Abstract
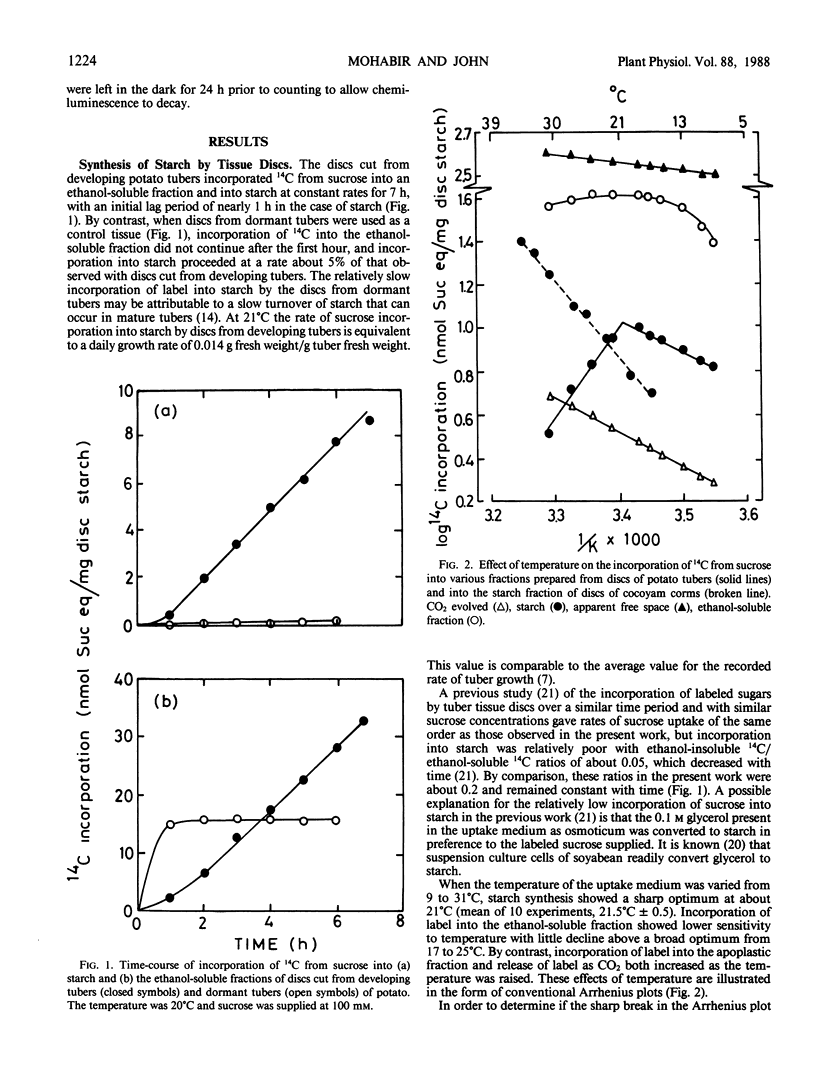
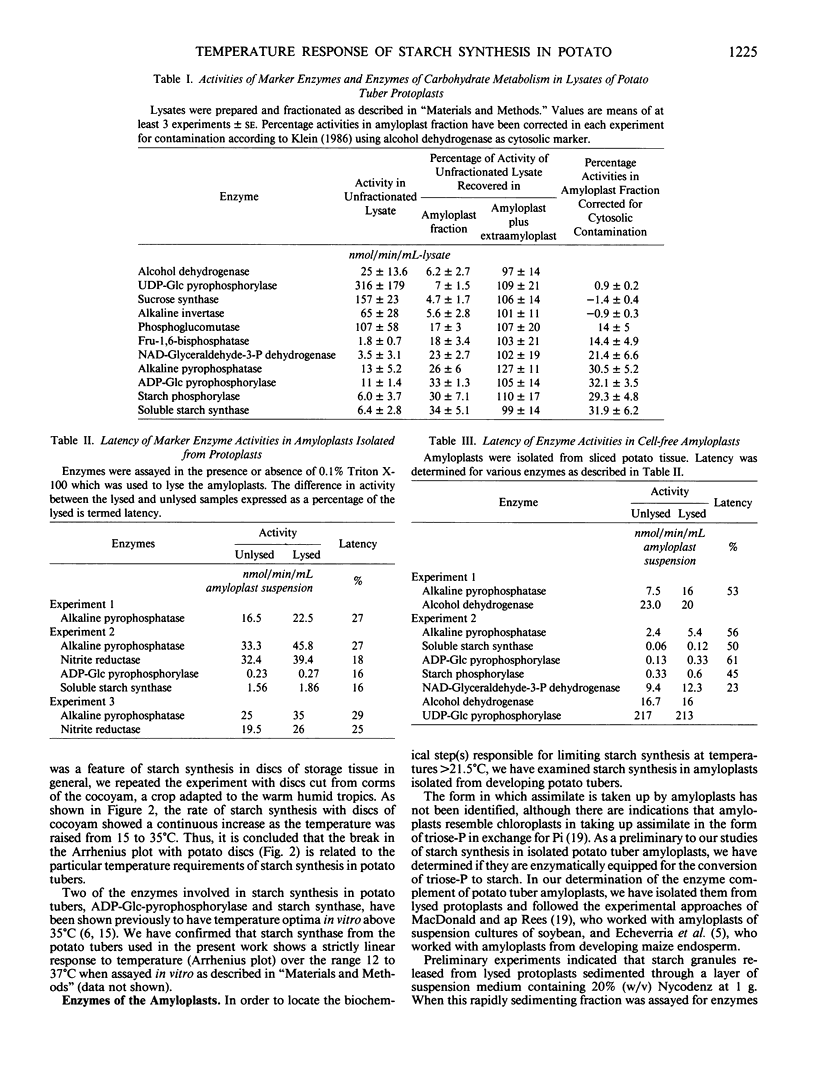
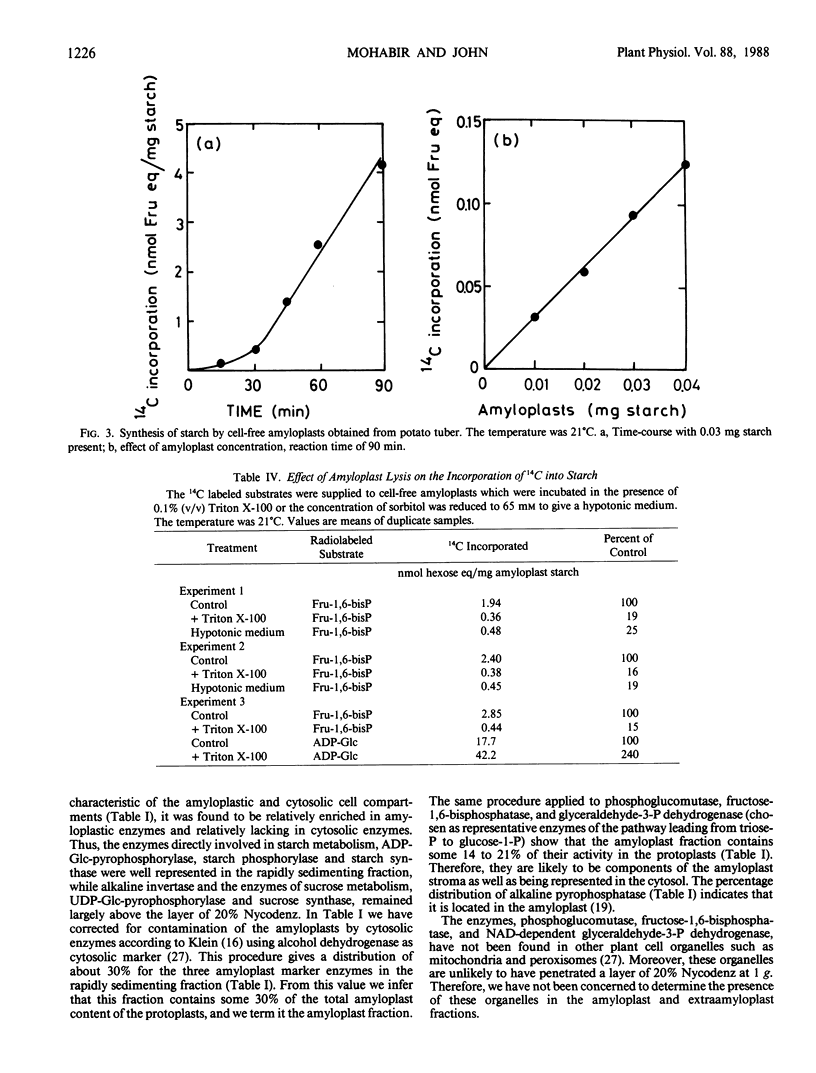
A sharp temperature optimum is observed at 21.5°C when the incorporation of [14C]sucrose into starch is measured with discs cut from developing tubers of potato (Solanum tuberosum L. cv Desirée). By contrast, increasing temperatures over the range 9 to 31°C only enhance release of 14C to respiratory CO2 and incorporation of 14C into the ethanolsoluble fraction. By comparison, starch synthesis in discs from developing corms of cocoyam (Colocasia esculenta L. Schott) is increased by raising the temperature from 15 to 35°C. The significance of a relatively low temperature optimum for starch synthesis in potato is discussed in relation to the yield limitations imposed by continuously high soil temperatures. Amyloplasts isolated from protoplasts prepared from developing potato tubers contain activities of alkaline pyrophosphatase, NAD-dependent glyceraldehyde-3-phosphate dehydrogenase, fructose-1,6-bisphosphatase, and phosphoglucomutase in addition to ADP-glucose-pyrophosphorylase, starch phosphorylase and starch synthase. Cell-free amyloplasts released by thinly slicing developing potato tubers