Abstract
Background and Objectives
For children with cerebral malaria, mortality is high, and in survivors, long-term neurologic and cognitive dysfunctions are common. While specific clinical factors are associated with death or long-term neurocognitive morbidity in cerebral malaria, the association of EEG features with these outcomes, particularly neurocognitive outcomes, is less well characterized.
Methods
In this prospective cohort study of 149 children age 6 months to 12 years who survived cerebral malaria in Kampala, Uganda, we evaluated whether depth of coma, number of clinical seizures, or EEG features during hospitalization were associated with mortality during hospitalization, short-term and long-term neurologic deficits, or long-term cognitive outcomes (overall cognition, attention, memory) over the 2-year follow-up.
Results
Higher Blantyre or Glasgow Coma Scores (BCS and GCS, respectively), higher background voltage, and presence of normal reactivity on EEG were each associated with lower mortality. Among clinical and EEG features, the presence of >4 seizures on admission had the best combination of negative and positive predictive values for neurologic deficits in follow-up. In multivariable modeling of cognitive outcomes, the number of seizures and specific EEG features showed independent association with better outcomes. In children younger than 5 years throughout the study, seizure number and presence of vertex sharp waves were independently associated with better posthospitalization cognitive performance, faster dominant frequency with better attention, and higher average background voltage and faster dominant background frequency with better associative memory. In children younger than 5 years at CM episode but 5 years or older at cognitive testing, seizure number, background dominant frequency, and the presence of vertex sharp waves were each associated with changes in cognition, seizure number and variability with attention, and seizure number with working memory.
Discussion
In children with cerebral malaria, seizure number is strongly associated with the risk of long-term neurologic deficits, while seizure number and specific EEG features (average background voltage, dominant rhythm frequency, presence of vertex sharp waves, presence of variability) are independently associated with cognitive outcomes. Future studies should evaluate the predictive value of these findings.
Introduction
Malaria kills more than 600,000 people annually, most of them were younger than 5 years.1 Infection may result in clinical syndromes ranging from asymptomatic parasitemia, uncomplicated malaria (a flu-like illness), to severe malaria with end-organ dysfunction. The most lethal form of severe disease is cerebral malaria (CM), defined as an otherwise unexplained coma in someone with asexual forms of Plasmodium parasitemia. Untreated, CM is universally fatal.2 With prompt and optimal treatment, mortality rates vary from 15% to 25%.3-5 Clinical seizures are very common both before and during hospitalization.
Multiple studies have established associations between biomarkers or clinical factors and mortality in pediatric CM. Clinical risk factors for death include deeper coma (Blantyre Coma Score [BCS, a measure of coma depth used in young children across Africa] ≤ 1), leukocytosis, hypoglycemia, hyperlactatemia, and papilledema.4-9 Diffuse brain swelling on brain MRI,5 low flow on transcranial Doppler ultrasound,10 and certain features on admission EEG5,11—focal or background slowing, low amplitudes, lack of reactivity, and absence of normal sleep architecture—are associated with death in children with CM.
In CM survivors, neurologic and cognitive deficits are common. At hospital discharge, 10%–30% of survivors have gross neurologic abnormalities.12 Deficits may resolve or appear after hospitalization. Long-term, 25%–50% have neurodevelopmental impairments13,14 and 9%–17% have epilepsy.15-17 Previously described neurodevelopmental sequelae include visual impairment, motor abnormalities, ataxia, language regression, cognitive impairment, and behavioral problems.11-29 Lower average voltage and lower variability of fast frequencies on admission EEG are associated with neurologic deficits at the time of discharge.11,30,31 In brain MRI acquired at 1 month postdischarge, atrophy and multifocal signal abnormalities are seen in children who had deficits at discharge.14,26
Although various clinical factors are associated long-term adverse outcomes in CM survivors—repeated seizures prior to or during hospitalization, admission depth of coma, higher maximum temperature, male sex, and the presence of focal neurologic deficits at hospital discharge13,14,17,26—comparatively few biomarkers have been investigated to this end. The association of EEG features with long-term cognitive outcomes has not been characterized to date. Knowledge of EEG features associated with adverse long-term cognitive outcomes may aid in identifying high-risk children most in need of cognitive training and rehabilitation. To evaluate whether admission EEG features are associated with mortality or adverse long-term neurologic or cognitive outcomes in children with CM, we compared clinical findings and EEG features on admission in a cohort of Ugandan children with CM to in-hospital mortality, short-term and long-term neurologic deficits, and long-term cognitive outcomes over a 2-year follow-up period.
Methods
Study Enrollment, Clinical Care, and Evaluation
Children age 6 months to 12 years with a diagnosis of CM (defined as coma with BCS ≤2 or Glasgow Coma Score [GCS] ≤8, blood smear positive for Plasmodium falciparum, and lack of alternative etiology for coma) admitted to Mulago Hospital (Kampala, Uganda) between 2008 and 2013 were eligible for inclusion in this prospective cohort study. Children were excluded if they had a history of developmental delay, cerebral palsy, head trauma, coma, malnutrition requiring hospitalization, or other chronic illness requiring persistent medical care. The history of seizures was not an exclusion criterion. Participants received specific and supportive care according to standard guidelines for severe malaria in Uganda at that time. Intravenous quinine (20 mg/kg loading dose followed by 10 mg/kg every 8 hours for a minimum 3 doses or until the child was able to take orally) was immediately administered on CM diagnosis. The BCS for children younger than 5 years4 or GCS for children older than 5 years was assessed by a clinician. Parents, caregivers, or nurses notified the study medical officers about clinical seizures after admission, and the number of these seizures was recorded. We defined a seizure as nonsuppressible, repeated tonic-clonic movements. Clinical seizures were treated with a maximum of 2 doses intravenous diazepam, followed by a loading dose of intravenous phenobarbital 15 mg/kg plus additional escalation if needed. Some children received antiseizure medication before EEG performance.
