Abstract
Progressive multifocal leukoencephalopathy (PML) is a severe infection of the CNS occurring in immunocompromised individuals in which large demyelinating lesions are induced by polyomavirus JC (JCV). In the absence of effective antiviral treatment, control of the infection relies on restoring anti-JCV immunity. Thus, particularly in long-standing immunocompromising conditions such as organ transplantation, lymphoproliferative disorders, or idiopathic lymphopenia, new strategies to boost anti-JCV immune responses are needed. Here, we report the case of a patient developing PML in the context of kidney transplantation who received recombinant human interleukin 7 to foster immune responses against JCV. We give an overview of the immunologic mechanisms underlying the development of PML and immune restoration within the CNS after JCV infection. Immunotherapeutic strategies developed based on current understanding of the disease hold promise in managing patients with PML.
Case Report
A 78-year-old man with end-stage renal failure underwent renal transplantation in July 2021. His immunosuppressive regimen included tacrolimus, mycophenolate mofetil, and prednisone. Six months after transplantation, the patient developed subtle production aphasia. Brain MRI demonstrated multifocal frontal subcortical T2 hyperintense/T1 hypointense lesions without contrast enhancement (Figure, A–C) that were initially mistaken as an ischemic stroke. After 5 days, symptoms worsened and progressive multifocal leukoencephalopathy (PML) was confirmed by detecting JC virus (JCV) in the CSF (200 copies/mL). Consequently, maintenance immunosuppression was minimized, and a total of 2 g/kg IV immunoglobulins were given to prevent transplant rejection. Twelve days after the first MRI, the patient exhibited significant psychomotor slowing, mild aphasia, and proportional right-sided hemiparesis prompting a second MRI which showed enlargement of PML lesions without contrast enhancement. Given severe lymphopenia (0.27 g/L) and the absence of signs of immune reconstitution, a recombinant human interleukin 7 (rh-IL-7, CYT107, RevImmune) to foster anti-JCV immune response was started 25 days after disease onset. This treatment allowed a rapid restoration of lymphocyte count (1.75 g/L) within 10 days from treatment initiation. In parallel, the transplanted kidney was removed to avoid acute rejection. The patient subsequently developed a febrile condition with caecal ischemia and agranulocytosis of unknown origin, treated empirically with piperacillin/tazobactam. A few days later, he developed severe anemia and abdominal CT scan showed hemorrhagic effusion in the nephrectomy lodge. Given predicted long-term neurologic sequelae and overall abdominal evolution, the family and medical team decided to transition to palliative care. The patient died 7 weeks after PML onset. Brain autopsy demonstrated a large white matter lesion in the left hemisphere showing demyelination, relative preservation of axons and dense reactive phagocytes (CD68+, microglia/macrophages) with astrocytic (GFAP+) reaction (Figure, D–H). The presence of JCV in characteristic nuclear inclusions of astrocytes and oligodendrocytes was confirmed by anti-SV40 immunostaining (Figure, I). Furthermore, in and around the lesion, an important perivascular and interstitial T-cell infiltrate was revealed (CD3+ immunostaining), which was accompanied by a moderate perivascular B-cell (CD20+) infiltrate (Figure, J and K). This considerable T-cell recruitment in the brain lesion may reflect the initiation of immune reconstitution.
Figure. Radiologic and Pathologic Features in PML.
Representative MRI images showing canonical PML presentation with T2 hyperintense (A) and T1 hypointense (B) lesion of the frontal subcortical white matter and partially involving U fibers. In diffusion-weighted images, the lesion presents a demyelination front (C). Hematoxylin and eosin (HE, D, the position of the inset on the bottom and gray matter/white matter limit are highlighted by dashed lines), Luxol fast blue periodic acid–Schiff (LUPAS, E), and antineurofilament immunostained (NF, F) histologic sections show extensive white matter demyelination with relative preservation of axons. Numerous reactive astrogliosis (GFAP, G) and foamy macrophages (CD68, H) are found within the lesion. JC virus inclusions in nuclei of oligodendrocytes (double arrow in D) and astrocytes (simple arrows in D, G) are highlighted by SV40 immunostaining (I, simple arrows). T-cell infiltration is seen in the perivascular and interstitial spaces (CD3, J), accompanied by perivascular B cells (CD20, K). Scale bars = 5 mm (D–F) and 50 μm (inset on the bottom in D, G–K). PML = progressive multifocal leukoencephalopathy.
