Summary
Background
Exercise can rapidly drop glucose in people with type 1 diabetes. Ubiquitous wearable fitness sensors are not integrated into automated insulin delivery (AID) systems. We hypothesised that an AID can automate insulin adjustments using real-time wearable fitness data to reduce hypoglycaemia during exercise and free-living conditions compared with an AID not automating use of fitness data.
Methods
Our study population comprised of individuals (aged 21–50 years) with type 1 diabetes from from the Harold Schnitzer Diabetes Health Center clinic at Oregon Health and Science University, OR, USA, who were enrolled into a 76 h single-centre, two-arm randomised (4-block randomisation), non-blinded crossover study to use (1) an AID that detects exercise, prompts the user, and shuts off insulin during exercise using an exercise-aware adaptive proportional derivative (exAPD) algorithm or (2) an AID that automates insulin adjustments using fitness data in real-time through an exercise-aware model predictive control (exMPC) algorithm. Both algorithms ran on iPancreas comprising commercial glucose sensors, insulin pumps, and smartwatches. Participants executed 1 week run-in on usual therapy followed by exAPD or exMPC for one 12 h primary in-clinic session involving meals, exercise, and activities of daily living, and 2 free-living out-patient days. Primary outcome was time below range (<3·9 mmol/L) during the primary in-clinic session. Secondary outcome measures included mean glucose and time in range (3·9–10 mmol/L). This trial is registered with ClinicalTrials.gov, NCT04771403.
Findings
Between April 13, 2021, and Oct 3, 2022, 27 participants (18 females) were enrolled into the study. There was no significant difference between exMPC (n=24) versus exAPD (n=22) in time below range (mean [SD] 1·3% [2·9] vs 2·5% [7·0]) or time in range (63·2% [23·9] vs 59·4% [23·1]) during the primary in-clinic session. In the 2 h period after start of in-clinic exercise, exMPC had significantly lower mean glucose (7·3 [1·6] vs 8·0 [1·7] mmol/L, p=0∙023) and comparable time below range (1·4% [4·2] vs 4·9% [14·4]). Across the 76 h study, both algorithms achieved clinical time in range targets (71·2% [16] and 75·5% [11]) and time below range (1·0% [1·2] and 1·3% [2·2]), significantly lower than run-in period (2·4% [2·4], p=0∙0004 vs exMPC; p=0∙012 vs exAPD). No adverse events occurred.
Interpretation
AIDs can integrate exercise data from smartwatches to inform insulin dosing and limit hypoglycaemia while improving glucose outcomes. Future AID systems that integrate exercise metrics from wearable fitness sensors may help people living with type 1 diabetes exercise safely by limiting hypoglycaemia.
Funding
JDRF Foundation and the Leona M and Harry B Helmsley Charitable Trust, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Introduction
Type 1 diabetes (type 1 diabetes) is an autoimmune disorder that results in the destruction of the insulin-producing β cells in the pancreas. People with type 1 diabetes cannot produce sufficient insulin to maintain normal glucose concentrations and therefore must take exogenous insulin through either injection or an insulin pump. Automated insulin delivery (AID) systems are now commercially available and comprise a continuous glucose monitor (CGM), an insulin pump, and a control algorithm to automate insulin delivery based on sensed glucose.1–4 Commercial systems are hybrid systems requiring the person to estimate the amount of carbohydrates in their meals and enter this information into the AID to calculate meal insulin. Newer systems under development are fully automated and do not require carbohydrate entry.5 Although commercial AIDs have shown benefit, there is still need for improvement as many people on AID do not yet achieve the target of HbA1c below 7·0% and hypoglycaemia can occur, especially during times of high physical activity.6
A remaining challenge in glucose management for people with type 1 diabetes is maintaining optimal glucose concentration during and following exercise to avoid hypoglycaemia.7,8 Glucose management during exercise is particularly challenging for people with type 1 diabetes because the glucose response depends on many factors including type of exercise, duration, intensity, amount of insulin acting at the time of exercise, time of day, and whether exercise is competitive. Aerobic exercise is particularly problematic as it can cause sharp drops in glucose, especially when performed shortly after a meal when insulin concentrations are high.9 Compounding the problem is that there is substantial variation in glucose response during exercise, even when the exercise is done by the same person across similar days when all conditions of eating, insulin dosing, and exercise are consistent.10 Consensus statements provide guidelines to people with type 1 diabetes to help them adjust insulin and carbohydrate intake before and during exercise if necessary.7,8 Some commercial AIDs offer the ability to change the glucose target before and during exercise, which can be helpful in avoiding hypoglycaemia during exercise.11 However, adjustment of the target is not automatic and requires the person to change the target, typically several hours in advance of exercise. Herein, we present results from a clinical study showing how an AID system can respond automatically to exercise quantified using a wrist-worn fitness watch.
Automating the detection of exercise using wearable sensors is now possible because of the ubiquity of commercial wearable sensors that quantify physical activity measures including heart rate and accelerometry. These sensors can be leveraged to compute various derived metrics including metabolic equivalent to task (MET), exercise start and stop, duration, intensity, and exercise type. Research has been done to assess the accuracy of these metrics12 which is critical for their potential use within AIDs that can then automatically adjust dosing in response to exercise.
Once physical activity has been quantified, it needs to be properly used within a control algorithm to adjust insulin dosing. Turksoy and colleagues13 showed in a small in-clinic trial that various physical activity metrics including energy expenditure and galvanic skin impedance from a SenseWear Pro 3 (Pittsburgh, PA, USA) could be used to inform an AID. Participants in their 60 h in-clinic study performed a variety of exercises while using this multiple-input AID. Participants maintained a time in range of 69·9% with low time in hypoglycaemia, although 16 (59%) of 27 aerobic exercise sessions required participants to consume carbohydrates during exercise sessions to avoid low glucose. De Boer and Breton also showed, in an in-clinic study, that using heart rate as an additional input to an AID could significantly reduce the percent time in low glucose (<3·9 mmol/L) during exercise compared with an AID that did not use heart rate.14 Garcia-Tirado and colleagues described an AID that anticipated exercise from past behavioural patterns to estimate exercise in the future that could occur at the same time, showing in an in-clinic study that the number of low glucose events could be reduced when the AID anticipates exercise.15 Our group has used exercise metrics from wearable sensors to inform an exercise-aware multihormone (insulin and glucagon) adaptive proportional derivative (exAPD) closed-loop control algorithm.16 This algorithm was used to reduce insulin and increase glucagon in response to the exercise onset. Clinical studies showed that use of this exAPD algorithm when used in dual-hormone mode (insulin and glucagon) could help reduce hypoglycaemia substantially compared with when it was used in single-hormone mode.17 The exAPD algorithm detected when METs exceeded a threshold of 4·0 then prompted the user to confirm exercise. After user confirmation, the exAPD would shut off insulin for 30 min, and then reduce insulin delivery by 50% over the next 60 min. While the exAPD algorithm described above is helpful in reducing insulin during and following exercise, it required the user to interact with the system by responding to a prompt and confirming exercise. The exAPD algorithm was unable to respond automatically to physical activity that is not typically considered exercise, such as housework, yardwork, commuting by bike or walking, etc, because the intensity and duration thresholds of such activities might be lower than the intensity threshold for exercise detection used in exAPD.
The normal functioning pancreas responds continuously to physical activity by reducing insulin secretion when physical activity increases to avoid hypoglycaemia. Herein, we describe an exercise-aware model predictive control algorithm (exMPC) that behaves more like the human pancreas by responding in real-time to increases or decreases in physical activity to modulate the amount of insulin delivered. The algorithm has been described in earlier publications18,19 and a brief overview is in the appendix (pp 2–7).
Methods
Study design and participants
This study was a randomised, single-centre crossover study.