EEG Acquisition and Interpretation
A 30-minute EEG, performed using the standard 10–20 system, was acquired after initial clinical stabilization, generally within 4 hours of hospital admission. Recordings were obtained on an XLTEC 32 channel machine (Natus Medical Corporation, Pleasanton, CA) with a sampling rate of 200 Hz. EEG tracings were interpreted in real time to identify electrographic seizures or nonconvulsive status epilepticus. After hospital discharge, research interpretations by visual inspection (used in this study) were performed by a board-certified pediatric neurologist, blinded to patient outcome, using Persyst software (Persyst Corporation, Prescott, AZ).
Neurologic and Cognitive Assessments
In survivors, physical and neurologic examinations, and/or neurocognitive testing, were performed at hospital discharge (neurologic examination), 1 week after discharge (neurocognitive testing only), and 6, 12, and 24 months later (all evaluations) (eFigure 1, links.lww.com/WNL/D28). We defined a neurologic deficit as the presence of a motor or cranial nerve abnormality, ataxia, movement, speech, or visual problems. Cognitive outcomes were assessed using standardized testing tools previously validated for evaluation in Ugandan children. In children younger than 5 years, we measured overall cognition as a composite score of the fine motor, visual reception, and receptive and expressive language scales on the Mullen Scales of Early Learning.e1 We assessed attention using the Early Childhood Vigilance Test.e2 Associative memory was assessed using the Color Object Association Test.e3
In children 5 years and older, we measured overall cognition using the Kaufman Assessment Battery for Children (KABC),e4 with a summary mental processing index as the primary outcome. Attention was assessed using the Test of Variables of Attention,e5 with D prime measure as the primary outcome. We used the KABC-2 subtest for sequential processing to assess working memory. Emotional stimulation in the home was measured by age-appropriate versions of the Home Observation for the Measurement of the Environment.e6
We calculated age-adjusted z-scores for neuropsychological testing outcomes using aged-matched community controls.32 Z-scores were calculated using the patient's score minus the mean community control score for a child's age. We computed standard deviations by fitting a quadratic mixed-effects model, including a random intercept for the child. Owing to repeated testing, correlations within an individual were based on time between visits, considering all visits for community control children.
Statistical Analysis
Descriptive statistics are presented as median values with interquartile range for continuous measures and proportions for categorical measures. We assessed the association of baseline demographic, clinical, or EEG features with in-hospital mortality by logistic regression. We assessed the association of seizure number, BCS/GCS, and EEG features with neurologic deficits over a 24-month follow-up period using logistic regression with Firth penalized likelihood proceduree7 because of a low number of neurologic deficits during follow-up. We assessed the association of EEG features with longitudinal changes in cognitive z-scores over time using a linear mixed-effects (LME) model adjusted for age, socioeconomic status, home environment z-score, and antiseizure medication administration before EEG, where observations within subject were correlated using a subject-specific intercept, and time points were treated as categorical variables.33 The LME models used a banded diagonal covariance matrix to model within-subject variance-covariance errors. We fitted the mixed model by restricted maximum likelihood and used Kenward-Roger approximations to estimate the denominator degrees of freedom.
To further characterize how coma score, seizure number, and EEG features related to neurologic deficit at testing time points, we evaluated negative and positive predictive values for each factor and area under the curve for nonparametric receiver operating characteristic curves. To determine whether EEG features and seizure number were independently associated with long-term cognitive outcomes, we constructed multivariable regression models that included the number of clinical seizures and EEG features associated with cognitive outcomes in univariable analysis, choosing p value of ≤0.05 as significant. To correct for multiplicity, the Benjamini-Hochberg false discovery rate proceduree8 was used. Analyses were performed using Stata version 16.1 (StataCorp, College Station, TX). Please see supplementary material for references to testing instruments and statistical tools.
Standard Protocol Approvals, Registrations, and Patient Consents
Parents or guardians of study participants gave written informed consent. Institutional Review Boards at Michigan State University, University of Minnesota, and Makerere University School of Medicine granted ethical approval.
Data Availability
Anonymized data not published within this article will be made available by request from a qualified investigator.
Results
Study Participant Enrollment, Follow-up, and Neurologic Deficits
Of the 269 children admitted with CM, 176 had EEGs (eFigure 1, links.lww.com/WNL/D28). Ninety-three children with CM did not have EEG analysis performed because they awakened before EEG performance or an EEG technician was not available, so an EEG was not performed, or because their level of consciousness during the EEG was not noted. Of the 176 children who had an EEG on admission, 27 died. All 149 survivors returned for 1-week postdischarge follow-up. At the time of hospital discharge, the median age of survivors was 3.5 years, 64% were male, and 41% (61/148) had abnormal neurologic examinations. The rate of abnormal neurologic examinations decreased to 7.5% (11/146), 4.8% (7/145), and 4.2% (6/142) at the 6-, 12-, and 24-month follow-up time points, respectively. Follow-up was outstanding, with 142 of the 149 survivors of CM (95.3%) followed all the way through 2-year follow-up. All children with neurologic deficits at later time points had deficits at prior time points.