Discussion
This case highlights challenges in treating PML, especially in patients with sustained immunosuppression. PML is a rare opportunistic infection occurring in immunocompromised individuals including those with HIV, idiopathic CD4+ lymphopenia, hematological malignancies, or receiving treatment with immunosuppressive/immunomodulatory agents.1 The clinical presentation of PML, as described in this case, is usually subacute and may encompass cognitive or speech impairment, motor or sensory dysfunction, or ataxia. The classical radiologic and pathologic presentation for PML is shown in Figure.1
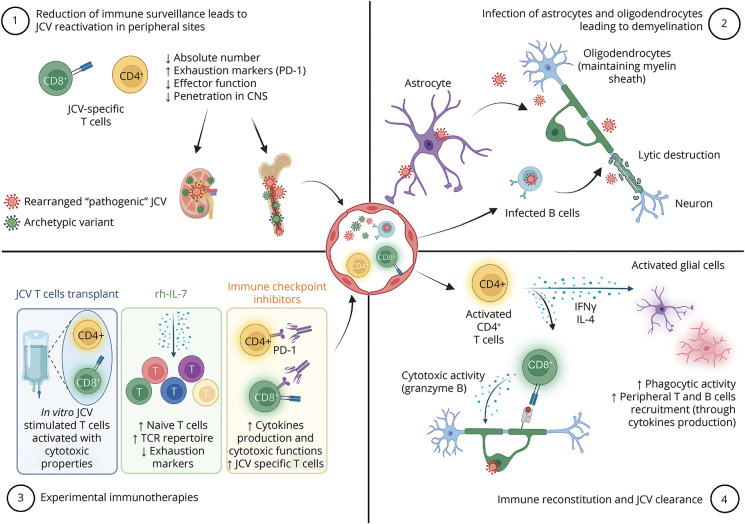
PML results from reactivation of JCV. The nonpathogenic form (archetype JCV) of the virus is transmitted from person to person and infects the oropharynx before spreading hematogenously to distant organs such as kidneys, bone marrow, or brain where it establishes latency/nonpathogenic persistent infection. In the context of immune suppression, the virus proliferates and accumulates sequence variations, leading to the creation of neurotropic rearranged (prototype) JCV. Then, after a second hematogenous spread or direct reactivation within the brain, this rearranged JCV can cause a lytic infection of brain cells. Oligodendrocytes, notably implicated in the generation and the maintenance of CNS myelin sheath, are particularly vulnerable, leading to extensive white matter involvement seen both on MRI and in postmortem pathology (Figure).1
Immunologic Mechanisms of Infection Control and Reactivation
Virus control relies primarily on cellular immunity as demonstrated in this case by the CD3+ T-cell infiltration (encompassing both CD4+ and CD8+ T cell subsets) seen at the edge of the lesion (Figure, J). Infiltration is dominated by CD8+ T cells, with cytotoxic granules (granzyme B) polarized toward JCV-infected CNS cells. CD8+ T cells' transmigration to the CNS seems to occur because of the CCR5 tissue-homing cue.2 CD8+ cytotoxic T lymphocytes are instrumental in JCV control because they are found in the vast majority of PML survivors.3
On the other hand, CD4+ T helper (Th) cells likely also play an important role as reactivation occurs mainly in patients with CD4 T-cell deficiencies. Th1, and to a lesser extent bifunctional Th1-Th2 cells, produce interferon-γ (IFN-γ) and IL-4 within the infected brain and promote viral clearance.4 Indeed, IFN-γ is a key regulator of the phagocytic activity of microglia and induces the expression of MHC class II and costimulatory molecules which allow them to function as antigen-presenting cells for infiltrating T cells. Furthermore, IFN-γ stimulates expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and chemokines such as CCL2, CXCL9, and CXCL10 by astrocytes, which may facilitate T cells recruitment to the brain. This instrumental pathway could be affected in some patients with PML who display abnormal CD4+ T-cell responses against JCV with a lack of IFN-γ production.5 The production of IL-4 is more unexpected and could participate in viral clearance by stimulating both humoral immunity and CD8+ T cells.4,6 IL-4 may also have a neuroprotective role by enhancing neuronal survival and recovery.7
Furthermore, as in other infectious diseases, T cells in PML may exhibit an exhausted phenotype. Exhaustion is a state of immune dysfunction characterized by the expression of inhibitory receptors such as the programmed cell death protein (PD)-1 leading to reduced cytokines production and cytotoxicity.8
The role of B cells in anti-JCV responses has been detailed elsewhere and will not be covered here.9
Immune Reconstitution Inflammatory Syndrome
To date, the best therapeutic option for PML relies on rapid restoration of immunity. Yet, immune response recovery is a double-edged sword because it may cause immune reconstitution inflammatory syndrome (IRIS) in a substantial proportion of patients. IRIS consists of a dysregulated anti-JCV immune response within the CNS, notably mediated by the cytotoxic activity of CD8+ T cells toward infected cells. It involves nonspecific tissue destruction induced by blood-brain barrier dysfunction, edema, and activation of macrophages and microglia.10 Clinically, IRIS is defined as a paradoxical deterioration in clinical status with specific MRI features not seen in PML (contrast enhancement, edema, mass effect). Management of PML-IRIS relies on corticosteroids. Targeted strategies to block the migration of CCR5+ CD8+ T cells have failed to demonstrate a beneficial effect.11
In transplant recipients, it is unclear whether rapid immune reconstitution could induce graft rejection. To prevent such acute rejection, we decided, in this case, to prophylactically explant the grafted kidney.
