Abstract
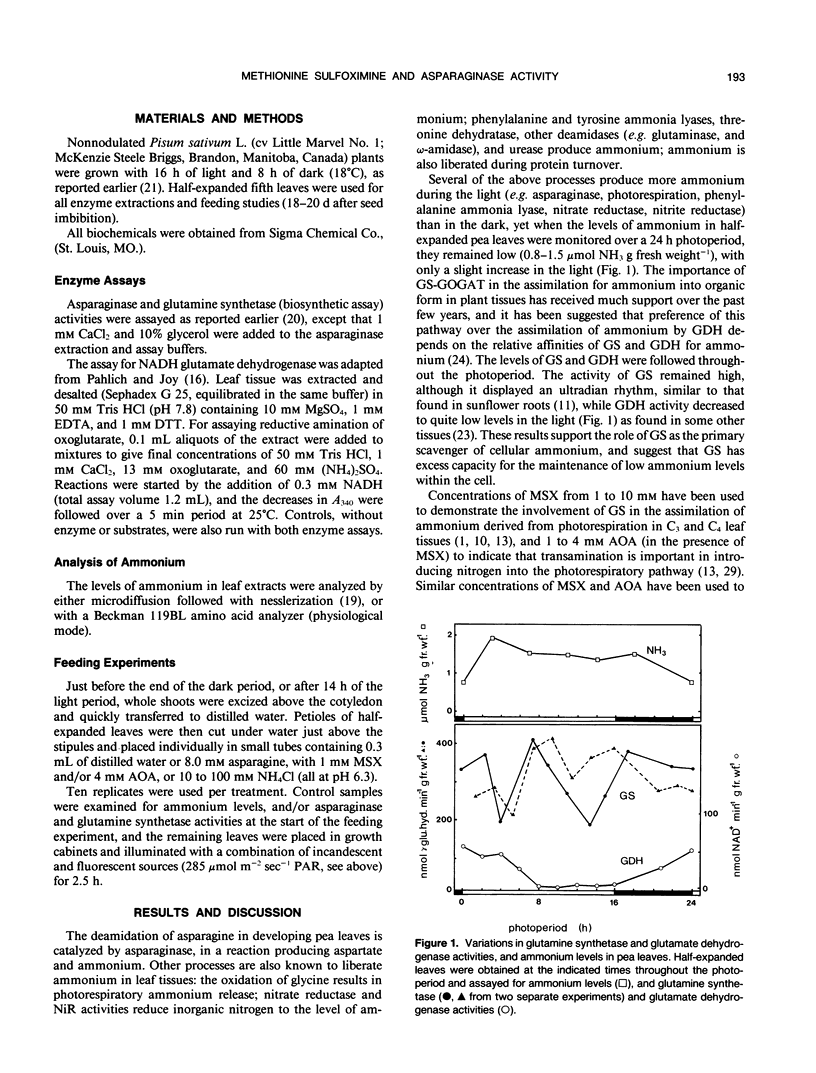
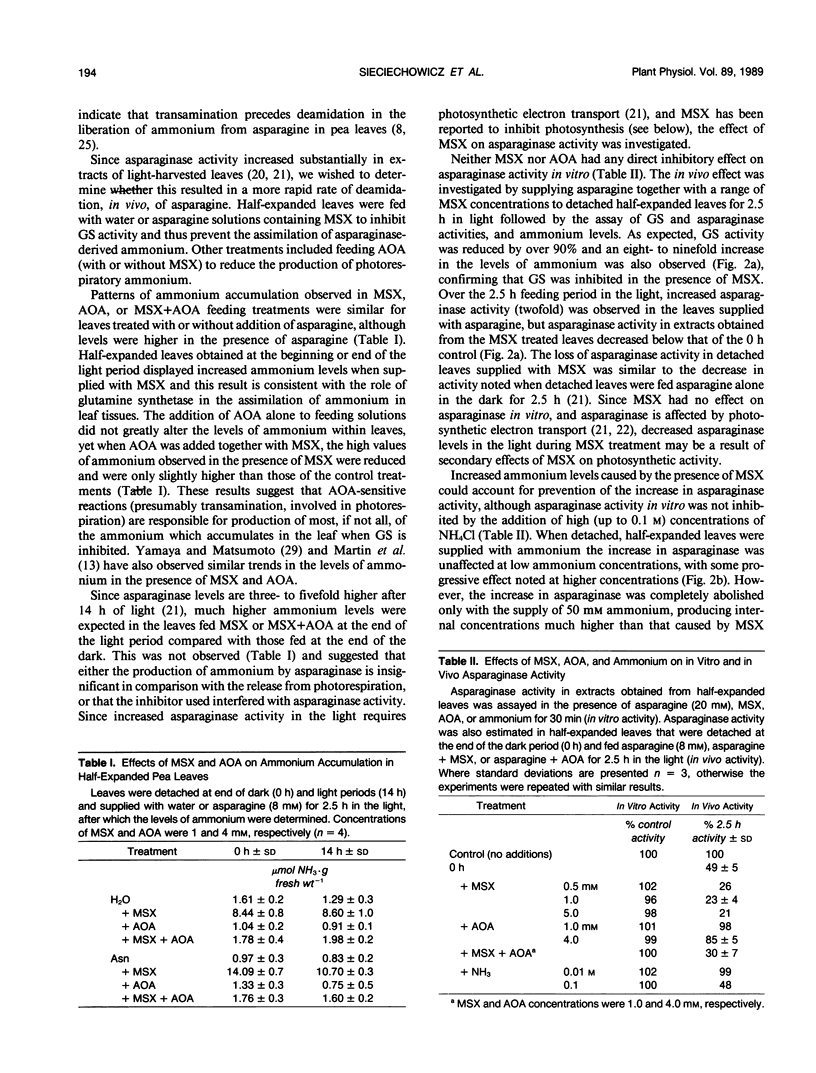
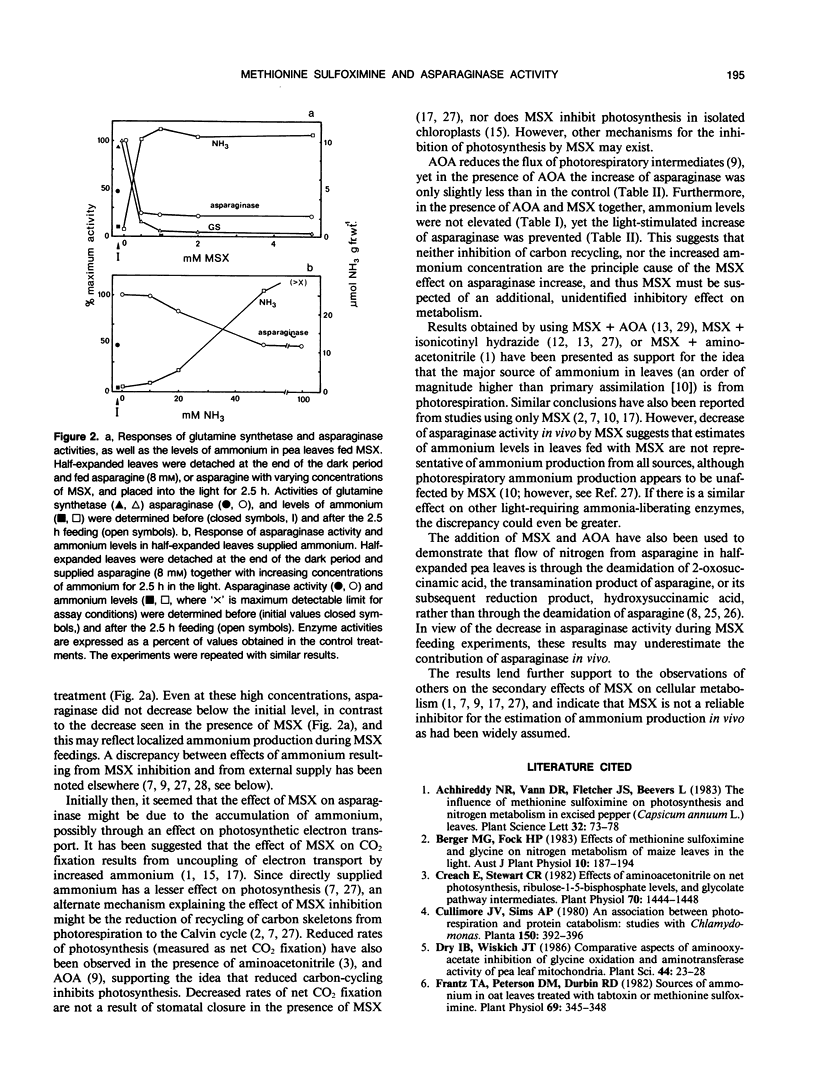
In developing leaves of Pisum sativum the levels of ammonium did not change during the light-dark photoperiod even though asparaginase (EC 3.5.1.1) did; asparaginase activity in detached leaves doubled during the first 2.5 hours in the light. When these leaves were supplied with 1 millimolar methionine sulfoximine (MSX, an inhibitor of glutamine synthetase, GS, activity) at the beginning of the photoperiod, levels of ammonium increased 8-to 10-fold, GS activity was inhibited 95%, and the light-stimulated increase in asparaginase activity was completely prevented, and declined to less than initial levels. When high concentrations of ammonium were supplied to leaves, the light-stimulated increase of asparaginase was partially prevented. However, it was also possible to prevent asparaginase increase, in the absence of ammonium accumulation, by the addition of MSX together with aminooxyacetate (AOA, which inhibits transamination and some other reactions of photorespiratory nitrogen cycling). AOA alone did not prevent light-stimulated asparaginase increase; neither MSX, AOA, or elevated ammonium levels inhibited the activity of asparaginase in vitro. These results suggest that the effect of MSX on asparaginase increase is not due solely to interference with photorespiratory cycling (since AOA also prevents cycling, but has no effect alone), nor to the production of high ammonium concentration or its subsequent effect on photosynthetic mechanisms. MSX must have