Association of Coma Score, Clinical Seizures, and EEG Features With Mortality
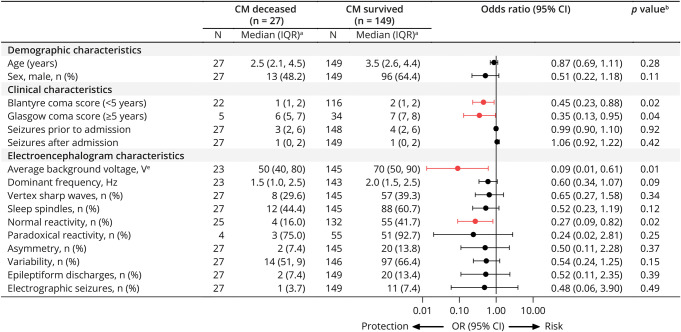
A higher BCS or GCS was associated with lower mortality (BCS, OR, 0.45, 95% CI 0.23, 0.88, p = 0.02; GCS, OR 0.35, 95% CI 0.13, 0.95, p = 0.04) (Figure 1). Among EEG features, higher average voltage (log10-transformed) (OR 0.05; 95% CI 0.01, 0.36; p = 0.003) and the presence of reactivity (OR 0.26, 95% CI 0.08, 0.81; p = 0.02) were associated with lower mortality.
Figure 1. Associations Between Demographic, Clinical, and EEG Characteristics and Mortality.
aExcept where noted, bLogistic regression model, cAdjusted for whether child was on antiseizure medication before EEG, dLog(base 10)-transformed for regression. Forest plot shows odds ratios and 95% CI of those who died vs survived, where red indicates a significant association between characteristic and protection of in-hospital mortality.
Associations of Clinical Seizures, Coma Score, and EEG Features With Neurologic Deficits Over Time
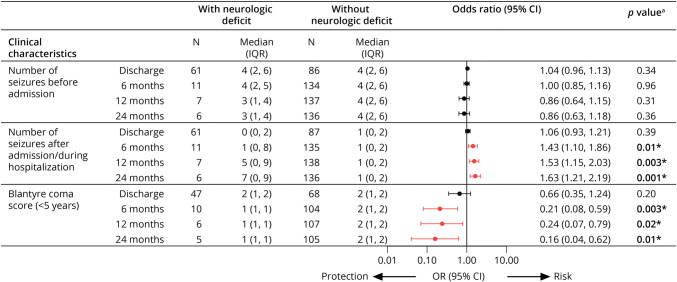
The number of seizures during admission was associated with neurologic deficits at 6, 12, and 24 months, with stronger associations over time (Figure 2). Having 4 or more seizures vs < 4 seizures was more strongly associated with neurologic deficits than a lower seizure number, whereas having 5 or more seizures did not discriminate better than have 4 or more seizures (eTable 1, links.lww.com/WNL/D28). A higher BCS was associated with a lower risk of neurologic deficits at the 6-, 12-, and 24-month time points (Figure 2). Too few older children (in whom GCS was used) had long-term neurologic deficits to allow evaluation of the association of GCS with neurologic deficits.
Figure 2. Association of Number of Seizures Before or During Admission or Blantyre Coma Score and Neurologic Deficits Over Time.
aBinary logistic regression using Firth penalized likelihood procedure. Red indicates significant association between clinical characteristic and protection or risk of neurologic deficit at each time point before correction for multiplicity. *Significant p value after correction for multiple comparisons using Benjamini-Hochberg false discovery rate approach.
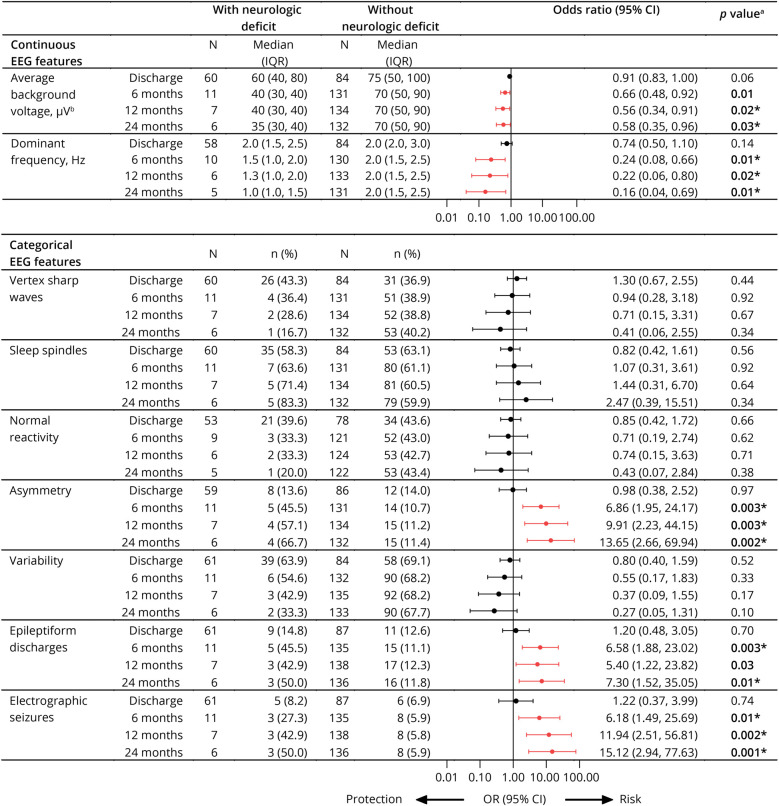
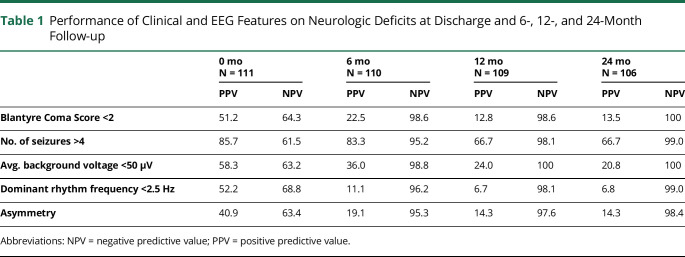