Immunotherapies in PML
Although restoration of anti-JCV immune responses is crucial for PML treatment, it remains challenging in patients with long-standing immunosuppression such as organ transplant recipients leading to a direct impact on prognosis.12 Understanding the immunologic mechanisms underlying PML opened a new era. Indeed, immunologic treatments/strategies have been repurposed to reinvigorate anti-JCV immune responses. In this case, we concomitantly withdrew immunosuppressing drugs and initiated rh-IL-7 hoping to allow immune reconstitution in the periphery and the CNS (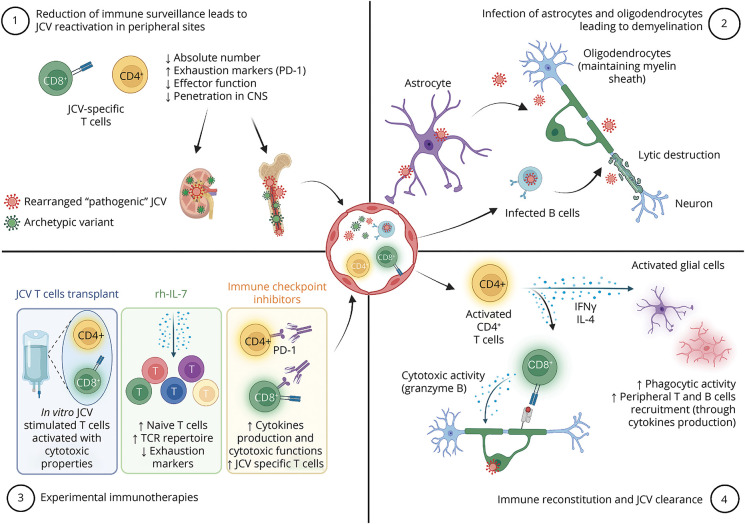 Graphical Abstract). Rh-IL-7, previously developed for sepsis, is a crucial cytokine for T-cell maturation that stimulates the proliferation of naive and memory T cells including JCV-specific ones. Rh-IL-7 might then improve survival and prognosis in PML. We favored IL-7 based on its safety, the main adverse effect being injection site reactions and transient flu-like symptoms, and a lower rate of IRIS (5.8%).13 Given the immune reconstitution observed in this case (both in the periphery and on CNS pathology), we could suggest that rh-IL-7 was sufficient to reinvigorate anti-JCV responses. However, our patient's poor outcome illustrates that time to introduce treatment is likely key to prevent severe neurologic damage.
Graphical Abstract). Rh-IL-7, previously developed for sepsis, is a crucial cytokine for T-cell maturation that stimulates the proliferation of naive and memory T cells including JCV-specific ones. Rh-IL-7 might then improve survival and prognosis in PML. We favored IL-7 based on its safety, the main adverse effect being injection site reactions and transient flu-like symptoms, and a lower rate of IRIS (5.8%).13 Given the immune reconstitution observed in this case (both in the periphery and on CNS pathology), we could suggest that rh-IL-7 was sufficient to reinvigorate anti-JCV responses. However, our patient's poor outcome illustrates that time to introduce treatment is likely key to prevent severe neurologic damage.
Two other immune strategies have been recently proposed. First immune checkpoint inhibitors (ICIs), which have expanding indications in cancer, may improve PML outcomes. The rationale relies on blocking PD-1 on JCV-specific T cells, thus stimulating their proliferation and clearance of the virus. A very low number of T cells at baseline and the phenotype of terminal exhaustion have been associated with treatment failure.12 Yet, ICIs were associated with frequent (30%) immune-related adverse events (i.e., colitis, pneumonitis) and IRIS (19%).14
Second, JCV-specific T-cell transfer has been the most promising strategy so far, but its availability remains limited to a few expert centers.12,15 In this treatment, mononuclear cells are obtained from HLA-matched healthy donors or the patient and expanded in vitro in the presence of JCV or BK polyomavirus antigenic peptides (both viruses share several immunogenic peptides). Transfer of in vitro stimulated T cells allows the reconstitution of a pool of anti-JCV T cells with cytotoxic capacities.