further inhibitory effects on metabolism. It is concluded that accumulation of ammonium in the presence of MSX may underestimate rates of ammonium turnover, since liberation of ammonium from systems such as asparaginase is reduced by the effects of MSX.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Créach E., Stewart C. R. Effects of aminoacetonitrile on net photosynthesis, ribulose-1,5-bisphosphate levels, and glycolate pathway intermediates. Plant Physiol. 1982 Nov;70(5):1444–1448. doi: 10.1104/pp.70.5.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz T. A., Peterson D. M., Durbin R. D. Sources of ammonium in oat leaves treated with tabtoxin or methionine sulfoximine. Plant Physiol. 1982 Feb;69(2):345–348. doi: 10.1104/pp.69.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. J., Weissman G. S. Rhythms in glutamine synthetase activity, energy charge, and glutamine in sunflower roots. Plant Physiol. 1982 Dec;70(6):1683–1688. doi: 10.1104/pp.70.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISTER A. Preparation of enzymatic reactions of the keto analogues of asparagine and glutamine. J Biol Chem. 1953 Feb;200(2):571–589. [PubMed] [Google Scholar]
- Martin F., Winspear M. J., Macfarlane J. D., Oaks A. Effect of Methionine Sulfoximine on the Accumulation of Ammonia in C(3) and C(4) Leaves : The Relationship between NH(3) Accumulation and Photorespiratory Activity. Plant Physiol. 1983 Jan;71(1):177–181. doi: 10.1104/pp.71.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlich E., Joy K. W. Glutamate dehydrogenase from pea roots: purification and properties of the enzyme. Can J Biochem. 1971 Jan;49(1):127–138. doi: 10.1139/o71-018. [DOI] [PubMed] [Google Scholar]