Among EEG features, lower average voltage, slower dominant frequency, and the presence of asymmetry, epileptiform discharges, or electrographic seizures were all associated with neurologic deficits at the 6-, 12-, and 24-month follow-up (Figure 3). Because clinical evaluations would rely on negative and positive predictive value of specific factors, we evaluated dichotomized values for BCS (<2 vs 2), seizure number (>4 vs ≤4), average background voltage (<50 vs ≥50 microvolt), dominant frequency (<2.5 vs ≥2.5 Hz), and asymmetry (yes vs no). Seizure number had the best positive predictive values for neurologic deficits in follow-up among all clinical or EEG features and had high negative predictive values like those of BCS or EEG features (Table 1).
Figure 3. Association Between EEG Features and Neurologic Deficits Over Time.
aBinary logistic regression using Firth penalized likelihood procedure, adjusted for antiseizure medication use before EEG. bRescaled as μV/10 in logistic regression model. Red indicates significant association between EEG feature and protection or risk of neurologic deficit at each time point before correction for multiplicity. *Significant p value after correction for multiple comparisons using Benjamini-Hochberg false discovery rate approach.
Table 1.
Performance of Clinical and EEG Features on Neurologic Deficits at Discharge and 6-, 12-, and 24-Month Follow-up
Association of the Number of Clinical Seizures and Coma Score With Cognitive Outcomes
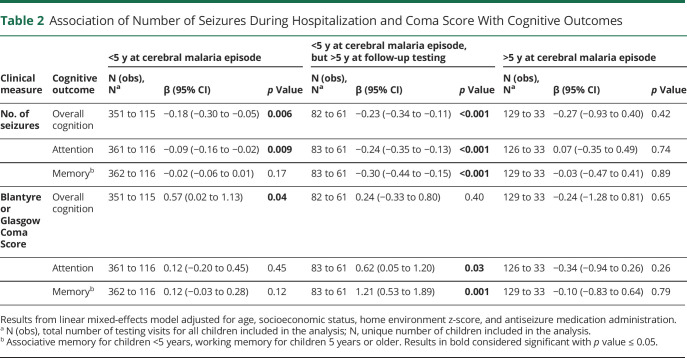
We evaluated whether clinical features (e.g., number of seizures, coma score) were associated with cognitive outcomes. Higher seizure number was associated with worse cognition and attention in children younger than 5 years at CM episode and worse cognition, attention, and associative memory in children younger than 5 years at CM episode but 5 years or older at testing time. Higher seizure number was not associated with any cognitive outcome in children 5 years or older at CM episode (Table 2). Cutoffs of 4+ or 5+ seizures (compared with <4 or <5 seizures, respectively) had similarly strong associations with cognitive outcomes in children younger than 5 years, and the associations with these cutoffs were stronger than with lower numbers of seizures (eTable 2, links.lww.com/WNL/D28).
Table 2.
Association of Number of Seizures During Hospitalization and Coma Score With Cognitive Outcomes
Higher admission BCS was associated with better overall cognitive ability in children younger than 5 years at CM episode, and better attention and memory in children younger than 5 years at CM episode but 5 years or older at testing time. Higher GCS was not associated with improved cognitive outcomes in any domain in children 5 years or older at CM episode (Table 2). In cognitive outcome evaluation, BCS and number of seizures were collinear, so an analysis of whether BCS and number of seizures were independently associated with cognitive outcomes was not possible.
