Abstract
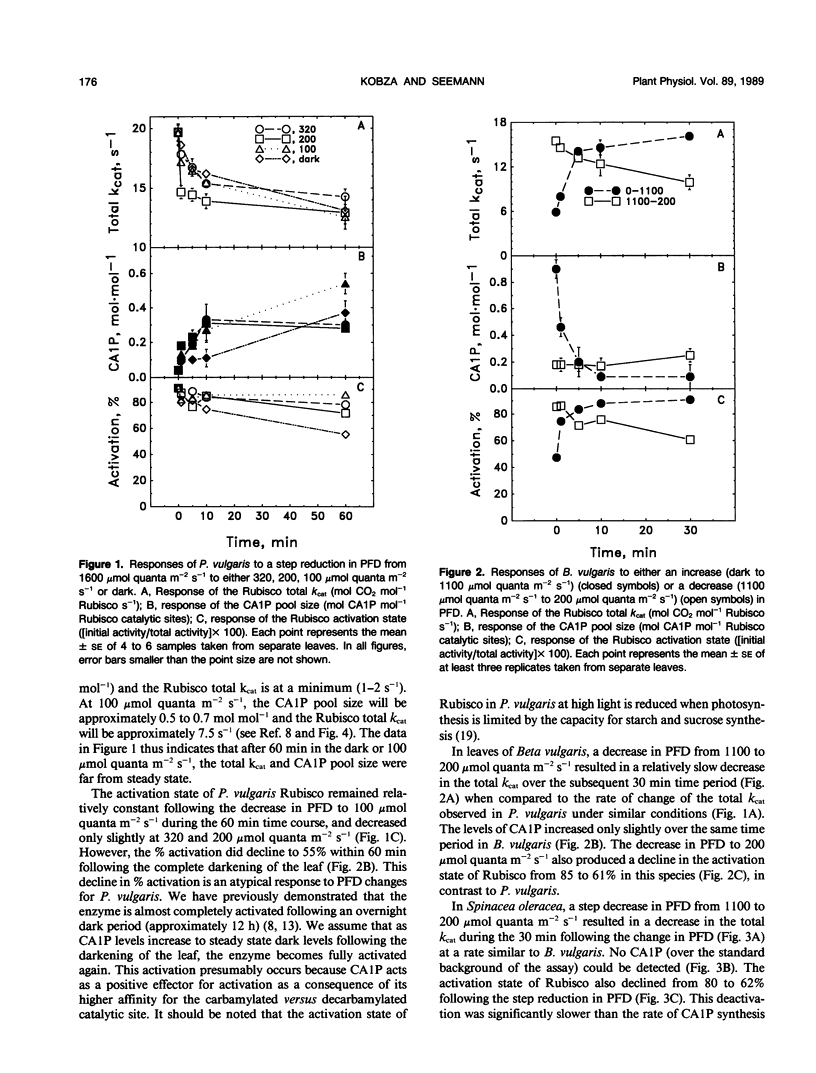
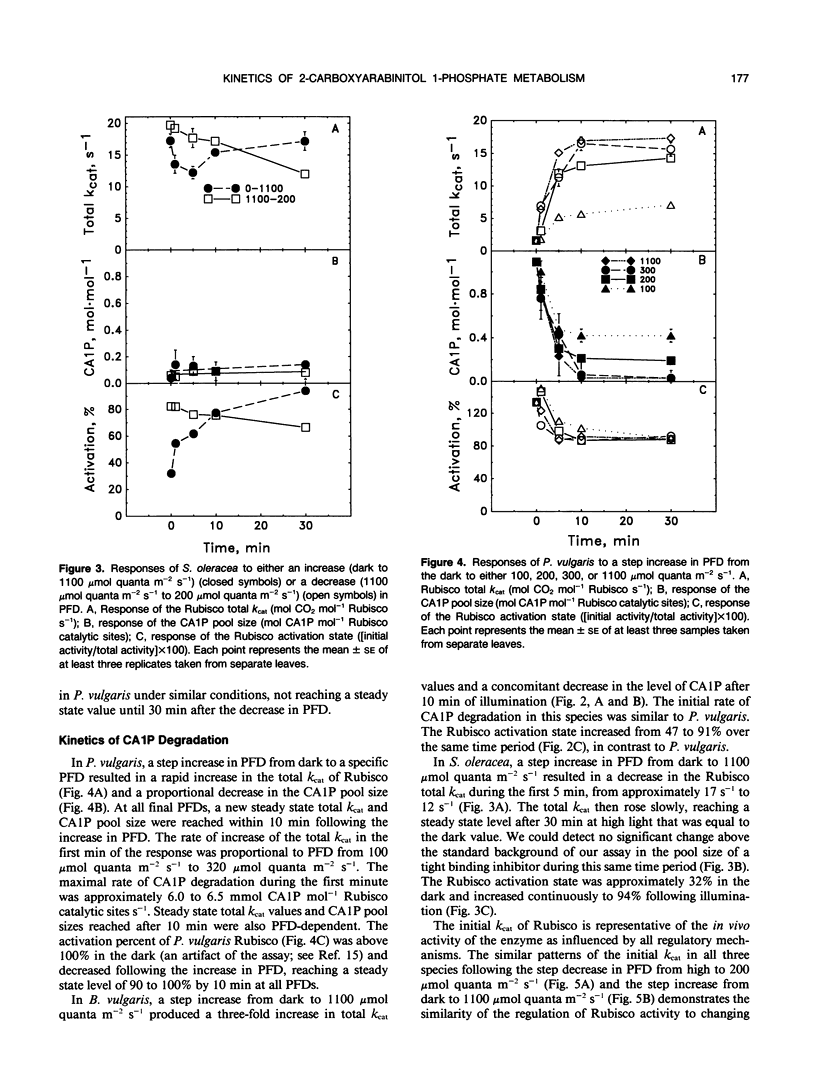
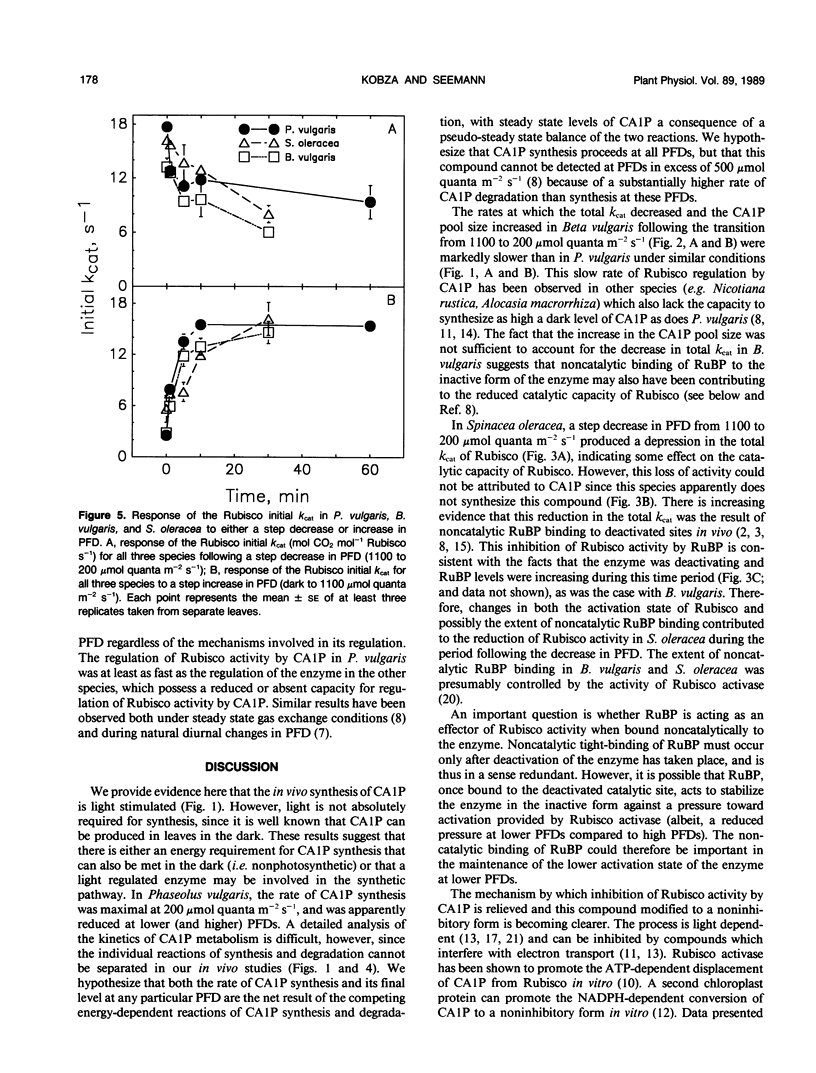
The light-dependent kinetics of the apparent in vivo synthesis and degradation of 2-carboxyarabinitol 1-phosphate (CA1P) were studied in three species of higher plants which differ in the extent to which this compound is involved in the light-dependent regulation of ribulose-1,5-bisphosphate carboxylase (Rubisco) activity. Detailed studies with Phaseolus vulgaris indicate that both the degradation and synthesis of this compound are light-stimulated, although light is absolutely required only for CA1P degradation. We hypothesize that the steady state level of CAIP at any particular photon flux density (PFD) represents a pseudo-steady state balance between ongoing synthesis and degradation of this compound. The rate of CA1P synthesis in P. vulgaris and the resultant reduction in the total catalytic constant of Rubisco were maximal at 200 micromoles quanta per square meter per second following a step decrease from a saturating PFD, and substantially faster than the rate of synthesis in the dark. Under these conditions an amount of CA1P equivalent to approximately 25% of the Rubisco catalytic site content was synthesized in less than 1 minute. The rate of synthesis was reduced at higher or lower PFDs. In Beta vulgaris, the rate of CA1P synthesis at 200 micromoles quanta per square meter per second was substantially slower than in P. vulgaris. In Spinacea oleracea, an apparent noncatalytic tight-binding of RuBP to deactivated sites on the enzyme was found to occur following a step decrease in PFD. When dark acclimated leaves of P. vulgaris were exposed to a step increase in PFD, the initial rate of CA1P degradation was also found to be dependent on PFD up to a maximum of approximately 300 to 400 micromoles quanta per square meter per second. The rate of degradation of this compound was similar in B. vulgaris. In S. oleracea, a step increase in PFD resulted in noncatalytic RuBP binding