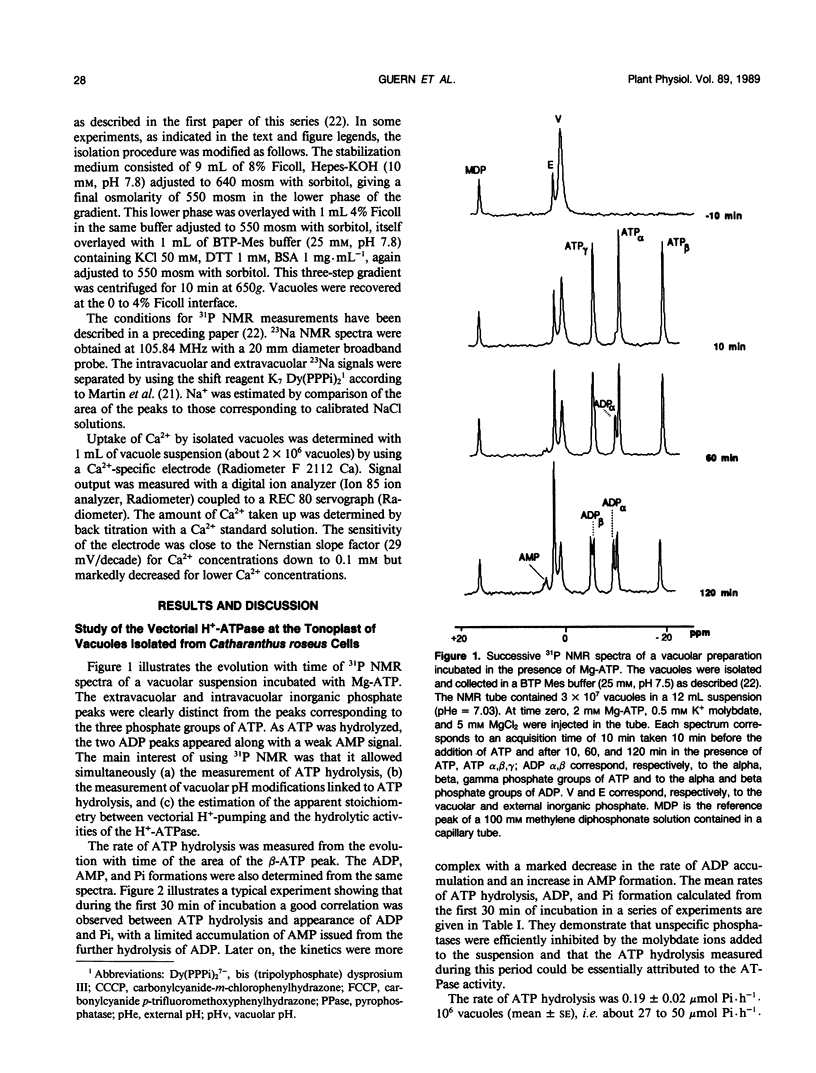
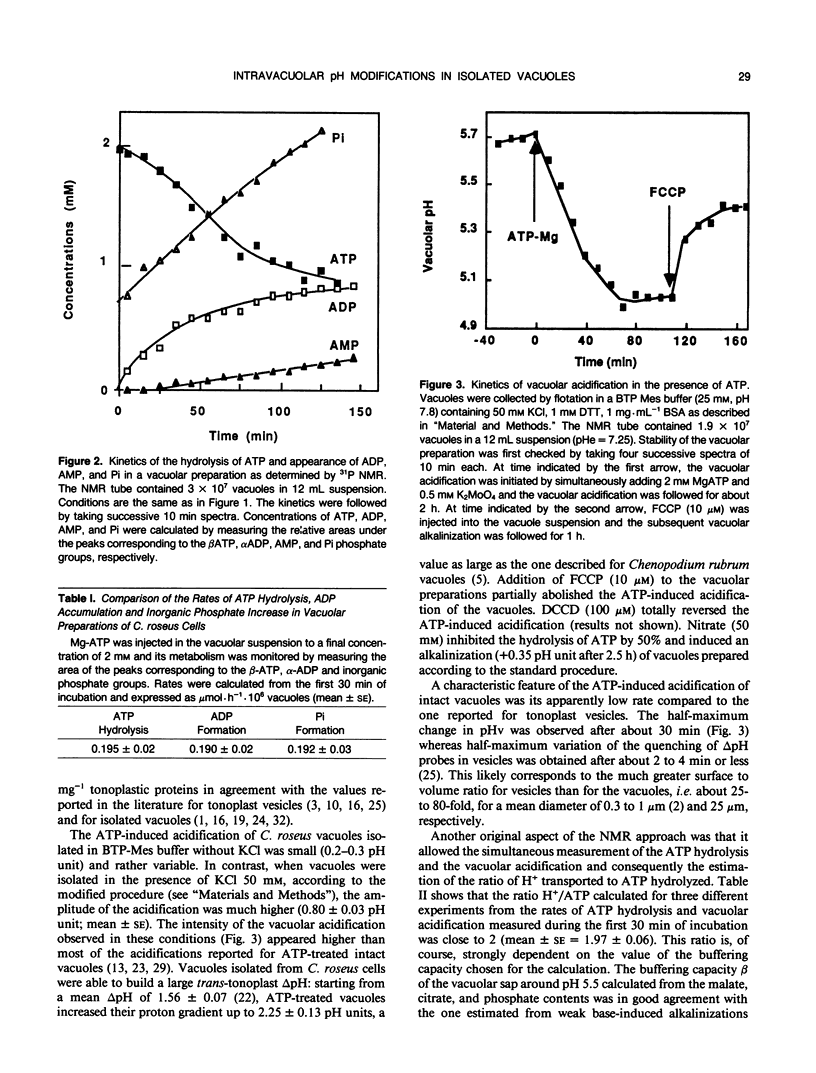
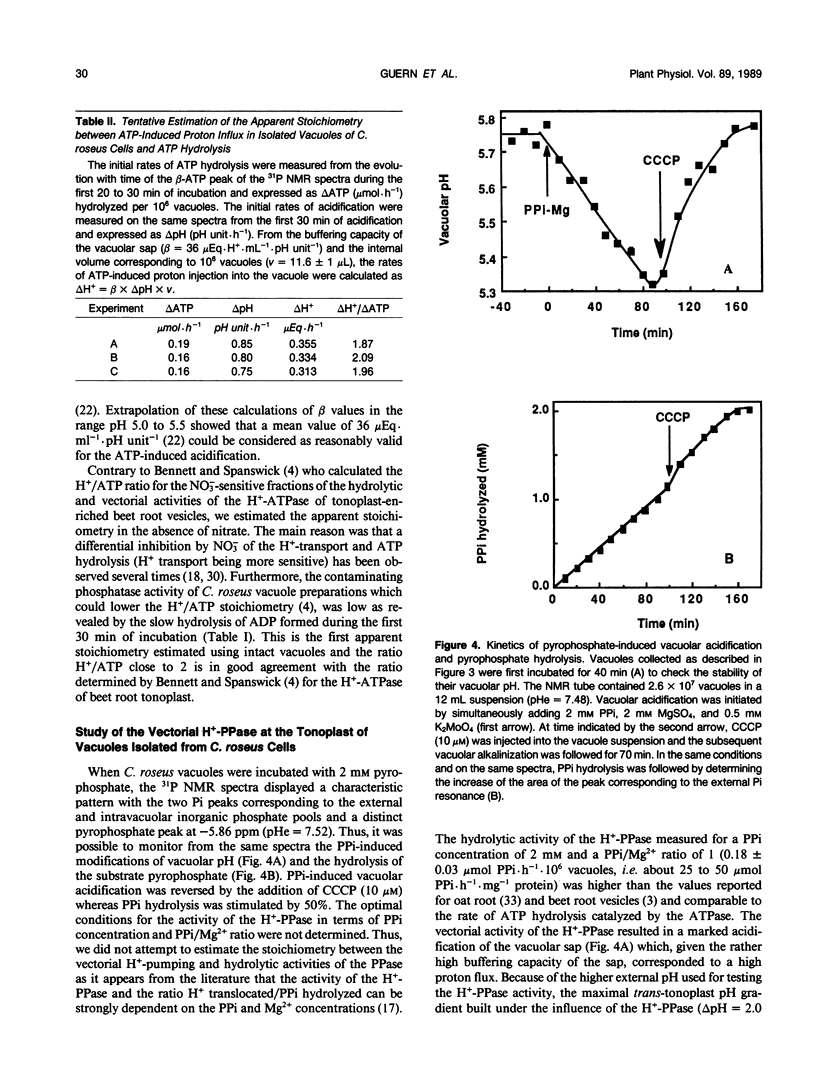
Abstract
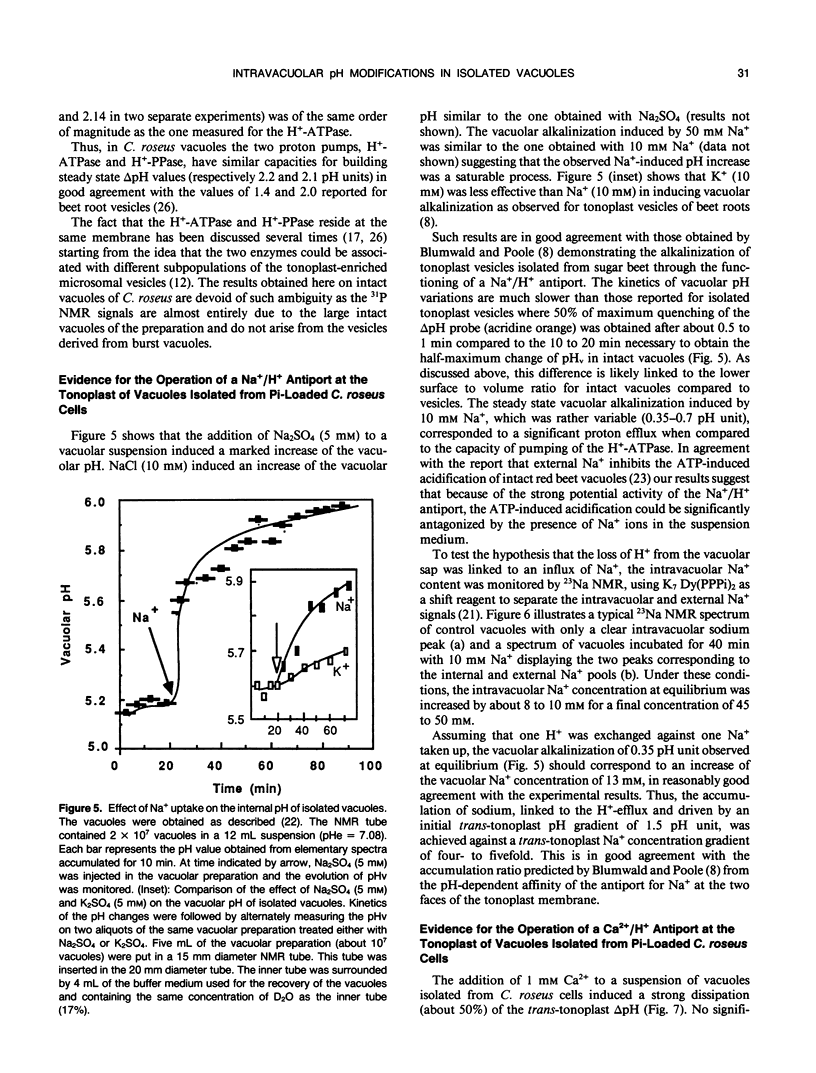
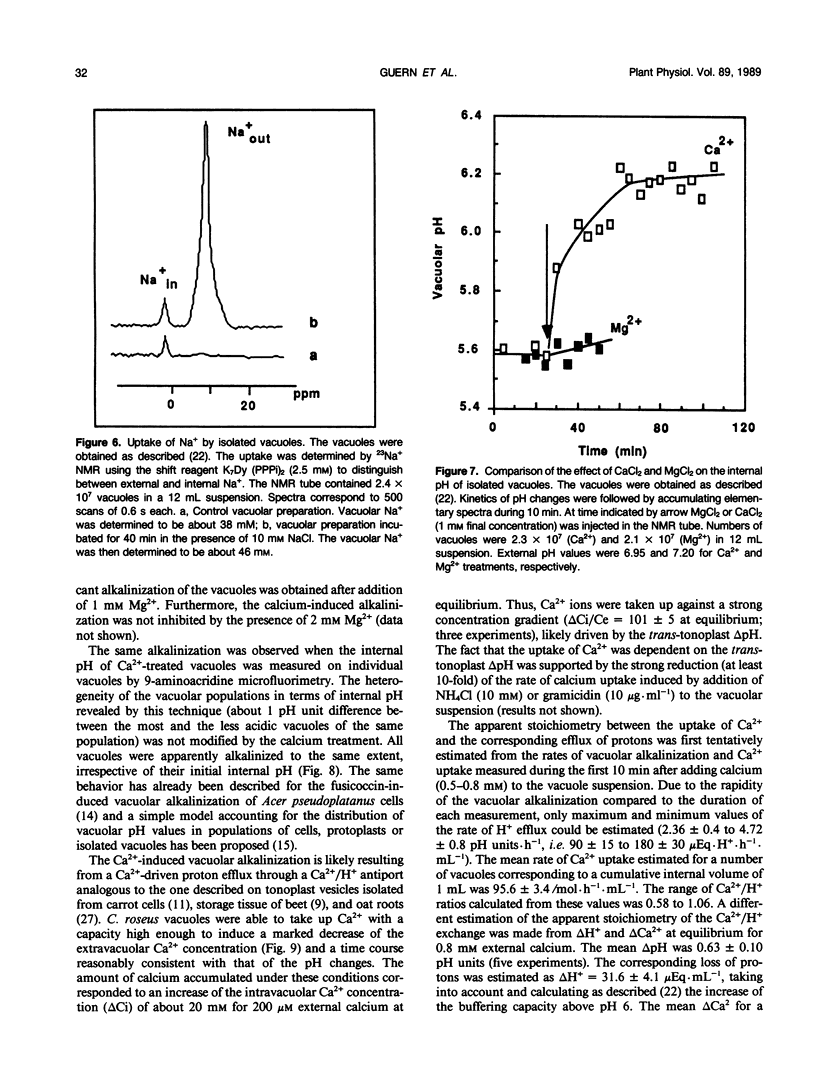
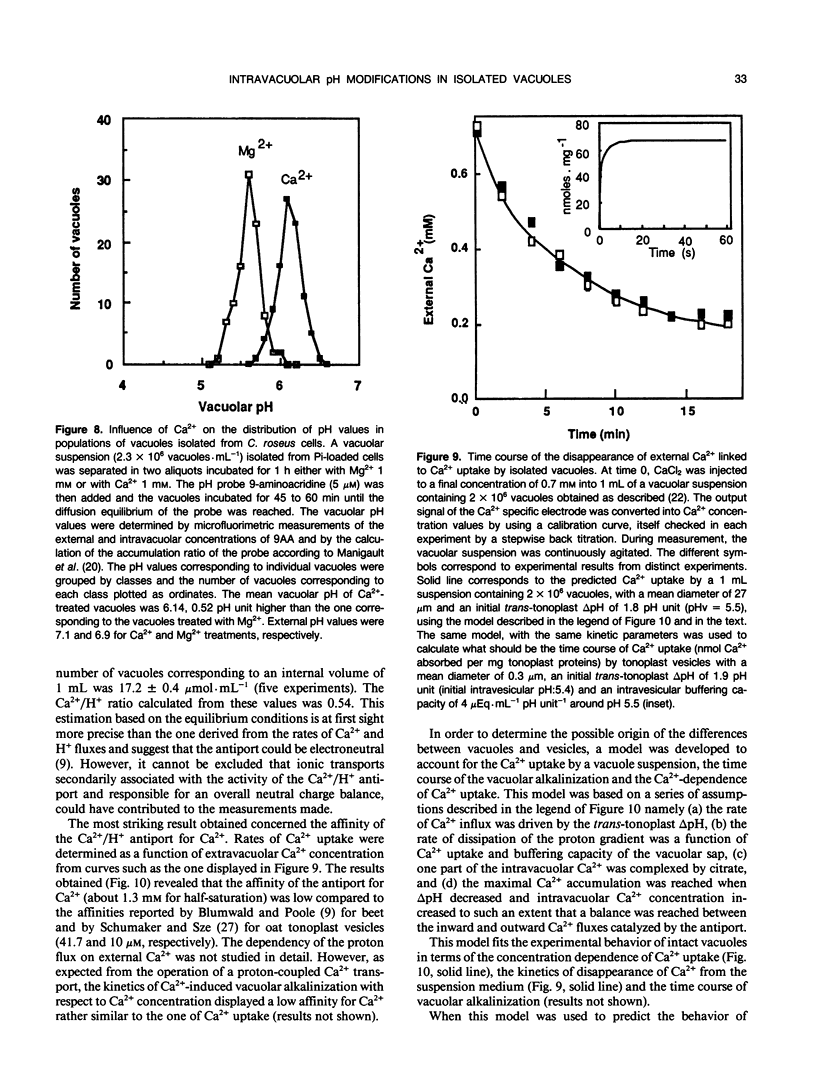
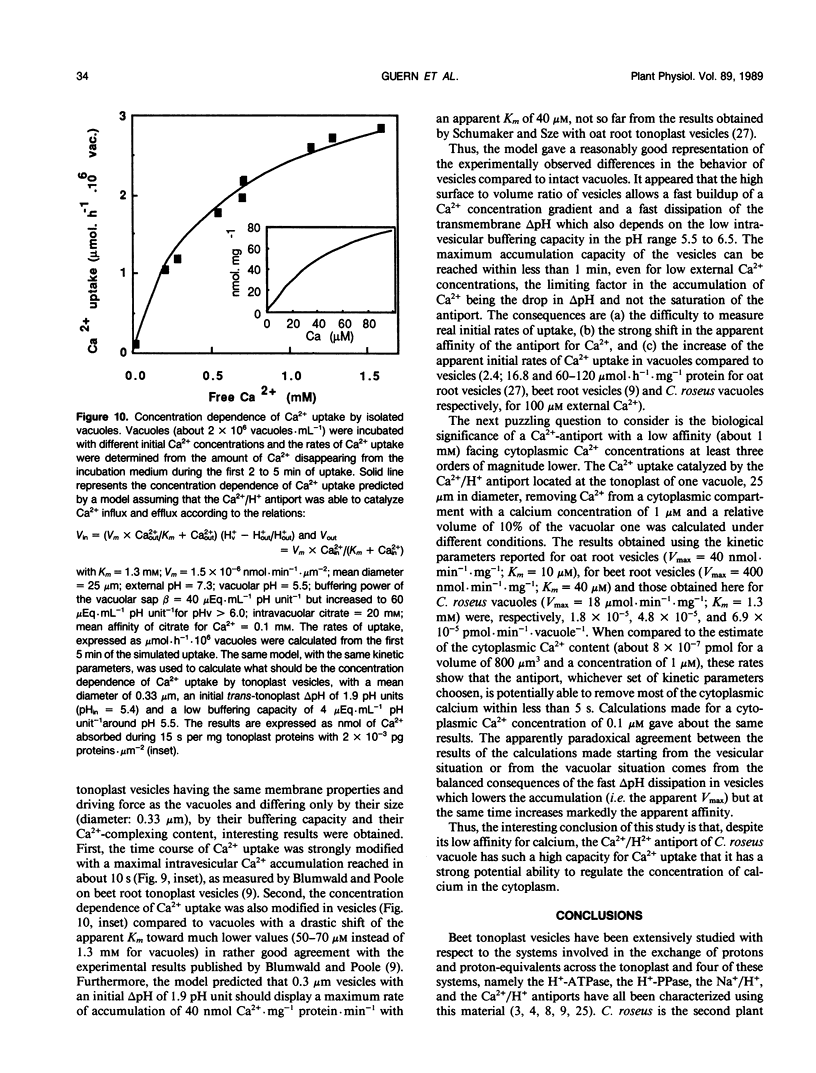
The vacuolar pH and the trans-tonoplast ΔpH modifications induced by the activity of the two proton pumps H+-ATPase and H+-PPase and by the proton exchanges catalyzed by the Na+/H+ and Ca2+/H+ antiports at the tonoplast of isolated intact vacuoles prepared from Catharanthus roseus cells enriched in inorganic phosphate (Y Mathieu et al 1988 Plant Physiol [in press]) were measured using the 31P NMR technique. The H+-ATPase induced an intravacuolar acidification as large as 0.8 pH unit, building a trans-tonoplast ΔpH up to 2.2 pH