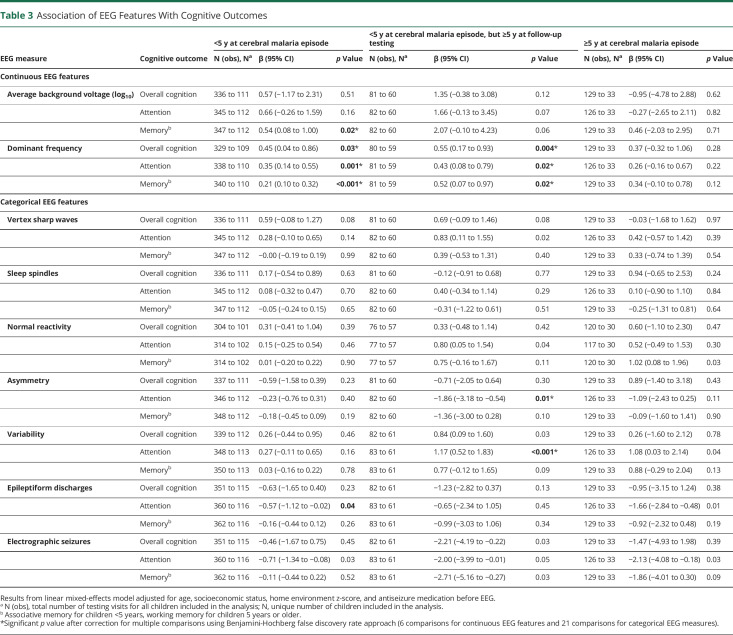
Association of EEG Features With Cognitive Outcomes
Finally, we evaluated associations of EEG features with cognitive outcomes. Among continuous EEG features, higher average background voltage and faster dominant frequency were associated with better cognitive outcomes in one or more domains in children younger than 5 years at the time of CM episode, whether tested at younger than 5 or 5 years or older at the time of follow-up testing. Neither amplitude nor frequency was associated with cognitive outcomes in children younger than 5 years at the time of CM episode (Table 3). Among categorical EEG variables, the presence of variability was associated with significantly higher attention scores, and asymmetry with significantly lower scores, in children younger than 5 years at CM episode but 5 years or older at the follow-up testing (Table 3).
Table 3.
Association of EEG Features With Cognitive Outcomes
Multivariable Model of Association of Clinical and EEG Features With Cognitive Outcomes
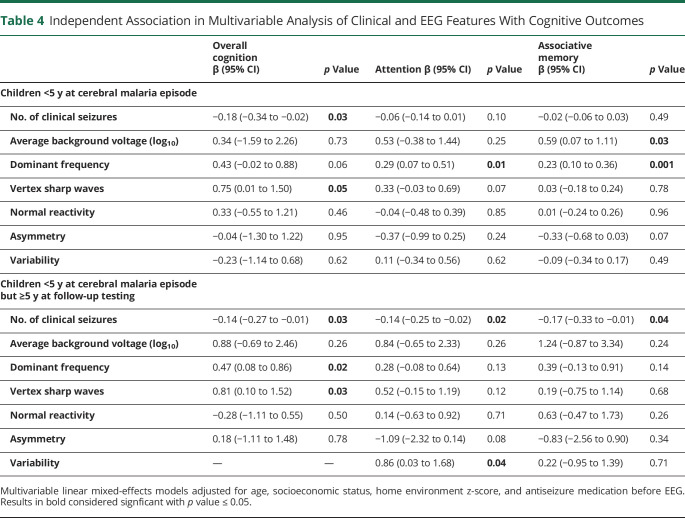
To determine whether clinical variables (seizure number) and EEG features were independently associated with cognitive outcomes, we conducted multivariable analysis, including all features associated in univariable analysis in the multivariable model (BCS was again not included because of collinearity with seizure number, and electrographic seizures and epileptiform discharges were not included because they were strongly associated with asymmetry). Among children younger than 5 years on admission and in follow-up, fewer clinical seizures and the presence of vertex sharp waves were independently associated with higher overall cognition scores, higher dominant frequency with better attention scores, higher average background voltage, and higher dominant frequency with better associative memory scores (Table 4).
Table 4.
Independent Association in Multivariable Analysis of Clinical and EEG Features With Cognitive Outcomes
Among children younger than 5 years on admission but 5 years or older in follow-up, fewer clinical seizures, faster dominant frequency, and the presence of vertex sharp waves were each independently associated with higher cognition scores. Fewer clinical seizures and the presence of variability were associated with higher scores on measures of attention. An increased number of seizures were associated with lower associative memory test scores (Table 4).
Multivariable models were not constructed for cognitive outcomes in children 5 years or older because the number of clinical seizures was not associated with any cognitive outcomes in this age group.
A schematic of clinical and EEG features that were associated with mortality, neurologic deficits over follow-up, or long-term cognition scores is presented in eFigure 2, links.lww.com/WNL/D28.
Discussion
In this study, we show for the first time that in children with CM, the clinical finding of the number of postadmission seizures and specific EEG features on admission are each independently associated with long-term cognitive outcomes, while seizure number alone, an easily assessable clinical parameter, is strongly associated with long-term neurologic deficits. Thus, the evaluation of postadmission number of seizures may be useful in identifying children at highest risk of neurologic deficits. Prediction of children at highest risk of cognitive impairment is more complex, but this study shows that specific EEG features (average background voltage, dominant rhythm frequency, presence of vertex sharp waves, presence of variability) and seizure number are each independently associated with specific cognitive outcomes. The study findings provide a basis for future studies that evaluate the predictive value of these clinical and EEG features for cognitive impairment. Such studies could potentially identify children at greatest risk of long-term cognitive impairment. Identification of these children, and the development of interventions that can decrease long-term impairment in these children, could substantially decrease the long-term neurocognitive burden of CM.