Participants were enrolled from the Harold Schnitzer Diabetes Health Center clinic at Oregon Health and Science University (OHSU). Inclusion criteria required diagnosis with type 1 diabetes for at least 1 year, age of 21–50 years, physically willing and able to perform aerobic exercise, current use of an insulin pump for at least 3 months with stable insulin pump settings for at least 2 weeks, living with a person aged 18 years or older, living within 40 miles of OHSU, baseline HbA1c of 10·0% or lower, and a total daily insulin requirement of less than 139 units per day, chosen to ensure that expected meal insulin could be fully delivered within 20 min. Exclusion criteria included females of childbearing age who were pregnant or intending to become pregnant, cardiovascular disease, renal insufficiency, liver failure, low haematocrit concentration, uncon trolled hyper tension, history of severe hypoglycaemia during past year, history of ketoacidosis during preceding 6 months, adrenal insufficiency, and active infection.
The first visit to the clinic was a screening visit that was within 12 weeks before the 1 week run-in period; participants were consented via written informed consent, screened for eligibility, and HbA1c was measured along with an EKG. After eligibility was confirmed, participants performed a 1 week run-in when they received training on using the Dexcom G6 CGM (Dexcom, San Diego, CA, USA). Following the 1 week run-in, participants arrived at the OHSU inpatient research unit to start the first 76 h treatment.
Complete protocol (Institutional Review Board protocol number 19973 and investigational device exemption (G200363) is provided in the appendix (p 7).
Procedures
Since the exMPC algorithm was a new algorithm that had not been previously tested in humans, for safety reasons and as requested by the US Food and Drug Administration (FDA), the first eight participants used the exMPC only during the 12 h daytime period (0700–1900 h) in the clinic on days 1–3. During the evening hours, these participants used the iPancreas system in open loop only. After safety was confirmed for the first eight participants using exMPC, the remaining partici pants used exMPC during days 2 and 3 under freeliving conditions. The primary in-clinic session was on day 3 for these first eight exMPC studies, and day 1 for the remaining exMPC studies and for all of the exAPD studies.
During the primary in-clinic session of the study, participants ate self-selected meals at approximately 0800 h, 1200 h, and 1700 h. Participants counted their own carbohydrates and entered these into the AID system using the iPancreas app; identical meals were consumed for the exMPC arm and the exAPD arms during the primary in-clinic session of the study. During the primary in-clinic session, participants performed activities of daily living at 1000 h that included vacuuming, washing dishes, folding laundry, etc, and performed a 30 min aerobic exercise video at 1500 h referred to herein as structured exercise. The exercise video included a 3 min warm-up and cool-down and workout designed to elicit a target heart rate of 70–80% of the age-predicted maximal heart rate. The hypothesis was that exMPC would lead to less percent time below range (time below range <3·9 mmol/L) than exAPD during the primary in-clinic session because exMPC was always using physical activity data to adjust the insulin and therefore responding to all activity during the day, not just structured exercise. Conversely, exAPD only adjusted insulin in response to structured exercise, but not throughout the day or during the activities of daily living. The exAPD also required the participant to respond to a prompt when physical activity exceeded 4·0 METs whereas the exMPC algorithm did not require any interactions from the participant in response to physical activity changes.
Participants were discharged from the clinic at approximately 2000 h. For days 2 and 3 of the interventional part of the study, participants used iPancreas at home under free-living conditions and were instructed to exercise on their own. On day 4 of the intervention, participants returned to the clinic to end the first arm of the study. After the first treatment visit, a washout period of 6 days to 10 weeks was done before performing the next 76 h intervention on either the exMPC or exAPD depending on the first intervention arm.
Outcomes
The primary outcome measure was the percent time below range during the 12 h primary in-clinic session. Primary and secondary outcome measures were also assessed during the entire 76 h study and also during the 2 h period immediately after the start of structured exercise on the primary in-clinic session. Glucose metrics were based on Dexcom G6 CGM data. Secondary outcome measures included the percent time in range (3·9–10 mmol/L), percent time above range (>10 mmol/L), total number of rescue carbohydrates required per day in response to hypoglycaemia (<3·9 mmol/L), mean glucose, percent time glucose was very low (<3·0 mmol/L), percent time sensed glucose was very high (>13·9 mmol/L) and mean amount of insulin per day.
Randomisation and masking
Participants were randomised using block randomisation with a block size of 4 to first use iPancreas running either exMPC or exAPD for the duration of the intervention. The study was non-blinded in that the study participants and the investigators knew the intervention that was being done.
Overview of iPancreas test platform
iPancreas (appendix p 41) is a modular, licensable, open access system to enable rapid prototyping of closed-loop and decision support algorithms and user interfaces for glucose management. The system (figure 1) comprises the custom iPancreas app running on a Samsung smartphone (Suwon-Si, Korea), a Dexcom G6 CGM, a research version Insulet Omnipod (Acton, MA, USA), a Polar M600 smartwatch (Kempele, Finland), and a custom-developed cloud monitoring and data acquisition repository running on Amazon Web Services (Seattle, WA, USA). CGM data and M600 heart rate and accelerometry data are received wirelessly by the phone. The control algorithm calculates the amount of insulin to deliver and sends this information wirelessly to the Omnipod. iPancreas includes a simple meal bolus calculator.
Figure 1: iPancreas system.
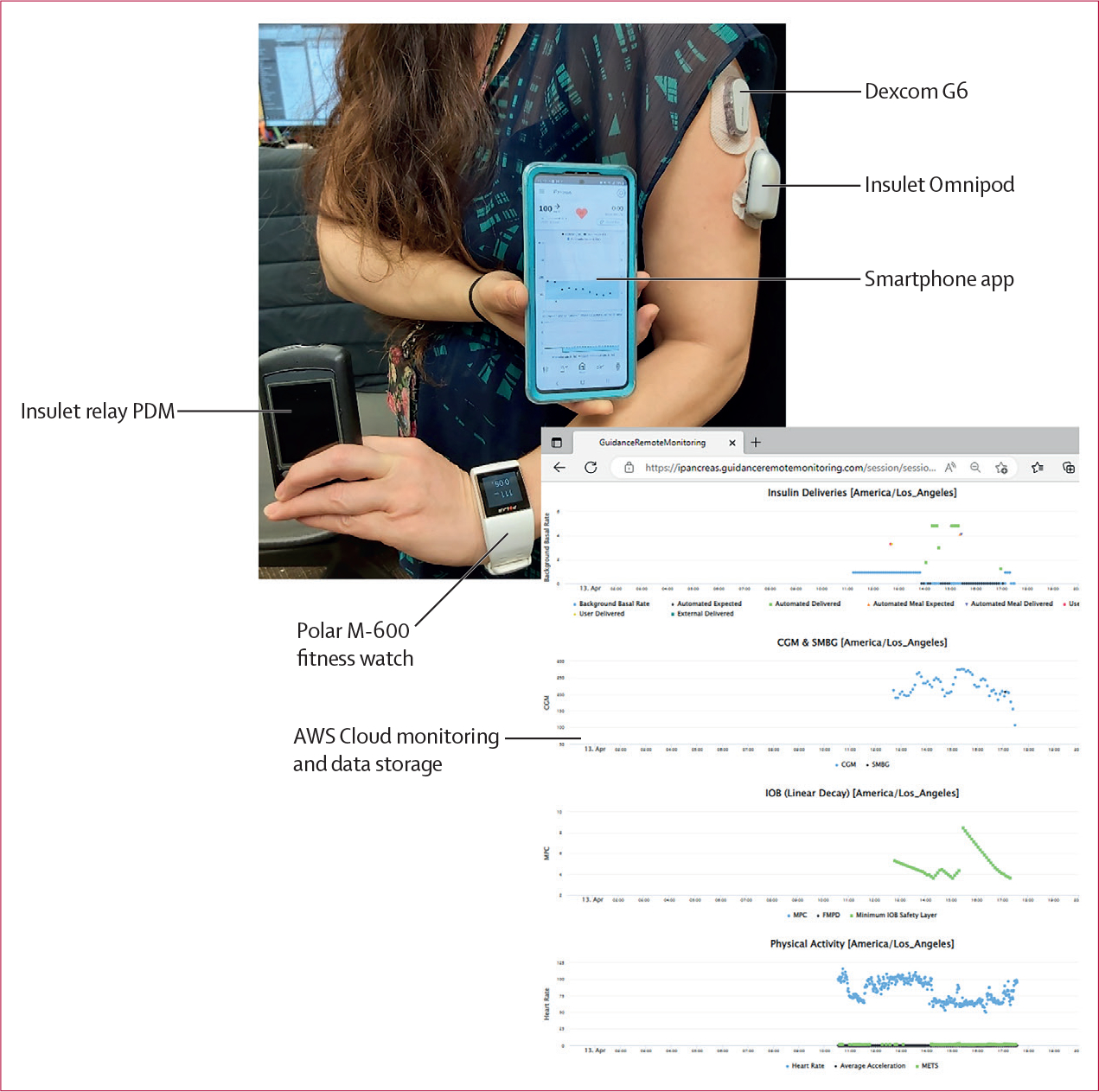
The system comprises a Dexcom G6 CGM, an Insulet Omnipod along with a relay PDM, a Polar M-600 smartwatch with heart rate and accelerometer sensors, and a Samsung smartphone running the exAPD or exMPC control algorithms. AWS=Amazon Web Services. exAPD=exercise-aware adaptive proportional derivative. exMPC=exercise-aware model predictive control. PDM=personal diabetes manager.