synthesize starch from [14C]triose-phosphate generated from [14C]fructose-1,6-bisphosphate in the reaction medium. This starch synthesis is inhibited by addition of 10 millimolar inorganic phosphate and requires amyloplast integrity, suggesting the operation of a triose-phosphate/inorganic phosphate exchange carrier at the amyloplast membrane. The temperature optimum at 21.5°C observed with tissue discs is not observed with amyloplasts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Echeverria E., Boyer C., Liu K. C., Shannon J. Isolation of amyloplasts from developing maize endosperm. Plant Physiol. 1985 Mar;77(3):513–519. doi: 10.1104/pp.77.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman R. B., Cardini C. E. Studies on the biosynthesis of starch. I. Isolation and properties of the soluble adenosine diphosphate glucose: starch glucosyltransferase of Solanum tuberosum. Arch Biochem Biophys. 1966 Sep 26;116(1):9–18. doi: 10.1016/0003-9861(66)90005-1. [DOI] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi C., Preiss J. Amylopectin degradation in pea chloroplast extracts. Plant Physiol. 1978 Feb;61(2):218–220. doi: 10.1104/pp.61.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S., Beevers H. Isolation and characterization of organelles from soybean suspension cultures. Plant Physiol. 1974 Feb;53(2):261–265. doi: 10.1104/pp.53.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greenberg E., Kuhn D. N., Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979 Aug;64(2):187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTIS H. G., LELOIR L. F. Measurement of UDP-enzyme systems. Methods Biochem Anal. 1962;10:107–136. doi: 10.1002/9780470110270.ch4. [DOI] [PubMed] [Google Scholar]
- Rijven A. H. Heat inactivation of starch synthase in wheat endosperm tissue. Plant Physiol. 1986 Jun;81(2):448–453. doi: 10.1104/pp.81.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos J. R., Lulai E. C., Knoper J. A. Translucent Tissue Defects in Solanum tuberosum L: I. Alterations in Amyloplast Membrane Integrity, Enzyme Activities, Sugars, and Starch Content. Plant Physiol. 1985 Jul;78(3):489–494. doi: 10.1104/pp.78.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos J. R., Preiss J. Pyrophosphorylases in Solanum tuberosum: III. PURIFICATION, PHYSICAL, AND CATALYTIC PROPERTIES OF ADPGLUCOSE PYROPHOSPHORYLASE IN POTATOES. Plant Physiol. 1982 Jun;69(6):1459–1466. doi: 10.1104/pp.69.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]