Previous studies of EEG findings in CM have focused on mortality and neurologic deficit outcomes. Our study findings are largely consistent with these studies which showed associations of lower average and maximal voltages, focal slowing, and a lack of reactivity with an increased likelihood of death.11 In other studies of EEG in CM, asymmetry and lower voltages were independently associated with an increased mortality risk.31 Similarly, we found, consistent with other studies, that lower average voltage was associated with neurologic deficits at discharge and/or in follow-up11,31 but did not find the association of sleep spindles with decreased odds of neurologic sequelae.31
EEG features did not provide substantially better negative or positive predictive value for the presence of neurologic deficits 6 months to 2 years after admission for CM than the simple measure of the number of clinical seizures postadmission. In this study, a cut-off threshold of ≥4 seizures during admission provided the greatest predictive value for long-term neurologic deficits. Future studies should determine whether this cutoff is consistent and replicated such that it could be used to identify children at risk for adverse neurologic outcomes.
Neurologic deficits decreased significantly from discharge (41%) to the 6-month follow-up (7.5%). Of the 10 children with deficits at 6 months, 6 still had deficits at 12 months (4.8%) and 5 at 24 months (4.2%). These findings are consistent with previous studies34 and suggest that most neurologic recovery in children with CM occurs within the first 6 months after acute illness. Foreknowledge of the small subset (4.2%) of CM survivors whose deficits persist for 2 years or more could help to identify those at highest need of early physical therapy or other neurologic rehabilitation.
Poor cognitive outcomes in children with CM younger than 5 years are strongly associated with increased numbers of clinical seizures during the index illness. An increased number of seizures are associated with worse long-term outcomes in all domains (overall cognition, attention, and working memory) in children younger than 5 years, but not in children 5 years or older. This suggests that the immature brain may be more sensitive to secondary injury from uncontrolled seizures. It is known that electrographic status epilepticus is associated with mortality in critically ill children without malaria35,36 and can compound neurologic injury.37-40 It is possible that identification of electrographic status epilepticus, early treatment of electrographic seizures, and aggressive reductions in seizure burden may be neuroprotective for long-term neurologic or cognitive sequelae, but this is currently unproven.
Admission EEG features were associated with cognitive outcomes in children younger than 5 years. Lower average EEG voltage was associated with significantly worse associative memory scores, and lower dominant frequency was associated with worse scores in all cognitive domains (cognitive ability, attention, and associative memory). These findings are consistent with findings in other comatose states related to diffuse brain injury (e.g., neonatal hypoxic-ischemic encephalopathy, cardiac arrest), in which low-voltage EEG is associated with unfavorable long-term neurocognitive or neurodevelopmental outcomes.41,42
In children younger than 5 years at the time of CM but 5 years or older at testing, the presence of state variability on EEG was associated with higher attention scores, while the presence of vertex waves was associated with better overall cognitive outcomes. In nonmalarial critical illness, the lack of normal sleep architecture or changes in state are associated with increased mortality in critically ill comatose patients both without43 and with44 acute cerebral injury. In our study, increased mortality risk was associated with attenuated voltage and lack of reactivity. The presence of vertex waves, a normal sleep element, and state variability were associated with improved long-term neurocognitive outcomes. Similarly, the presence of state variability and sleep architecture are biomarkers of the severity of acute cerebral injury due to CM. However, sleep may have a neuroprotective effect during CM leading to better long-term outcomes. It is possible that sleep states allow clearance of excitotoxic signal molecules from active neurons and neurotoxic substances from neuronal damage.45,46
Limitations of our study include the short (30-minute) duration of EEG recordings, which may fail to capture more transient EEG features. Continuous EEG monitoring, unavailable at our research site, would likely improve the sensitivity and specificity of clinical seizure detection, detect subclinical seizures, and quantify seizure burden.47,48 We did not include EEG or neuropsychological follow-up for children who were admitted in coma but recovered consciousness before EEG acquisition. EEG studies were interpreted by a pediatric neurologist with experience in children with CM. Although this avoids concerns with inter-rater reliability, it is possible that the use of multiple EEG interpreters with adjudicated reads may have changed our study's findings. Study strengths included rigorous inclusion and exclusion criteria; detailed and appropriate clinical, neurologic, and cognitive evaluation; outstanding follow-up through 2 years after the CM episode (>95%); and high quality of the EEG testing, with interpretation by a board-certified neurologist.
Most EEG use in Africa is in large referral hospitals, such as the one where our study was performed. For EEG to be useful clinically, trained technologists, equipment, and interpreters must all be available. If EEG equipment and personnel are available, EEG acquisition and interpretation may provide a benefit to children with CM by identifying electrographic-only seizures, facilitating early treatment. In the short-term, the benefit of additional EEG testing is likely to be limited to the few African centers with the equipment, trained technicians, and pediatric neurologist to interpret the EEG. Continued EEG studies in children with CM will nonetheless be important to better define brain physiology during this disease and to evaluate predictive value of study findings for long-term neurologic deficits and cognitive impairment. As costs of EEG equipment decrease and the workforce of technicians and pediatric neurologists in Africa increases, cost-benefit analysis of EEG testing for identification of at-risk children with CM should be performed.
In summary, this study shows that in children with CM, the number of postadmission seizures and specific EEG features on admission are independently associated with long-term cognitive outcomes, while seizure number alone, an easily assessable clinical parameter, is strongly associated with long-term neurologic deficits. Future studies should evaluate the predictive value of these findings to determine whether they can be useful in identification of children who survive CM who are at the highest risk of neurologic deficits or cognitive impairment and allow for evaluation of early interventions, once developed, to prevent or decrease the severity of these complications.
Acknowledgment
We thank the Persyst corporation for providing software used in EEG interpretations in our study.