Overview of the AID algorithms
The exMPC algorithm18–21 (appendix p 2) is a traditional MPC algorithm that uses a linearised version of a compartment model comprising nonlinear differential equations that represent kinetics and dynamics of subcutaneously delivered insulin, carbohydrates, and exercise. The insulin kinetics and dynamics models and the carbohydrate absorption model are described by Hovorka and colleagues22 and Wilinska and colleagues.23 The exercise model is described by Hernandez and colleagues24 and describes how glucose uptake and endogenous glucose production are impacted by METs. We calculated METs using accelerometer and heart rate data (appendix p 6). The METs data is provided as an input to the control algorithm every 5 min when a new insulin delivery micro-bolus is calculated.
The exAPD algorithm17,20,21 is a proportional-derivative control algorithm that includes a fading history of past CGM to calculate the amount of insulin that should be delivered every 5 min.25 Because the exAPD algorithm does not include a model of metabolism, exercise metrics such as heart rate and accelerometry cannot be easily included as a continuous input. Instead, the exAPD algorithm calculates METs (appendix p 6), and if METs exceeds a threshold of 4·0, then the person using iPancreas will be prompted to confirm exercise. After accepting the prompt, insulin is turned off for 30 min and then reduced by 50% for 1 h.
Artificial intelligence (AI)-augmented safety layer
Both exAPD and exMPC use a safety layer built into iPancreas. This includes an AI-based predictive low glucose suspend algorithm that uses a long-short-term memory (LSTM) neural network26 glucose forecasting algorithm to automatically shut off insulin if CGM is 3·9–7·77 mmol/L and predicted to drop below 5 mmol/L within 30 min (appendix p 5–6). In addition, the maximum insulin delivery rate is limited to four times the user’s typical basal insulin infusion rate. Finally, maximum insulin delivery is limited such that insulin on board never exceeds 35% of the total daily insulin requirement.
Statistical analysis
For the primary outcome of percent time with glucose less than 3·9 mmol/L, we anticipated a mean paired difference of 1·2 (SD 2·5) with a kurtotic, double-exponential distribution, based on previously published, closed-loop study data20 and simulations using the OHSU simulator.27 A sample of 24 participants provided 80% power at the 0∙05 level of significance to detect a difference of that size or larger using a two-sided t test with an adjustment for the distribution. For normally distributed secondary outcomes, we had over 80% power to detect differences of 0·6 SD or greater using a two-sided one-sample t test for the mean difference at the 0∙05 significance level. Power calculations were done using Power Analysis and Sample Size Software (version 14; NCSS, Kaysville, UT, USA).
The primary study endpoint was percent of time with CGM below range (time below range, <3·9 mmol/L) during the primary in-clinic session. The hypothesis to be tested was whether the exMPC decreases time below range compared with the exAPD algorithm. For this, as well as for secondary endpoints, we used an intention-to-treat analysis whereby all available data were included if the participant completed the primary in-clinic session. Mean paired differences were calculated as the differences between the two sample means with SD of the differences
where is the sample correlation between measurements in the two arms. Two-sided p values were calculated using a panel model for each endpoint with a random intercept for participant and indicator variables for the intervention, the sequence of the intervention (exMPC first or second, as a measure of potential carryover effects), and period (first or second). For the primary endpoint, which had non-normally distributed residuals, the standard errors for this model were calculated using bootstrap methods where participants were resampled with 1000 replications. For endpoints that were counts, we used negative binomial models. Other non-normally distributed outcomes were transformed or bootstrapped, depending on the fit of the transformation. Analyses were completed using Matlab (Mathworks, Natick MA; version 21b) and Stata/IC (Stata Statistical Software, College Station TX; version 16.1).
This trial is registered with ClinicalTrials.gov, NCT04771403.
Role of the funding source
Funders did not have a role in the study design, data collection, interpretation of results, or the writing of the manuscript.
Results
Between April 13, 2021, and Oct 3, 2022, 27 adults (18 females) with type 1 diabetes from the Harold Schnitzer Diabetes Health Center clinic at OHSU were recruited into the study (table 1). Of the 27 participants screened, 25 (16 females) participated in the study (figure 2). 24 of 25 participants completed the exMPC arm and 22 of 25 participants completed the exAPD arm (figure 2). Reasons for not completing an arm included a software error that occurred and was fixed early on in the study (n=2), pump occlusion (n=1), and a participant withdrew from the study before starting the arm (n=1). There were no serious adverse events. The washout period was not correlated with time below range or time in range.
Table 1:
Study participant demographics at baseline
| Participants (n=25) | |
|---|---|
|
| |
| Age, years | 34·4 (8·8) |
| Weight, kg | 78·8 (13·5) |
| Sex at birth | |
| Male | 9 (36%) |
| Female | 16 (64%) |
| Race | |
| American Indian or Alaska native | 1 (4%) |
| White | 22 (88%) |
| More than one race | 2 (8%) |
| Ethnicity | |
| Hispanic | 0 |
| Non-Hispanic | 25 (100%) |
| HbA1c | 6·4% (0·6) |
| HbA1c, mmol/mol | 49·5 (4·9) |
| Diabetes duration, years | 22·8 (9·7) |
| AID users | 11 (44%) |
| CGM users | 24 (96%) |
Data are mean (SD) or n (%). AID=automated insulin delivery. CGM=continuous glucose monitoring. HbA1c=glycated haemoglobin A1c.
Figure 2: Trial profile.
