Abstract
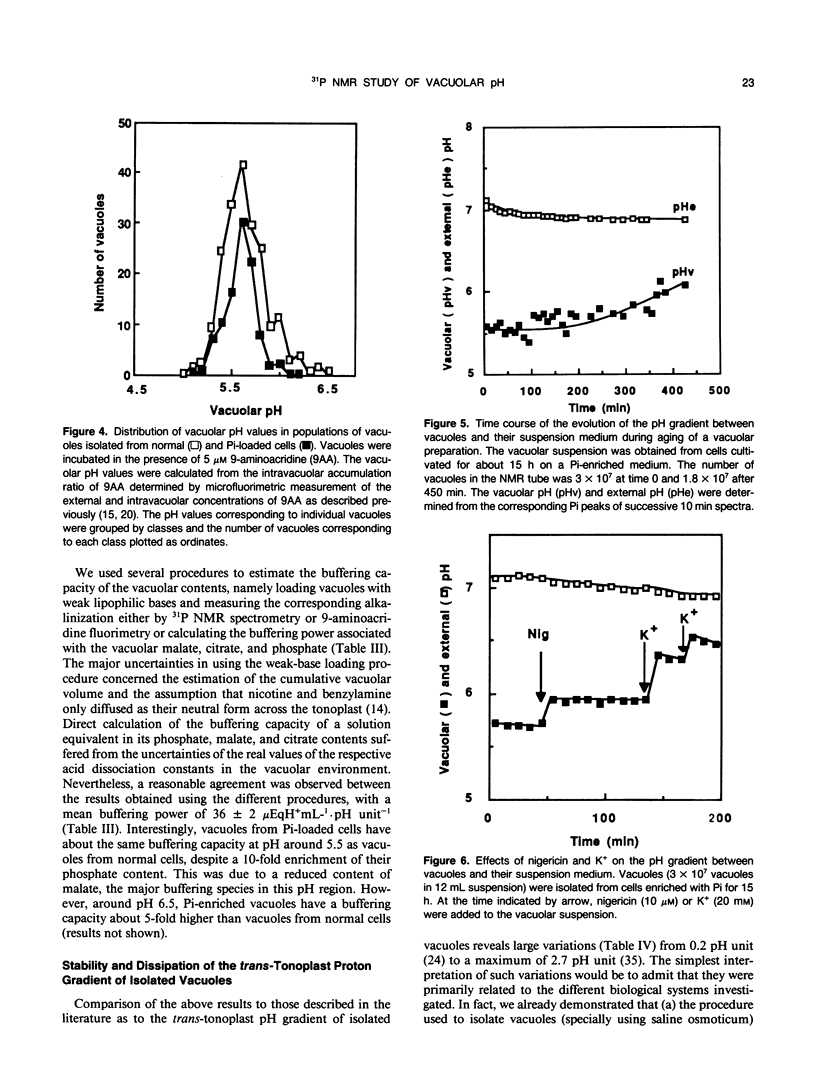
For the first time, the 31P nuclear magnetic resonance technique has been used to study the properties of isolated vacuoles of plant cells, namely the vacuolar pH and the inorganic phosphate content. Catharanthus roseus cells incubated for 15 hours on a culture medium enriched with 10 millimolar inorganic phosphate accumulated large amounts of inorganic phosphate in their vacuoles. Vacuolar phosphate ions were largely retained in the vacuoles when protoplasts were prepared from the cells and vacuoles isolated from the protoplasts. Vacuolar inorganic phosphate concentrations up to 150 millimolar were routinely obtained. Suspensions prepared with 2 to 3 × 106 vacuoles per milliliter from the enriched C. roseus cells have an internal pH value of 5.50 ± 0.06 and a mean trans-tonoplast ΔpH of 1.56 ± 0.07. Reliable determinations of vacuolar and external pH could be made by using accumulation times as low as 2 minutes. These conditions are suitable to follow the kinetics of H+ exchanges at the tonoplast. The 31P nuclear magnetic resonance technique also offered the possibility of monitoring simultaneously the stability of the trans-tonoplast pH and phosphate gradients. Both appeared to be reasonably stable over several hours. The buffering capacity of the vacuolar sap around pH 5.5 has been estimated by several procedures to be 36 ± 2 microequivalents per milliliter per pH unit. The increase of the buffering capacity due to the accumulation of phosphate in the vacuoles is, in large part, compensated by a decrease of the intravacuolar malate content.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumwald E., Poole R. J. Kinetics of Ca/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris L. Plant Physiol. 1986 Mar;80(3):727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Na/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985 May;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Nitrate storage and retrieval in Beta vulgaris: Effects of nitrate and chloride on proton gradients in tonoplast vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3683–3687. doi: 10.1073/pnas.82.11.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodelius P., Vogel H. J. A phosphorus-31 nuclear magnetic resonance study of phosphate uptake and storage in cultured Catharanthus roseus and Daucus carota plant cells. J Biol Chem. 1985 Mar 25;260(6):3556–3560. [PubMed] [Google Scholar]
- Foyer C., Walker D., Spencer C., Mann B. Observations on the phosphate status and intracellular pH of intact cells, protoplasts and chloroplasts from photosynthetic tissue using phosphorus-31 nuclear magnetic resonance. Biochem J. 1982 Feb 15;202(2):429–434. doi: 10.1042/bj2020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J., Mathieu Y., Pean M., Pasquier C., Beloeil J. C., Lallemand J. Y. Cytoplasmic pH Regulation in Acer pseudoplatanus Cells: I. A P NMR Description of Acid-Load Effects. Plant Physiol. 1986 Nov;82(3):840–845. doi: 10.1104/pp.82.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Proton transport in isolated vacuoles from corn coleoptiles. Plant Physiol. 1985 May;78(1):104–109. doi: 10.1104/pp.78.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E., Schramm M. J., Kaiser G., Kaiser W. M., Heber U. Transport of anions in isolated barley vacuoles : I. Permeability to anions and evidence for a cl-uptake system. Plant Physiol. 1986 Apr;80(4):895–901. doi: 10.1104/pp.80.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Nishimura M. pH in Vacuoles Isolated from Castor Bean Endosperm. Plant Physiol. 1982 Sep;70(3):742–744. doi: 10.1104/pp.70.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986 Sep 15;261(26):12172–12178. [PubMed] [Google Scholar]
- Thom M., Komor E. Electrogenic proton translocation by the ATPase of sugarcane vacuoles. Plant Physiol. 1985 Feb;77(2):329–334. doi: 10.1104/pp.77.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Leigh R. A., Kaestner K. H., Sze H. Electrogenic h-pumping pyrophosphatase in tonoplast vesicles of oat roots. Plant Physiol. 1986 Jun;81(2):497–502. doi: 10.1104/pp.81.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]