Conclusion
PML is an interesting model to illustrate how understanding a pathology's underlying immune mechanisms may lead to the design of innovative therapeutic strategies. Although these strategies offer new perspectives for the treatment of PML, it is not the long-awaited panacea as demonstrated in our patient. Large case series tend to suggest it might be beneficial, especially in patients with a grim prognosis.13-15 Unfortunately, the availability of these treatments is still limited to expert centers. Finally, international controlled clinical trials are needed to ascertain efficacy and safety.
Acknowledgment
We would like to thank the patient's family for their help and RevImmune for providing CYT107 for compassionate use.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021;17(1):37-51. doi: 10.1038/s41582-020-00427-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-Blondel G, Bauer J, Uro-Coste E, et al. Therapeutic use of CCR5 antagonists is supported by strong expression of CCR5 on CD8+ T cells in progressive multifocal leukoencephalopathy-associated immune reconstitution inflammatory syndrome. Acta Neuropathol. 2015;129(3):463-465. doi: 10.1007/s00401-015-1383-6 [DOI] [PubMed] [Google Scholar]
- 3.Du Pasquier RA. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127(9):1970-1978. doi: 10.1093/brain/awh215 [DOI] [PubMed] [Google Scholar]
- 4.Aly L, Yousef S, Schippling S, et al. Central role of JC virus-specific CD4+ lymphocytes in progressive multi-focal leucoencephalopathy-immune reconstitution inflammatory syndrome. Brain. 2011;134(9):2687-2702. doi: 10.1093/brain/awr206 [DOI] [PubMed] [Google Scholar]
- 5.Jelcic I, Jelcic I, Kempf C, et al. Mechanisms of immune escape in central nervous system infection with neurotropic JC virus variant: neurotropic JCV. Ann Neurol. 2016;79(3):404-418. doi: 10.1002/ana.24574 [DOI] [PubMed] [Google Scholar]
- 6.Lee A, Park SP, Park CH, et al. IL-4 induced innate CD8+ T cells control persistent viral infection. PLoS Pathog. 2015;11(10):e1005193. doi: 10.1371/journal.ppat.1005193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh JT, Hendrix S, Boato F, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125(2):699-714. doi: 10.1172/jci76210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan CS, Bord E, Broge TA, et al. Increased program cell death-1 expression on T lymphocytes of patients with progressive multifocal leukoencephalopathy. J Acquir Immun Defic Syndr. 2012;60(3):244-248. doi: 10.1097/qai.0b013e31825a313c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durali D, de Goër de Herve M-G, Gasnault J, Taoufik Y. B cells and progressive multifocal leukoencephalopathy: search for the missing link. Front Immunol. 2015;6:241. doi: 10.3389/fimmu.2015.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer J, Gold R, Adams O, Lassmann H. Progressive multifocal leukoencephalopathy and immune reconstitution inflammatory syndrome (IRIS). Acta Neuropathol. 2015;130(6):751-764. doi: 10.1007/s00401-015-1471-7 [DOI] [PubMed] [Google Scholar]
- 11.Bernard-Valnet R, Moisset X, Maubeuge N, et al. CCR5 blockade in inflammatory PML and PML-IRIS associated with chronic inflammatory diseases' treatments. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1097. doi: 10.1212/nxi.0000000000001097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard‐Valnet R, Koralnik IJ, Du Pasquier R. Advances in treatment of progressive multifocal leukoencephalopathy. Ann Neurol. 2021;90(6):865-873. doi: 10.1002/ana.26198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lajaunie R, Mainardi I, Gasnault J, et al. Outcome of progressive multifocal leukoencephalopathy treated by interleukin‐7. Ann Neurol. 2022;91(4):496-505. doi: 10.1002/ana.26307 [DOI] [PubMed] [Google Scholar]
- 14.Boumaza X, Bonneau B, Roos‐Weil D, et al. Progressive multifocal leukoencephalopathy treated by immune checkpoint inhibitors. Ann Neurol. 2022;93(2):257-270. doi: 10.1002/ana.26512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortese I, Beck ES, Al-Louzi O, et al. BK virus-specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: an open-label, single-cohort pilot study. Lancet Neurol. 2021;20(8):639-652. doi: 10.1016/s1474-4422(21)00174-5 [DOI] [PMC free article] [PubMed] [Google Scholar]