to Rubisco followed by an apparent release of RuBP and activation of the enzyme. The in vivo rate of change of Rubisco activity in response to an increase or decrease in PFD was similar between species despite the differences between species in the mechanisms used for the regulation of this enzyme's activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry J. A., Lorimer G. H., Pierce J., Seemann J. R., Meek J., Freas S. Isolation, identification, and synthesis of 2-carboxyarabinitol 1-phosphate, a diurnal regulator of ribulose-bisphosphate carboxylase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):734–738. doi: 10.1073/pnas.84.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A., Portis A. R. Protein-bound ribulose bisphosphate correlates with deactivation of ribulose bisphosphate carboxylase in leaves. Plant Physiol. 1988 May;87(1):244–249. doi: 10.1104/pp.87.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. R., Seemann J. R. Differences between Wheat Genotypes in Specific Activity of Ribulose-1,5-bisphosphate Carboxylase and the Relationship to Photosynthesis. Plant Physiol. 1984 Apr;74(4):759–765. doi: 10.1104/pp.74.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobza J., Seemann J. R. Mechanisms for light-dependent regulation of ribulose-1,5-bisphosphate carboxylase activity and photosynthesis in intact leaves. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3815–3819. doi: 10.1073/pnas.85.11.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988 Jan;86(1):293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Anderson J. C. Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol. 1987 Sep;85(1):66–71. doi: 10.1104/pp.85.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Berry J. A., Freas S. M., Krump M. A. Regulation of ribulose bisphosphate carboxylase activity in vivo by a light-modulated inhibitor of catalysis. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8024–8028. doi: 10.1073/pnas.82.23.