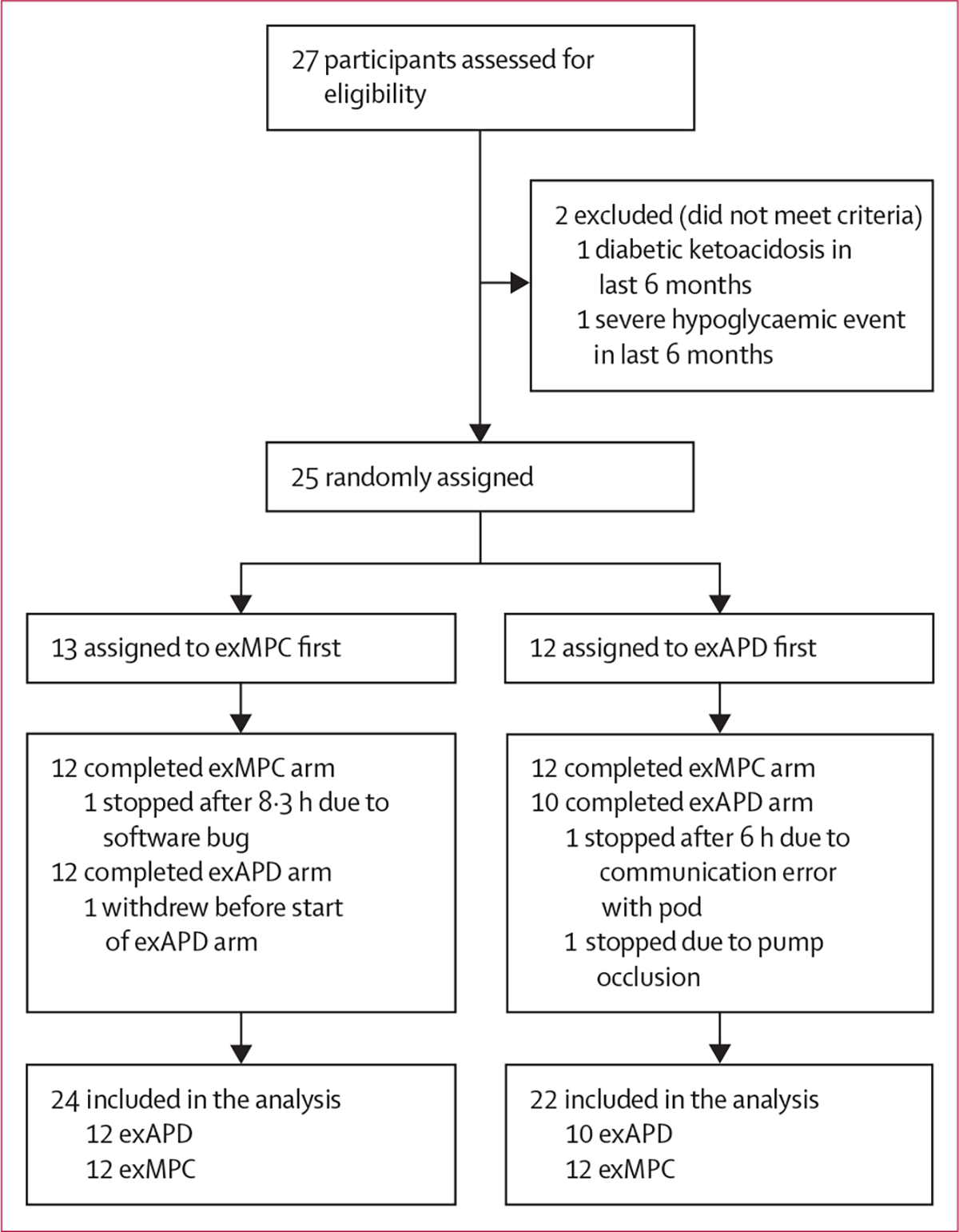
exAPD=exercise-aware adaptive proportional derivative. exMPC=exercise-aware model predictive control.
Both exMPC and exAPD were similarly effective at using exercise data to prevent low glucose. For the primary outcome measure, participants using exMPC had similar time below range compared with participants using exAPD during the primary in-clinic session (1·3% vs 2·5%, difference −1·2 [SD 7·3]; p=0∙46; table 2). Time in range was similar for participants using the exMPC compared with the exAPD during the primary in-clinic session (63·2% vs 59·4%, difference 3·8 [29·8]; p=0∙49). Table 2 shows that there was no significant effect of the sequence of participants performing exMPC versus exAPD arms first. However, the order of performing the intervention regardless of intervention type as indicated by the column labelled period, was significant for time in range (increased in period 2) and time above range (decreased in period 2), indicating that the participants might have benefited from learning in the first period. Figure 3 shows that the exMPC algorithm tended to respond more to the activities of daily living during the morning time by turning down insulin whereas exAPD did not adjust insulin based on the activities of daily living since it was only designed to respond to prolonged vigorous exercise including the structured, user-confirmed exercise video. The automated insulin on board calculated in the 2 h before the start of exercise was comparable for exAPD and exMPC (appendix p 44).
Table 2:
Outcome metrics comparing the exAPD algorithm versus the exMPC algorithm during the primary in-clinic session
| exAPD | exMPC | Difference | p value (exAPD vs exMPC) | p value (sequence) | p value (period) | |
|---|---|---|---|---|---|---|
|
| ||||||
| % time below range (<3·9 mmol/L)* | 2·5 (7·0) | 1·3 (2·9) | −1·2 (7·3) | 0·46 | 0·50 | 0·22 |
| % time in range (3·9–10·0 mmol/L) | 59·4 (23·1) | 63·2 (23·9) | 3·8 (29·8) | 0·49 | 0·41 | 0·047 |
| % high glucose (>10 mmol/L) | 38·1 (21·4) | 35·5 (24·4) | −2·6 (28·4) | 0·63 | 0·34 | 0·012 |
| % very low glucose (<3·0 mmol/L) | 0·27 (0·87) | 0 | ·· | ·· | ·· | ·· |
| % very high glucose (>13·9 mmol/L) | 8·0 (15·8) | 7·5 (11·4) | −0·5 (16·0) | 0·87 | 0·65 | 0·24 |
| Mean glucose (mmol/L) | 9·4 (1·6) | 9·2 (1·6) | −0·2 (1·8) | 0·63 | 0·59 | 0·15 |
| Rescue carbohydrate (count)† | 1·09 (2·16) | 0·58 (0·93) | −0·51 (2·36) | 0·33 | 0·31 | 0·33 |
| Insulin (units)‡ | 21·2 (8·0) | 21·4 (10·0) | 0·3 (4·7) | 0·94 | 0·60 | 0·60 |
Data are mean (SD) or difference (SD). The p value for sequence indicates the significance of the sequence of doing the exAPD verus exMPC first. The p value for the period indicates whether the order of doing the intervention, regardless of the type, was significant. exAPD=exercise-aware adaptive proportional derivative. exMPC=exercise-aware model predictive control.
p values estimated using bootstrapped standard errors.
p values from negative binomial (count) regression model.
p values from mixed effects regression model on insulin units.
Figure 3: CGM data, insulin, and METs during the primary in-clinic session (0700–1900 h).
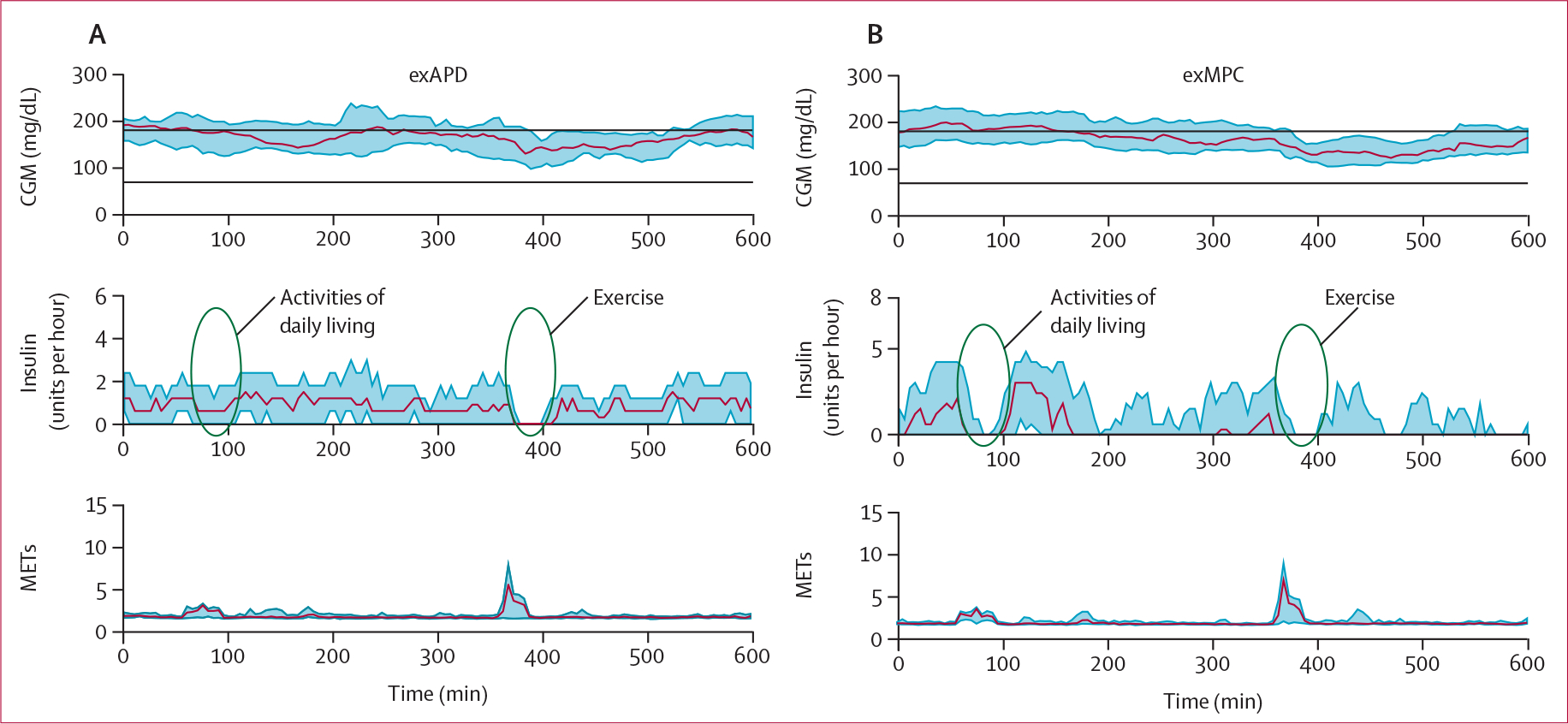
Middle plots show how the exMPC tended to reduce insulin earlier in the day when activities of daily living were taking place, whereas, the exAPD only shut off insulin when the structured exercise took place later in the day. CGM=continuous glucose monitor. exAPD=exercise-aware adaptive proportional derivative. exMPC=exercise-aware model predictive control. MET=metabolic equivalent to task.