units. The hydrolysis of the phosphorylated substrate and the vacuolar acidification were monitored simultaneously to estimate kinetically the apparent stoichiometry between the vectorial proton pumping and the hydrolytic activity of the H+-ATPase. A ratio of H+ translocated/ATP hydrolyzed of 1.97 ± 0.06 (mean ± standard error) was calculated. Pyrophosphate-treated vacuoles were also acidified to a significant extent. The H+-PPase at 2 millimolar PPi displayed hydrolytic and vectorial activities comparable to those of the H+-ATPase, building a steady state ΔpH of 2.1 pH units. Vacuoles incubated in the presence of 10 millimolar Na+ were alkalinized by 0.4 to 0.8 pH unit. It has been shown by using 23Na NMR that sodium uptake was coupled to the H+ efflux and occurred against rather large concentration gradients. For the first time, the activity of the Ca2+/H+ antiport has been measured on isolated intact vacuoles. Ca2+ uptake was strongly inhibited by NH4Cl or gramicidin. Vacuoles incubated with 1 millimolar Ca2+ were alkalinized by about 0.6 pH unit and this H+ efflux was associated to a Ca2+ uptake as demonstrated by measuring the external Ca2+ concentration with a calcium specific electrode. Steady state accumulation ratios of Ca2+ as high as 100 were reached for steady state external concentrations about 200 micromolar. The rate of Ca2+ uptake appeared markedly amplified in intact vacuoles when compared to tonoplast vesicles but the antiport displayed a much lower affinity for calcium. The different behavior of intact vacuoles compared to vesicles appears mainly to be due to differences in the surface to volume ratio and in