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Kirschbaum M. U., Sharkey T. D., Pearcy R. W. Regulation of Ribulose-1,5-Bisphosphate Carboxylase Activity in Alocasia macrorrhiza in Response to Step Changes in Irradiance. Plant Physiol. 1988 Sep;88(1):148–152. doi: 10.1104/pp.88.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Sharkey T. D. Salinity and Nitrogen Effects on Photosynthesis, Ribulose-1,5-Bisphosphate Carboxylase and Metabolite Pool Sizes in Phaseolus vulgaris L. Plant Physiol. 1986 Oct;82(2):555–560. doi: 10.1104/pp.82.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Binding of a Phosphorylated Inhibitor to Ribulose Bisphosphate Carboxylase/Oxygenase during the Night. Plant Physiol. 1985 Aug;78(4):839–843. doi: 10.1104/pp.78.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Parry M. A., Gutteridge S., Keys A. J. Species variation in the predawn inhibition of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1986 Dec;82(4):1161–1163. doi: 10.1104/pp.82.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Seemann J. R., Berry J. A. Regulation of Ribulose-1,5-Bisphosphate Carboxylase Activity in Response to Changing Partial Pressure of O(2) and Light in Phaseolus vulgaris. Plant Physiol. 1986 Jul;81(3):788–791. doi: 10.1104/pp.81.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streusand V. J., Portis A. R. Rubisco Activase Mediates ATP-Dependent Activation of Ribulose Bisphosphate Carboxylase. Plant Physiol. 1987 Sep;85(1):152–154. doi: 10.1104/pp.85.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu C. V., Allen L. H., Bowes G. Effects of Light and Elevated Atmospheric CO(2) on the Ribulose Bisphosphate Carboxylase Activity and Ribulose Bisphosphate Level of Soybean Leaves. Plant Physiol. 1983 Nov;73(3):729–734. doi: 10.1104/pp.73.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu J. C., Allen L. H., Bowes G. Dark/Light modulation of ribulose bisphosphate carboxylase activity in plants from different photosynthetic categories. Plant Physiol. 1984 Nov;76(3):843–845. doi: 10.1104/pp.76.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]