Low glucose (<3·9 mmol/L) occurred during the 2 h after the start of structured exercise during the primary in-clinic session for three participants in each of the exMPC and the exAPD arm. Table 3 shows that the duration of the low glucose during and following structured exercise was similar for exMPC compared with exAPD (1·4% vs 4·9%, difference −3·5 [15·4]; p=0∙29). Participants using exMPC had a significantly lower mean CGM compared with exAPD during this 2 h window after structured exercise in the primary in-clinic session (7·3 vs 8·0 mmol/L, difference −0·8 [1·4]; p=0∙023) and had similar time in very low glucose (0 for exMPC vs 0·57% for exAPD). One participant in the exAPD arm had a very low glucose (<3·0 mmol/L) in the 2 h after the start of structured exercise whereas none of the participants experienced this in the exMPC arm. Participants using the exMPC had better glucose outcomes following in-clinic structured exercise as indicated by a significantly lower mean glucose (figure 4). Figure 4 also shows that although the exAPD shut off insulin completely when structured exercise was detected by the algorithm and accepted by the user prompt, the exMPC algorithm did not completely shut off insulin for all participants as the exercise data were just one input to the algorithm, and in certain cases, a complete shut-off of insulin was not necessarily indicated to maintain optimal glucose outcomes. Insulin shut-off could have been caused by either the LSTM or the exercise detection. The carbohydrate intake before and during exercise was not different between the exMPC and exAPD arms for in-clinic exercise (appendix pp 42–43).
Table 3:
Outcome metrics comparing exAPD algorithm versus the exMPC algorithm in the 2 h after structured exercise started on the in-clinic day
| exAPD | exMPC | Difference | p value (exAPD vs exMPC) | p value (sequence) | p value (period) | |
|---|---|---|---|---|---|---|
|
| ||||||
| % time below range (<3·9 mmol/L)* | 4·9 (14·4) | 1·4 (4·2) | −3·5 (15·4) | 0·29 | 0·13 | 0·11 |
| % time in range (3·9–10·0 mmol/L)* | 81 (22) | 87 (17) | 6 (26) | 0·25 | 0·70 | >0·99 |
| % high glucose (>10 mmol/L)* | 14·2 (20·2) | 11·5 (16·6) | −2·7 (21·4) | 0·50 | 0·29 | 0·30 |
| % very low glucose (<3·0 mmol/L) | 0·57 (2·67) | 0 | ·· | ·· | ·· | ·· |
| % very high glucose (>13·9 mmol/L) | 1·14 (3·68) | 0 | ·· | ·· | ·· | ·· |
| Mean glucose (mmol/L) | 8·0 (1·7) | 7·3 (1·6) | −0·8 (1·4) | 0·023 | 0·34 | 0·64 |
| Rescue carbohydrate (count) | 0·27 (0·55) | 0·21 (0·41) | −0·06 (0·77) | |||
| Insulin (units)† | 1·5 (0·9) | 1·7 (2·0) | 0·2 (1·6) | 0·60 | 0·90 | 0·95 |
Data are mean (SD) or difference (SD). The p value for sequence indicates the significance of the sequence of doing the exAPD versus exMPC first. The p value for the period indicates whether the order of doing the intervention, regardless of the type, was significant. exAPD=exercise-aware adaptive proportional derivative. exMPC=exercise-aware model predictive control.
p values estimated using bootstrapped standard errors.
p values from mixed effects regression model on insulin units.
Figure 4: CGM data, insulin, and METs during the 2 h after the start of the primary in-clinic session structured exercise.
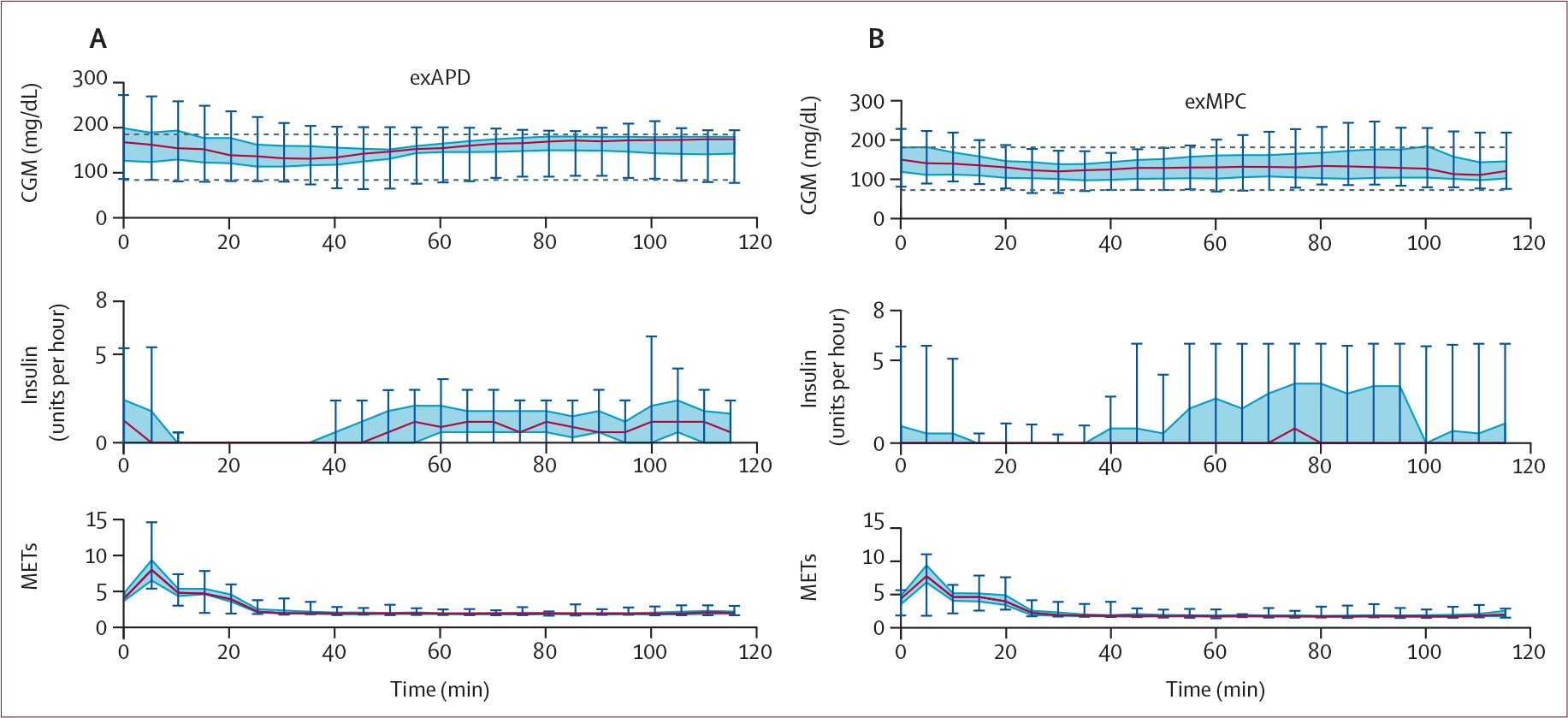
The exercise-aware model predictive control (exMPC) algorithm data are shown on the right panel and the exercise-aware adaptive proportional derivative (exAPD) algorithm data are shown on the left panel. Notice that the exAPD shuts off insulin completely once structured exercise is detected while the exMPC will only shut off insulin completely if necessary. CGM=continuous glucose monitor. MET=metabolic equivalent to task.