the rates of dissipation of the pH gradient. Despite its low affinity, the Ca2+/H+ antiport has a high potential capacity to regulate cytoplasmic concentration of calcium.
Full text
PDF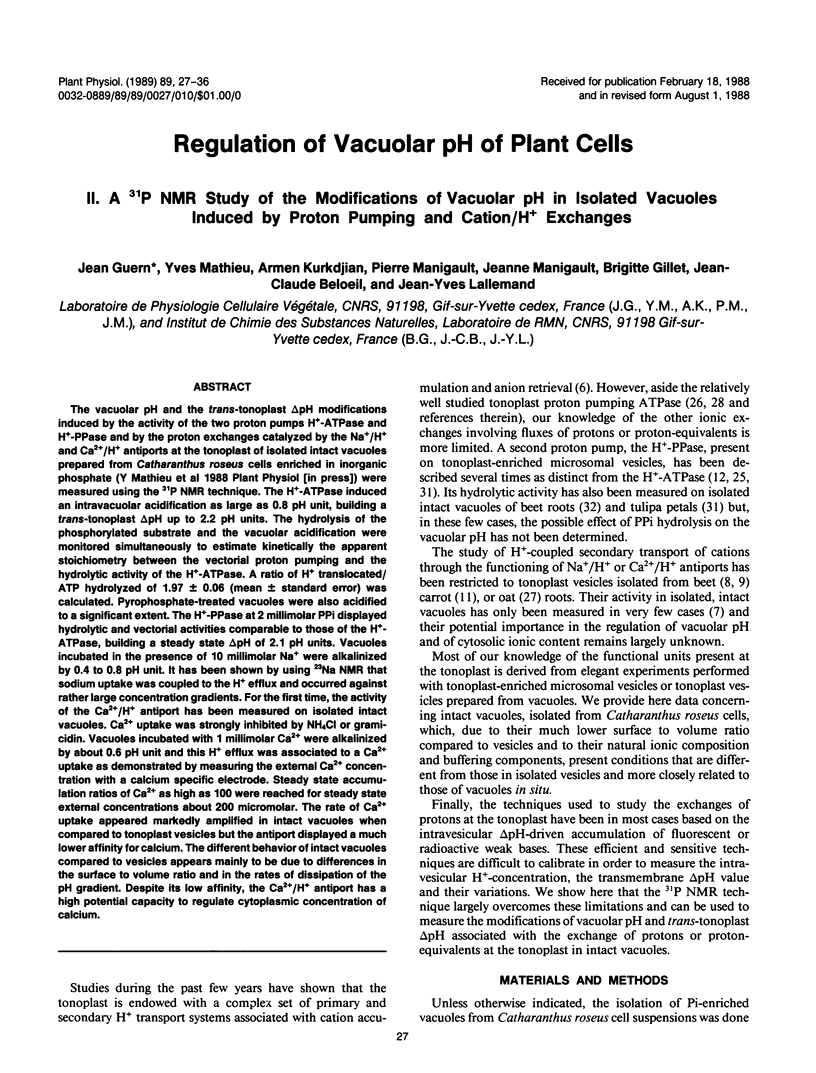


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: II. H/ATP Stoichiometry of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):545–548. doi: 10.1104/pp.74.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentrup F. W., Gogarten-Boekels M., Hoffmann B., Gogarten J. P., Baumann C. ATP-dependent acidification and tonoplast hyperpolarization in isolated vacuoles from green suspension cells of Chenopodium rubrum L. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2431–2433. doi: 10.1073/pnas.83.8.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Cragoe E. J., Poole R. J. Inhibition of na/h antiport activity in sugar beet tonoplast by analogs of amiloride. Plant Physiol. 