Glossary
- BCS
Blantyre Coma Score
- CM
cerebral malaria
- GCS
Glasgow Coma Score
- KABC
Kaufman Assessment Battery for Children
- LME
linear mixed-effects
Appendix. Authors
Study Funding
Funding was provided by National Institute of Neurological Disorders and Stroke grant R01NS055349.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.World Malaria Report 2021. World Health Organization; 2021. [Google Scholar]
- 2.Idro R, Ndiritu M, Ogutu B, et al. . Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA. 2007;297(20):2232-2240. doi. 10.1001/jama.297.20.2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Seidlein L, Olaosebikan R, Hendriksen ICE, et al. . Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8):1080-1090. doi. 10.1093/cid/cis034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71(265):441-459. [PubMed] [Google Scholar]
- 5.Seydel KB, Kampondeni SD, Valim C, et al. . Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372(12):1126-1137. doi. 10.1056/nejmoa1400116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chimalizeni Y, Kawaza K, Molyneux M, Taylor T. The platelet Count in cerebral malaria, is it useful to the clinician? Am J Trop Med Hyg. 2010;83(1):48-50. doi. 10.4269/ajtmh.2010.09-0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewallen S, Bakker H, Taylor TE, Wills BA, Courtright P, Molyneux ME. Retinal findings predictive of outcome in cerebral malaria. Trans R Soc Trop Med Hyg. 1996;90(2):144-146. doi. 10.1016/S0035-9203(96)90116-9 [DOI] [PubMed] [Google Scholar]
- 8.Newton CRJC, Valim C, Krishna S, et al. . The prognostic value of measures of acid/base balance in pediatric falciparum malaria, compared with other clinical and laboratory parameters. Clin Infect Dis. 2005;41(7):948-957. doi. 10.1086/432941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haldar K, Murphy SC, Milner DA, Taylor TE. Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu Rev Pathol Mech Dis. 2007;2(1):217-249. doi. 10.1146/annurev.pathol.2.010506.091913 [DOI] [PubMed] [Google Scholar]
- 10.O'Brien NF, Mutatshi Taty T, Moore-Clingenpeel M, et al. . Transcranial Doppler ultrasonography provides insights into neurovascular changes in children with cerebral malaria. J Pediatr. 2018;203:116-124.e3. doi. 10.1016/j.jpeds.2018.07.075 [DOI] [PubMed] [Google Scholar]
- 11.Postels DG, Wu X, Li C, et al. . Admission EEG findings in diverse paediatric cerebral malaria populations predict outcomes. Malar J. 2018;17(1):208. doi. 10.1186/s12936-018-2355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postels DG, Taylor TE, Molyneux M, et al. . Neurologic outcomes in retinopathy-negative cerebral malaria survivors. Neurology. 2012;79(12):1268-1272. doi. 10.1212/WNL.0b013e31826aacd4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John CC, Bangirana P, Byarugaba J, et al. . Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122(1):e92-e99. doi. 10.1542/peds.2007-3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langfitt JT, McDermott MP, Brim R, et al. . Neurodevelopmental impairments 1 year after cerebral malaria. Pediatrics. 2019;143(2):e20181026. doi. 10.1542/peds.2018-1026 [DOI] [PubMed] [Google Scholar]
- 15.Carter JA, Neville BGR, White S, et al. . Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45(8):978-981. doi. 10.1111/j.0013-9580.2004.65103.x [DOI] [PubMed] [Google Scholar]
- 16.Postels D, Birbeck G. Children with retinopathy-negative cerebral malaria. Pediatr Infect Dis J. 2011;30(11):953-956. doi. 10.1097/inf.0b013e3182271c69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birbeck GL, Molyneux ME, Kaplan PW, et al. . Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9(12):1173-1181. doi. 10.1016/S1474-4422(10)70270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holding PA, Stevenson J, Peshu N, Marsh K. Cognitive sequelae of severe malaria with impaired consciousness. Trans R Soc Trop Med Hyg. 1999;93(5):529-534. doi. 10.1016/S0035-9203(99)90368-1 [DOI] [PubMed] [Google Scholar]
- 19.Idro R, Carter JA, Fegan G, Neville BGR, Newton CRJC. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child. 2005;91(2):142-148. doi. 10.1136/adc.2005.077784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boivin MJ, Bangirana P, Byarugaba J, et al. . Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119(2):e360-e366. doi. 10.1542/peds.2006-2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Idro R, Kakooza-Mwesige A, Balyejjussa S, et al. . Severe neurological sequelae and behaviour problems after cerebral malaria in Ugandan children. BMC Res Notes. 2010;3(1):104. doi. 10.1186/1756-0500-3-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boivin MJ, Gladstone MJ, Vokhiwa M, et al. . Developmental outcomes in Malawian children with retinopathy-positive cerebral malaria. Trop Med Int Health. 2011;16(3):263-271. doi. 10.1111/j.1365-3156.2010.02704.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bangirana P, Opoka RO, Boivin MJ, et al. . Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis. 2014;59(3):336-344. doi. 10.1093/cid/ciu293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boivin MJ, Vokhiwa M, Sikorskii A, Magen JG, Beare NAV. Cerebral malaria retinopathy predictors of persisting neurocognitive outcomes in Malawian children. Pediatr Infect Dis J. 2014;33(8):821-824. doi. 10.1097/INF.0000000000000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, John CC. Neurocognitive domains affected by cerebral malaria and severe malarial anemia in children. Learn Individ Differ. 2016;46:38-44. doi. 10.1016/j.lindif.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brim R, Magen J, Taylor T, et al. . Cognitive outcomes and psychiatric symptoms of retinopathy-positive cerebral malaria: cohort description and baseline results. Am J Trop Med Hyg. 2017;97(1):225-231. doi. 10.4269/ajtmh.17-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boivin MJ, Mohanty A, Sikorskii A, Vokhiwa M, Magen JG, Gladstone M. Early and middle childhood developmental, cognitive, and psychiatric outcomes of Malawian children affected by retinopathy positive cerebral malaria. Child Neuropsychol. 2019;25(1):81-102. doi. 10.1080/09297049.2018.1451497 [DOI] [PubMed] [Google Scholar]