Across the entire 76 h study (table 4), exMPC and exAPD performed comparably in terms of time in range (71·2% vs 75·5%, difference −4·3 [16·4]; p=0∙13) and time below range (0·96% vs 1·30%, difference −0·33 [1·92]; p=0∙47). The use of exMPC over the full study duration required similar rescue carbohydrates compared with exAPD (0·65 for exMPC vs 1·03 per day for exAPD; p=0∙14).
Table 4:
Outcome metrics comparing the exAPD algorithm versus the exMPC algorithm across the entire 76 h study
| exAPD | exMPC | Difference | P value (exAPD vs exMPC) | P value (sequence) | P value (period) | |
|---|---|---|---|---|---|---|
|
| ||||||
| % time below range (<3·9 mmol/L)* | 1·30 (2·16) | 0·96 (1·21) | −0·33 (1·92) | 0·47 | 0·53 | 0·98 |
| % time in range (3·9–10·0 mmol/L) | 75·5 (10·7) | 71·2 (16·1) | −4·3 (16·4) | 0·13 | 0·28 | 0·81 |
| % high glucose (>10 mmol/L) | 23·2 (10·9) | 27·8 (16·0) | 4·6 (16·5) | 0·10 | 0·31 | 0·80 |
| % very low glucose (<3·0 mmol/L) | 0·13 (0·33) | 0·05 (0·15) | −0·08 (0·33) | |||
| % very high glucose (>13·9 mmol/L)* | 4·5 (5·7) | 7·0 (10·9) | 2·5 (11·6) | 0·27 | 0·17 | 0·92 |
| Mean glucose (mmol/L)† | 8·5 (0·9) | 8·8 (1·3) | 0·32 (1·3) | 0·17 | 0·34 | 0·56 |
| Rescue carbohydrate (count per day)‡ | 1·03 (1·34) | 0·65 (0·84) | −0·38 (1·15) | 0·14 | 0·17 | 0·59 |
| Insulin (units per day)§ | 40·8 (16·0) | 44·6 (22·6) | 3·8 (11·4) | 0·25 | 0·61 | 0·162 |
Data are mean or difference (SD). The p value for sequence indicates the significance of the sequence of doing the exAPD versus exMPC first. The p value for the period indicates whether the order of doing the intervention, regardless of the type, was significant. exAPD=exercise-aware adaptive proportional derivative. exMPC=exercise-aware model predictive control.
p values estimated using bootstrapped standard errors.
p values from mixed effects regression model on inverse-transformed glucose (mmol).
p values from negative binomial (count) regression model.
p values from model with log (insulin per day) as outcome.
During the free-living exercise sessions at home (appendix pp 42–43), there was no statistically significant difference between the glucose change for exMPC (−21·5 mg/dL [43·8]) versus exAPD (−13·4 mg/dL [27·2]; p=0∙56). CGM at the start and end of exercise was comparable between the in-home exMPC and exAPD exercise sessions. Carbohydrate intake before in-home exercise was higher for exMPC compared with exAPD (7·7 g [8·5] vs 1·0 [3·7]; p=0∙01). No low glucose (<3·9 mmol/L) was observed during the in-home exercise for any participants on either algorithm.
In a post-hoc analysis, we considered the hypothesis that participants using either exMPC or exAPD would have better glucose outcomes compared with their usual care since many participants did not use an AID in their usual care. We compared glucose metrics from the intervention periods with the run-in week when the participants used their own insulin pump and CGM. Notably many participants (n=11) were using commercial AID as their current therapy during run-in. Participants had lower time below range compared with the run-in period (2·4% run-in, p=0∙002 comparing run-in with exMPC of 0·96% and p=0∙019 comparing run-in with exAPD of 1·3%). Time in range was higher for both algorithms compared with run-in but only significant for exAPD (69·2%, p=0∙52 comparing exMPC of 71·2% and p=0∙036 comparing exAPD of 75·5%).
Discussion
Results indicate that the exMPC and exAPD algorithms, that both make use of exercise metrics, yielded comparable glucose outcomes. The exMPC yielded similar time in range and time below range compared with the exAPD during the primary in-clinic session. During the two 2 h after the start of the primary in-clinic session structured exercise period, the exMPC algorithm had better performance than the exAPD in terms of a significantly lower mean glucose without significant concomitant increases in time below range or very low glucose. The exMPC algorithm did not require any interaction from the participant in response to exercise, whereas the exAPD algorithm required the user to respond to the exercise announcement prompts. In this way, exMPC presumably required a lower burden than exAPD.
This is the first study whereby exercise metrics (ie, heart rate and accelerometry) were used as continuous inputs to an AID system to modify insulin dosing under free-living, real-world settings. Previously, the exAPD algorithm was used in both single and dual-hormone closed-loop studies in in-clinic and outpatient free-living conditions. However, the adjustment to the insulin and glucagon dosing was done only after the user responded to a prompt indicating that exercise had been initiated. The exMPC algorithm did not require a user prompt and could respond throughout the day to exercise events. This enabled adjustment of insulin dosing even in response to activities of daily living (figure 3). The inhome results (table 4) indicate that both the exMPC and exAPD performed well and comparably under free-living conditions and on average both were able to keep participants above 70% time in range and less than 4% time below range as recommended by the American Diabetes Association.28 There were no significant differences between the glucose outcomes for the two algorithms during the in-home exercise portions of the study and no low glucose observed during any of the inhome exercise sessions.
Early work on integrating exercise metrics into AID by Turksoy and colleagues13 and De Boer and Breton14 was done within an in-clinic setting. Other work assessed commercial AIDs during exercise. Breton and colleagues evaluated Control-IQ during a ski camp.29 Control-IQ and other commercial AIDs do not use physical activity as an input. They provide the option for adjusting the target glucose before exercise and during exercise to reduce insulin delivery, which can help avoid exercise-induced hypo glycaemia if done in advance.6,11 However, people often forget to make adjustments in advance or make inappropriate adjustments. Furthermore, people might be active throughout the day, but they might not consider any of these activities to be exercise. An AID system like exMPC that can automatically adjust insulin throughout all activities in the day could be helpful to people struggling with glucose management. Glucose has been shown to drop substantially even if basal insulin is suspended at the start of exercise30 and so anticipation of exercise could be critical for future applications.15
Results indicate that use of exercise metrics collected from a commercially available wrist-worn fitness monitor can be used effectively by either exMPC or exAPD as a continuous input in an AID to achieve clinical glucose outcome targets28 for people with type 1 diabetes. Limitations of this study are first that it was a short study. Future studies will evaluate the system over a longer period of time. In addition, the study was powered for 24 participants, but only 22 participants completed the exAPD arm. Furthermore, the SD of the outcome measures was larger in the study than the ones used to power the study. This was probably because a simulator was used to estimate the variance for the power analysis, which can yield smaller variance estimates than real-world data. The small number of participants and the larger variance could partially explain why statistically significant differences might not have been observed in the primary or secondary outcome measures assuming that these differences exist. Second, the performance of the algorithms on primary in-clinic structured exercise sessions was only evaluated on a single type of exercise (aerobic) and was only 30 minutes in duration. However, the system performed well during the free-living portion of the study when participants were instructed to perform exercise on their own on days 2 or 3 of the study. In the future, it will be important to evaluate the exMPC under a variety of exercise types (resistance, aerobic, interval), durations, intensities, and under fasting versus non-fasting states.31 Although the current study was not powered to explore factors contributing to glucose changes, a study by Riddell and colleagues32 provides an analysis on a large cohort of people with type 1 diabetes (n=497) exercising under free-living conditions. They identify baseline glucose, rate of change of glucose before exercise, insulin-on-board at start of exercise and other factors related to changes in glucose during aerobic, resistance, and interval exercise. Third, for safety reasons required by the FDA, we needed to include an in-clinic evaluation period for the exMPC, which potentially introduced noise into the findings because these first eight participants did the exercise on the day 3 in-clinic session. When evaluating the impact of doing the first eight exMPC participants on day 3, we found that these participants had higher time in range and lower time below range than the following 16 participants who did the in-clinic exercise on day 1; the p value did not reach significance (p=0∙063). Future studies will not require this type of a study design. Fourth, the study population from this study was generally well controlled with a mean HbA1c of 6·4%. Future studies will need to evaluate these algorithms in a more broadly representative population. A final limitation is that the results presented here are for only one type of fitness watch, the Polar M600. In a previous study,12 we found that accuracy of different fitness watches including the Garmin and the Fitbit watches, were comparable. If fitness watches are to be used in future closed-loop systems, it will be important to carefully assess the accuracy of heart rate and accelerometer data.