1987 Sep;85(1):30–33. doi: 10.1104/pp.85.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Kinetics of Ca/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris L. Plant Physiol. 1986 Mar;80(3):727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Na/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985 May;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Thornley W. R., Wyse R. E. Membrane transport in isolated vesicles from sugarbeet taproot : I. Isolation and characterization of energy-dependent, h-transporting vesicles. Plant Physiol. 1985 Aug;78(4):865–870. doi: 10.1104/pp.78.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. R., Sze H. Calcium transport in tonoplast and endoplasmic reticulum vesicles isolated from cultured carrot cells. Plant Physiol. 1986 Feb;80(2):549–555. doi: 10.1104/pp.80.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson A., Fichmann J., Spear D., Taiz L. Pyrophosphate-driven proton transport by microsomal membranes of corn coleoptiles. Plant Physiol. 1985 Sep;79(1):159–164. doi: 10.1104/pp.79.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew R. R., Spanswick R. M. Characterization of Anion Effects on the Nitrate-Sensitive ATP-Dependent Proton Pumping Activity of Soybean (Glycine max L.) Seedling Root Microsomes. Plant Physiol. 1985 Feb;77(2):352–357. doi: 10.1104/pp.77.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Martin J. B., Klein G., Satre M. 23Na NMR study of intracellular sodium ions in Dictyostelium discoideum amoeba. Arch Biochem Biophys. 1987 May 1;254(2):559–567. doi: 10.1016/0003-9861(87)90138-x. [DOI] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986 Sep 15;261(26):12172–12178. [PubMed] [Google Scholar]
- Thom M., Komor E. Electrogenic proton translocation by the ATPase of sugarcane vacuoles. Plant Physiol. 1985 Feb;77(2):329–334. doi: 10.1104/pp.77.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S. I., Nagahashi G., Brouillette J. N. Proton pumping kinetics and origin of nitrate inhibition of tonoplast-type H+-ATPase. Arch Biochem Biophys. 1987 Aug 1;256(2):625–637. doi: 10.1016/0003-9861(87)90620-5. [DOI] [PubMed] [Google Scholar]
- Wang Y., Leigh R. A., Kaestner K. H., Sze H. Electrogenic h-pumping pyrophosphatase in tonoplast vesicles of oat roots. Plant Physiol. 1986 Jun;81(2):497–502. doi: 10.1104/pp.81.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]