- 28.Ngoungou EB, Dulac O, Poudiougou B, et al. . Epilepsy as a consequence of cerebral malaria in area in which malaria is endemic in Mali, west Africa. Epilepsia. 2006;47(5):873-879. doi. 10.1111/j.1528-1167.2006.00524.x [DOI] [PubMed] [Google Scholar]
- 29.Christensen SS, Eslick GD. Cerebral malaria as a risk factor for the development of epilepsy and other long-term neurological conditions: a meta-analysis. Trans R Soc Trop Med Hyg. 2015;109(4):233-238. doi. 10.1093/trstmh/trv005 [DOI] [PubMed] [Google Scholar]
- 30.Andrews A, Zelleke T, Harrar D, Izem R, Gai J, Postels D. Theta-alpha variability on admission EEG is associated with outcome in pediatric cerebral malaria. J Clin Neurophysiol. 2021;40(2):136-143. doi. 10.1097/WNP.0000000000000865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews A, Zelleke T, Izem R, et al. . Using EEG in resource-limited areas: comparing qualitative and quantitative interpretation methods in cerebral malaria. Pediatr Neurol. 2022;126:96-103. doi. 10.1016/j.pediatrneurol.2021.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergemann TL, Bangirana P, Boivin MJ, Connett JE, Giordani BJ, John CC. Statistical approaches to assess the effects of disease on neurocognitive function over time. J Biom Biostat. 2012;Suppl 7:7310. doi. 10.4172/2155-6180.S7-016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conroy AL, Tran TM, Bond C, et al. . Plasma amino acid concentrations in children with severe malaria are associated with mortality and worse long-term kidney and cognitive outcomes. J Infect Dis. 2022;226(12):2215-2225. doi. 10.1093/infdis/jiac392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. J Pediatr. 1997;131(1):125-129. doi. 10.1016/s0022-3476(97)70135-5 [DOI] [PubMed] [Google Scholar]
- 35.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. . Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;41(1):215-223. doi. 10.1097/CCM.0b013e3182668035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagenman KL, Blake TP, Sanchez SM, et al. . Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014;82(5):396-404. doi. 10.1212/WNL.0000000000000082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vespa PM, Miller C, McArthur D, et al. . Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35(12):2830-2836. doi. 10.1097/00003246-200712000-00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchida TN, Barkovich AJ, Bollen AW, Hart AP, Ferriero DM. Childhood status epilepticus and excitotoxic neuronal injury. Pediatr Neurol. 2007;36(4):253-257. doi. 10.1016/j.pediatrneurol.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 39.Shah DK, Wusthoff CJ, Clarke P, et al. . Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99(3):F219-F224. doi. 10.1136/archdischild-2013-305206 [DOI] [PubMed] [Google Scholar]
- 40.Struck AF, Westover MB, Hall LT, Deck GM, Cole AJ, Rosenthal ES. Metabolic correlates of the ictal-interictal continuum: FDG-PET during continuous EEG. Neurocrit Care. 2016;24(3):324-331. doi. 10.1007/s12028-016-0245-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awal MA, Lai MM, Azemi G, Boashash B, Colditz PB. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: a structured review. Clin Neurophysiol. 2016;127(1):285-296. doi. 10.1016/j.clinph.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 42.Sandroni C, D'Arrigo S, Cacciola S, et al. . Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46(10):1803-1851. doi. 10.1007/s00134-020-06198-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knauert MP, Gilmore EJ, Murphy TE, et al. . Association between death and loss of stage N2 sleep features among critically ill patients with delirium. J Crit Care. 2018;48:124-129. doi. 10.1016/j.jcrc.2018.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abend NS, Dlugos DJ, Clancy RR. A review of long-term EEG monitoring in critically ill children with hypoxic–ischemic encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol. 2013;30(2):134-142. doi. 10.1097/WNP.0b013e3182872af9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoué S, Honda K, Komoda Y. Sleep as neuronal detoxification and restitution. Behav Brain Res. 1995;69(1-2):91-96. doi. 10.1016/0166-4328(95)00014-k [DOI] [PubMed] [Google Scholar]
- 46.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354(6315):1004-1008. doi. 10.1126/science.aah4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62(10):1743-1748. doi. 10.1212/01.WNL.0000125184.88797.62 [DOI] [PubMed] [Google Scholar]
- 48.Schreiber JM, Zelleke T, Gaillard WD, Kaulas H, Dean N, Carpenter JL. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012;17(1):31-38. doi. 10.1007/s12028-012-9715-z [DOI] [PubMed] [Google Scholar]
- eReferences are available at: links.lww.com/WNL/D77 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from a qualified investigator.