- Platt S. G., Anthon G. E. Ammonia accumulation and inhibition of photosynthesis in methionine sulfoximine treated spinach. Plant Physiol. 1981 Mar;67(3):509–513. doi: 10.1104/pp.67.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Deal L., Haworth P., Jamieson G. C., Reuter C. C., Ericson M. C. Amino Acid Metabolism of Lemna minor L. : I. Responses to Methionine Sulfoximine. Plant Physiol. 1986 Dec;82(4):1057–1062. doi: 10.1104/pp.82.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieciechowicz K., Ireland R. J., Joy K. W. Diurnal variation of asparaginase in developing pea leaves. Plant Physiol. 1985 Feb;77(2):506–508. doi: 10.1104/pp.77.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Joy K. W., Ireland R. J. Amino Acid metabolism in pea leaves : utilization of nitrogen from amide and amino groups of [N]asparagine. Plant Physiol. 1984 Apr;74(4):822–826. doi: 10.1104/pp.74.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Joy K. W., Ireland R. J. Utilization of the amide groups of asparagine and 2-hydroxysuccinamic Acid by young pea leaves. Plant Physiol. 1984 Jul;75(3):527–530. doi: 10.1104/pp.75.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K. A., Givan C. V., Keys A. J. Glutamic Acid metabolism and the photorespiratory nitrogen cycle in wheat leaves: metabolic consequences of elevated ammonia concentrations and of blocking ammonia assimilation. Plant Physiol. 1984 May;75(1):60–66. doi: 10.1104/pp.75.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]


