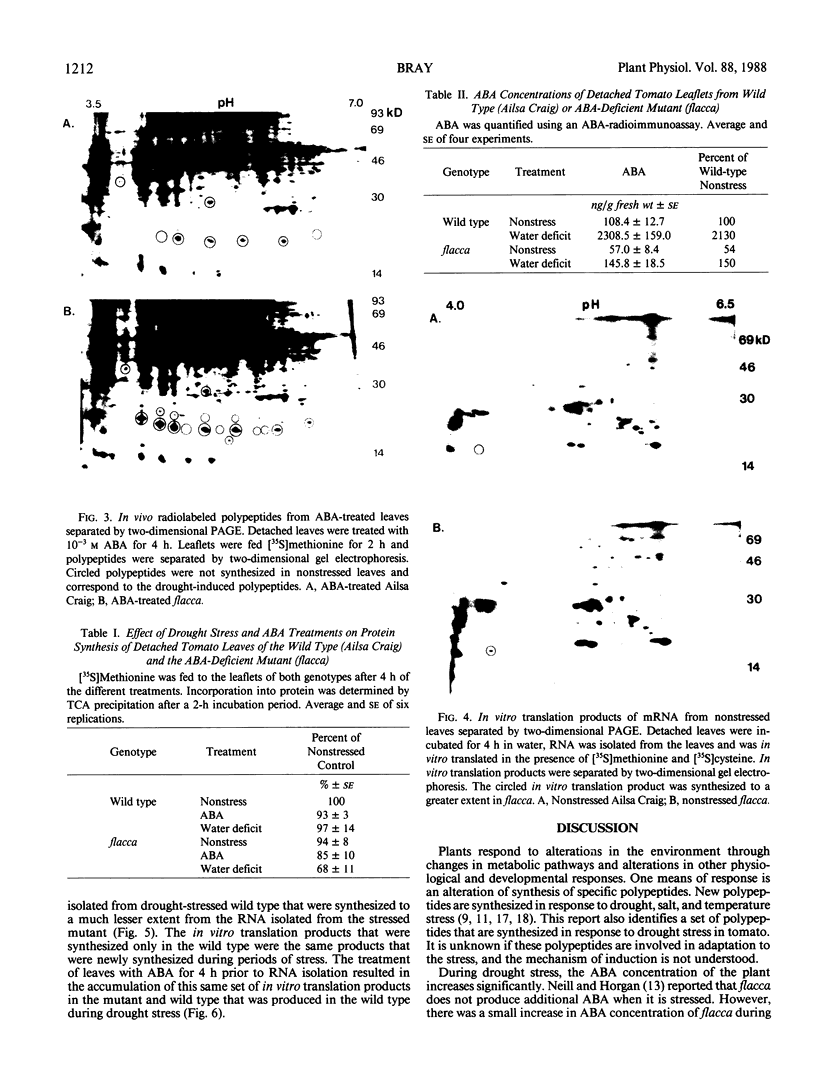
Drought stress triggers abscisic acid (ABA) biosynthesis resulting in ABA accumulation. The ABA-deficient tomato mutant, flacca (Lycopersicon esculentum Mill. cv Ailsa Craig), does not synthesize ABA in response to drought stress. This mutant has been used to distinguish polypeptides and in vitro translation products that are synthesized during drought stress in response to elevated ABA levels from those that are induced directly by altered water relations. A set of polypeptides and in vitro translation products was synthesized during drought stress in the wild type. These polypeptides and in vitro translation products were synthesized to a lesser extent in the drought-stressed ABA-deficient mutant. Treatment of flacca with ABA resulted in the synthesis of the drought-stress-induced polypeptides and in vitro translation products. These results support the hypothesis that many of the polypeptides that are synthesized during drought are regulated by alterations in ABA concentration. Similarly, the mRNA population was altered by ABA during drought stress.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford K. J. Water Relations and Growth of the flacca Tomato Mutant in Relation to Abscisic Acid. Plant Physiol. 1983 May;72(1):251–255. doi: 10.1104/pp.72.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray E. A., Beachy R. N. Regulation by ABA of beta-Conglycinin Expression in Cultured Developing Soybean Cotyledons. Plant Physiol. 1985 Nov;79(3):746–750. doi: 10.1104/pp.79.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier Y. Changes in the Electrophoretic Patterns of the Soluble Proteins of Winter Wheat and Rye following Cold Acclimation and Desiccation Stress. Plant Physiol. 1983 Feb;71(2):400–403. doi: 10.1104/pp.71.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa R. S., Cleland R. E. Water stress and protein synthesis: I. Differential inhibition of protein synthesis. Plant Physiol. 1975 Apr;55(4):778–781. doi: 10.1104/pp.55.4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick P., Dvorák J. Gene induction and repression by salt treatment in roots of the salinity-sensitive Chinese Spring wheat and the salinity-tolerant Chinese Spring x Elytrigia elongata amphiploid. Proc Natl Acad Sci U S A. 1987 Jan;84(1):99–103. doi: 10.1073/pnas.84.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila J. J., Papp J. E., Schultz G. A., Bewley J. D. Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic Acid, and wounding. Plant Physiol. 1984 Sep;76(1):270–274. doi: 10.1104/pp.76.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Hanson A. D., Chandler P. C. Water stress enhances expression of an alpha-amylase gene in barley leaves. Plant Physiol. 1986 Feb;80(2):350–359. doi: 10.1104/pp.80.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Ramagopal S. Differential mRNA transcription during salinity stress in barley. Proc Natl Acad Sci U S A. 1987 Jan;84(1):94–98. doi: 10.1073/pnas.84.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopal S. Salinity stress induced tissue-specific proteins in barley seedlings. Plant Physiol. 1987 Jun;84(2):324–331. doi: 10.1104/pp.84.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Bracker C. A., Hasegawa P. M., Handa A. K., Buckel S., Hermodson M. A., Pfankoch E., Regnier F. E., Bressan R. A. Characterization of osmotin : a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987 Oct;85(2):529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Handa A. K., Hasegawa P. M., Bressan R. A. Proteins Associated with Adaptation of Cultured Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M. Abnormal stomatal behavior in wilty mutants of tomato. Plant Physiol. 1966 Oct;41(8):1387–1391. doi: 10.1104/pp.41.8.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M., Nevo Y. Abnormal stomatal behavior and root resistance, and hormonal imbalance in three wilty mutants of tomato. Biochem Genet. 1973 Mar;8(3):291–300. doi: 10.1007/BF00486182. [DOI] [PubMed] [Google Scholar]