Supplementary Material
Research in context.
Evidence before this study
Before this study, there had not been a study showing that exercise metrics including heart rate and accelerometry collected from wearable fitness sensors can be incorporated into an automated insulin delivery (AID) system under free-living conditions. We reviewed publications in the area of incorporation of wearable fitness data into AID and automated multihormone delivery systems. We searched publicly available databases including PubMed without excluding by language or by date using the search terms “type 1 diabetes”, “exercise”, and “automated insulin delivery” or “artificial pancreas”. PubMed returned a total of 165 manuscripts, of which the majority were either review articles, studies done on commercial AID systems that do not incorporate exercise metrics as inputs to their algorithms, in silico mathematical models of exercise metabolism, or glucose forecasting models designed to work during exercise and evaluated post hoc. There has been preliminary work on incorporating heart rate, accelerometry and other measures from fitness wearables as continuous inputs into AID reported by our group as well as Breton, De Boer, Garcia-Tirado and colleagues at the University of Virginia, and Turksoy, Cinar and colleagues at the Illinois Institute of Technology. The preliminary studies by Breton, De Boer, Garcia-Tirado and by Turksoy and Cinar were all done in an in-clinic setting under prescribed conditions including fixed meal times, exercise types, durations, and intensities. De Boer and colleagues showed that the percent time less than 3·9 mmol/L could be significantly reduced during an in-clinic study of a heart-rate informed AID system compared with a standard AID (0·5 +/− 2·1% vs 7·4 +/− 12·5%, p=0·028). Turksoy and Cinar also showed, in their in-clinic studies, that heart rate and other exercise metrics could be incorporated into an AID to yield high time in range and low time in hypoglycaemia, though carbohydrate intake was required in 59% of the exercise sessions to avoid hypoglycaemia. Garcia-Tirado, Breton and colleagues described a new AID that used previous imposed exercise behavioural patterns done at specific times over several weeks to determine if the AID system could then anticipate and respond to exercise when it occurred in the future at these same times. The intervention when exercise was anticipated was done in an in-clinic study whereby they showed that the system could reduce hypoglycaemia compared with if the system did not anticipate the exercise and adjust dosing in advance. Although these studies showed a potential benefit of incorporating exercise data into the AID dosing and decision-making, there had not yet been a study done under free-living conditions whereby exercise metrics were used to inform control decisions and modify insulin dosing. In our previous work, we had incorporated exercise as a metric for adjusting insulin dosing during exercise, but it required the user to acknowledge a prompt when exercise was detected by a wearable fitness sensor.
Therefore, these preliminary systems were designed to work only during structured exercise periods as opposed to continuously throughout the day and especially during increased activity during daily living such as housework or yardwork. Thus, before the current study, there had not yet been a study showing that an AID system receiving continuous fitness data for adjusting insulin dosing is effective at maintaining clinical targets for glucose outcomes both during exercise and under free-living real-world conditions.
Added value of this study
Results from the study presented in this manuscript show for the first time that exercise metrics collected from a wearable fitness sensor can be effectively used as an additional input into an AID system. Results indicate that during a two-hour period following the start of exercise, an AID that automatically incorporates real-time exercise metrics into dosing decisions can improve glucose outcomes compared with a system that requires a user prompt and makes adjustments to insulin dosing that are rule-based (eg, shut insulin off for a period of time and then reduce insulin delivery if exercise is detected and acknowledged by the user). Results across the full 76 h study period which included two days of free-living indicate that an AID that uses exercise metrics as a real-time input for calculating automated insulin dosing can help achieve clinical targets for glucose outcomes.
Implications of all the available evidence
This study provides evidence that can support the development of next-generation exercise-aware commercial AID systems. These exercise-aware AID systems might ultimately leverage the ubiquity of wearable fitness sensors for informing AIDs during an active lifestyle. An exercise-aware AID could ultimately help people living with type 1 diabetes improve their overall health through exercise while maintaining safety and improved glucose outcomes during and following exercise. An exercise-aware AID could also provide benefit for people with type 1 diabetes who are living an active lifestyle who might struggle with glucose management during physical activity that is not traditionally considered exercise (eg, housework, yardwork, commuting).
Acknowledgments
We would like to thank our study participants who dedicated their time to support research in diabetes. And we would like to thank Tomas Walker and Andy Balo from Dexcom for their support on integrating the Dexcom G6 into iPancreas. We would like to thank Trang Ly from Insulet Corporation for their support on integrating the Insulet Omnipod into iPancreas. This study was supported by a joint grant from JDR Foundation and the Leona M and Harry B Helmsley Charitable Trust (2-SRA-2017-502-M-B) and by grants from the National Institutes of Health (NIH)–National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK1225833-01 and 1R01DK120367-01) and NIH training grant (1F30DK128914-01).
Footnotes
Declaration of interests
PGJ and JRC have a financial interest in Pacific Diabetes Technologies, a company that might have a commercial interest in the results of this research and technology. JRC also reports advisory board participation for Zealand Pharma, Novo Nordisk, Insulet, and AstraZeneca. PGJ reports advisory board participation for Eli Lilly. PGJ and JRC have received research funding at their institution from Dexcom. All other authors declare no competing interests.
Contributor Information
Peter G Jacobs, Artificial Intelligence for Medical Systems Lab, Department of Biomedical Engineering, Center for Health and Healing, Oregon Health and Science University, Portland, OR, USA.
Navid Resalat, Artificial Intelligence for Medical Systems Lab, Department of Biomedical Engineering, Center for Health and Healing, Oregon Health and Science University, Portland, OR, USA.
Wade Hilts, Artificial Intelligence for Medical Systems Lab, Department of Biomedical Engineering, Center for Health and Healing, Oregon Health and Science University, Portland, OR, USA.
Gavin M Young, Artificial Intelligence for Medical Systems Lab, Department of Biomedical Engineering, Center for Health and Healing, Oregon Health and Science University, Portland, OR, USA.
Joseph Leitschuh, Artificial Intelligence for Medical Systems Lab, Department of Biomedical Engineering, Center for Health and Healing, Oregon Health and Science University, Portland, OR, USA.
Joseph Pinsonault, Artificial Intelligence for Medical Systems Lab, Department of Biomedical Engineering, Center for Health and Healing, Oregon Health and Science University, Portland, OR, USA.
Joseph El Youssef, Harold Schnitzer Diabetes Health Center, Oregon Health and Science University, Portland, OR, USA.
Deborah Branigan, Harold Schnitzer Diabetes Health Center, Oregon Health and Science University, Portland, OR, USA.
Virginia Gabo, Harold Schnitzer Diabetes Health Center, Oregon Health and Science University, Portland, OR, USA.
Jae Eom, Harold Schnitzer Diabetes Health Center, Oregon Health and Science University, Portland, OR, USA.
Katrina Ramsey, Oregon Clinical and Translational Research Institute Biostatistics and Design Program, Oregon Health and Science University, Portland, OR, USA.
Robert Dodier, Artificial Intelligence for Medical Systems Lab, Department of Biomedical Engineering, Center for Health and Healing, Oregon Health and Science University, Portland, OR, USA.
Clara Mosquera-Lopez, Artificial Intelligence for Medical Systems Lab, Department of Biomedical Engineering, Center for Health and Healing, Oregon Health and Science University, Portland, OR, USA.
Leah M Wilson, Harold Schnitzer Diabetes Health Center, Oregon Health and Science University, Portland, OR, USA.
Jessica R Castle, Harold Schnitzer Diabetes Health Center, Oregon Health and Science University, Portland, OR, USA.
Data sharing
Our group at Oregon Health and Science University maintains a data repository for all data sets generated from our clinical studies. These data are de-identified and made available for any group interested in using them by signing a data sharing agreement to enable further research and discovery. The data include time-matched continuous glucose monitor, insulin, accelerometry, and heart rate data as well as a data dictionary that fully describes the data in the dataset. The data have been added to our repository and will be available at the time of publication of this manuscript. Access to the data is done by contacting the corresponding author (jacobsp@ohsu.edu) and making a request for data.
References
- 1.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016; 316: 1407–08. [DOI] [PubMed] [Google Scholar]
- 2.Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021; 44: 1630–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019; 381: 1707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ware J, Boughton CK, Allen JM, et al. Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit Health 2022; 4: e245–55. [DOI] [PubMed] [Google Scholar]
- 5.Tsoukas MA, Majdpour D, Yale J-F, et al. A fully artificial pancreas versus a hybrid artificial pancreas for type 1 diabetes: a single-centre, open-label, randomised controlled, crossover, non-inferiority trial. Lancet Digit Health 2021; 3: e723–32. [DOI] [PubMed] [Google Scholar]
- 6.Wilson LM, Jacobs PG, Riddell MC, Zaharieva DP, Castle JR. Opportunities and challenges in closed-loop systems in type 1 diabetes. Lancet Diabetes Endocrinol 2022; 10: 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moser O, Riddell MC, Eckstein ML, et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia 2020; 63: 2501–20. [DOI] [PubMed] [Google Scholar]
- 8.Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol 2017; 5: 377–90. [DOI] [PubMed] [Google Scholar]
- 9.Yardley JE, Kenny GP, Perkins BA, et al. Resistance versus aerobic exercise: acute effects on glycemia in type 1 diabetes. Diabetes Care 2013; 36: 537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyler N, Mosquera-Lopez C, Young G, El Youssef J, Castle J, Jacobs P. Quantifying the impact of physical activity on future glucose trends using machine learning. iScience 2022; 25: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paldus B, Morrison D, Zaharieva DP, et al. A randomized crossover trial comparing glucose control during moderate-intensity, high-intensity, and resistance exercise with hybrid closed-loop insulin delivery while profiling potential additional signals in adults with type 1 diabetes. Diabetes Care 2022; 45: 194–203. [DOI] [PubMed] [Google Scholar]
- 12.Reddy RK, Pooni R, Zaharieva DP, et al. Accuracy of wrist-worn activity monitors during common daily physical activities and types of structured exercise: evaluation study. JMIR Mhealth Uhealth 2018; 6: e10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turksoy K, Hajizadeh I, Hobbs N, et al. Multivariable artificial pancreas for various exercise types and intensities. Diabetes Technol Ther 2018; 20: 662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBoer MD, Cherñavvsky DR, Topchyan K, Kovatchev BP, Francis GL, Breton MD. Heart rate informed artificial pancreas system enhances glycemic control during exercise in adolescents with T1D. Pediatr Diabetes 2017; 18: 540–46. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Tirado J, Brown SA, Laichuthai N, et al. Anticipation of historical exercise patterns by a novel artificial pancreas system reduces hypoglycemia during and after moderate-intensity physical activity in people with type 1 diabetes. Diabetes Technol Ther 2021; 23: 277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs PG, Resalat N, El Youssef J, et al. Incorporating an exercise detection, grading, and hormone dosing algorithm into the artificial pancreas using accelerometry and heart rate. J Diabetes Sci Technol 2015; 9: 1175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson LM, Jacobs PG, Ramsey KL, et al. Dual-hormone closed-loop system using a liquid stable glucagon formulation versus insulinonly closed-loop system compared with a predictive low glucose suspend system: an open-label, outpatient, single-center, crossover, randomized controlled trial. Diabetes Care 2020; 43: 2721–29. [DOI] [PubMed] [Google Scholar]
- 18.Resalat N, El Youssef J, Reddy R, Jacobs PG. Design of a dual-hormone model predictive control for artificial pancreas with exercise model. Ann Int Conf IEEE Eng Med Biol Soc 2016; 2016: 2270–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resalat N, Youssef JE, Reddy R, Jacobs PG. Evaluation of model complexity in model predictive control within an exercise-enabled artificial pancreas. IFAC-PapersOnLine 2017; 50: 7756–61. [Google Scholar]
- 20.Castle JR, El Youssef J, Wilson LM, et al. Randomized outpatient trial of single and dual-hormone closed-loop systems that adapt to exercise using wearable sensors. Diabetes Care 2018; 41: 1471–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs PG, El Youssef J, Reddy R, et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab 2016; 18: 1110–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovorka R, Canonico V, Chassin LJ, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas 2004; 25: 905–20. [DOI] [PubMed] [Google Scholar]
- 23.Wilinska ME, Chassin LJ, Schaller HC, Schaupp L, Pieber TR, Hovorka R. Insulin kinetics in type-I diabetes: continuous and bolus delivery of rapid acting insulin. IEEE Trans Biomed Eng 2005; 52: 3–12. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Ordoñez M, Campos-Delgado DU. An extension to the compartmental model of type 1 diabetic patients to reproduce exercise periods with glycogen depletion and replenishment. J biomechanics 2008; 41: 744–52. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs PG, El Youssef J, Castle J, et al. Automated control of an adaptive bihormonal, dual-sensor artificial pancreas and evaluation during inpatient studies. IEEE Trans Biomed Eng 2014; 61: 2569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosquera-Lopez C, Jacobs PG. Incorporating glucose variability into glucose forecasting accuracy assessment using the new glucose variability impact index and the prediction consistency index: an LSTM case example. J Diabetes Sci Technol 2023; 16: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resalat N, El Youssef J, Tyler N, Castle J, Jacobs PG. A statistical virtual patient population for the glucoregulatory system in type 1 diabetes with integrated exercise model. PloS one 2019; 14: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care 2022; 45 (suppl 1): S83–96. [DOI] [PubMed] [Google Scholar]
- 29.Breton MD, Chernavvsky DR, Forlenza GP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care 2017; 40: 1644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaharieva D, Yavelberg L, Jamnik V, Cinar A, Turksoy K, Riddell MC. The effects of basal insulin suspension at the start of exercise on blood glucose levels during continuous versus circuit-based exercise in individuals with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Technol Ther 2017; 19: 370–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yardley JE. Reassessing the evidence: prandial state dictates glycaemic responses to exercise in individuals with type 1 diabetes to a greater extent than intensity. Diabetologia 2022; 65: 1994–99. [DOI] [PubMed] [Google Scholar]
- 32.Riddell MC, Li Z, Gal RL, et al. Examining the acute glycemic effects of different types of structured exercise sessions in type 1 diabetes in a real-world setting: the type 1 diabetes and exercise initiative (T1DEXI). Diabetes Care 2023; 46: 704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our group at Oregon Health and Science University maintains a data repository for all data sets generated from our clinical studies. These data are de-identified and made available for any group interested in using them by signing a data sharing agreement to enable further research and discovery. The data include time-matched continuous glucose monitor, insulin, accelerometry, and heart rate data as well as a data dictionary that fully describes the data in the dataset. The data have been added to our repository and will be available at the time of publication of this manuscript. Access to the data is done by contacting the corresponding author (jacobsp@ohsu.edu) and making a request for data.


