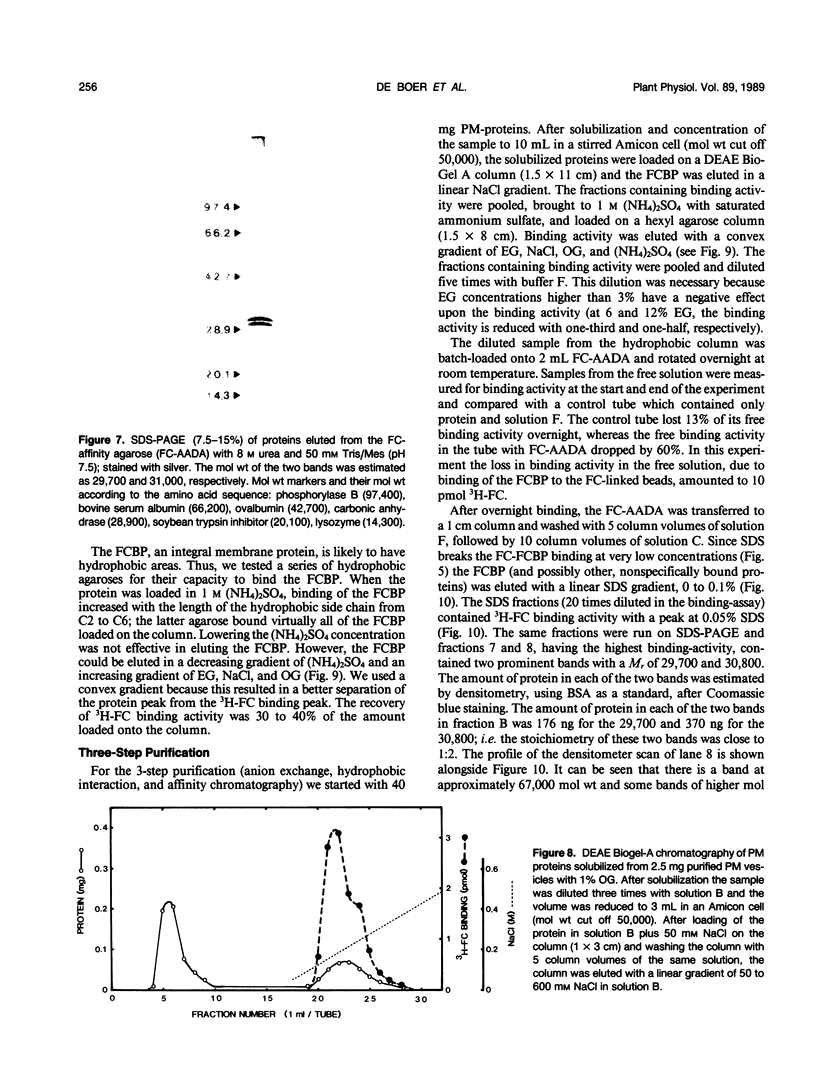
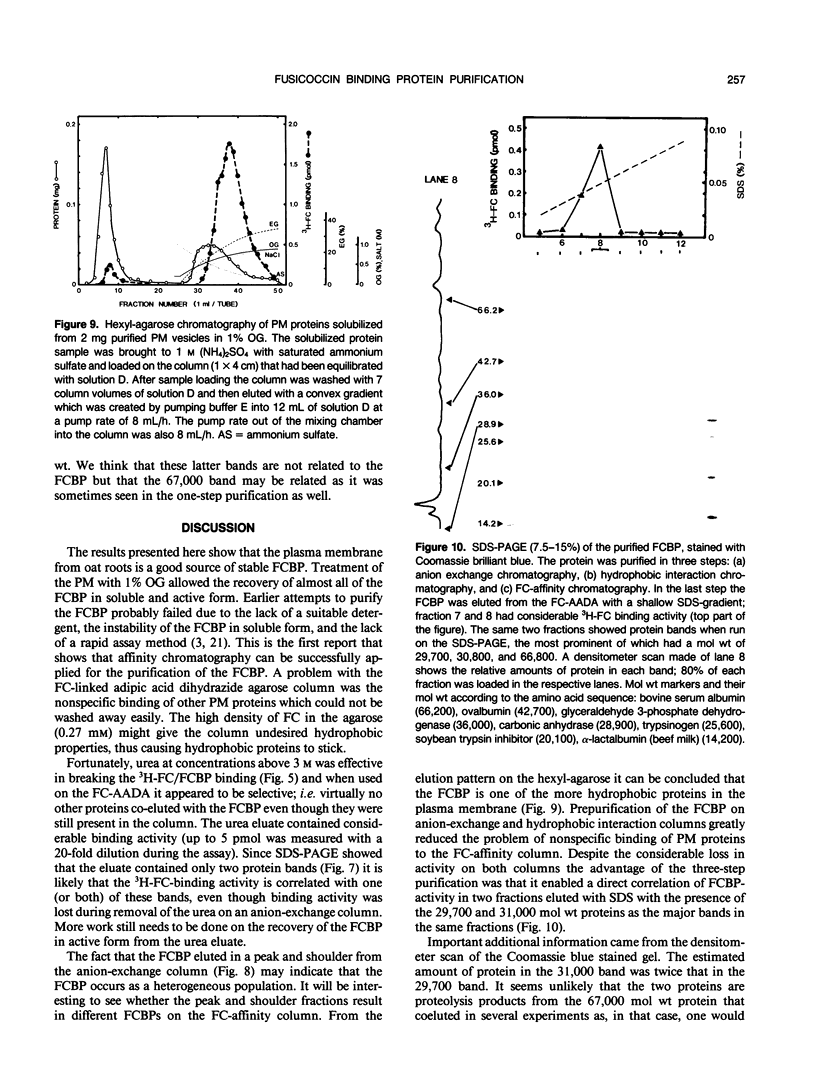
Abstract
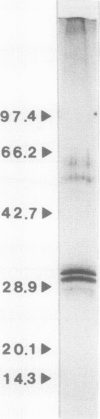
Fusicoccin (FC), a fungal phytotoxin, stimulates the H+-ATPase located in the plasma membrane (PM) of higher plants. The first event in the reaction chain leading to enhanced H+-efflux seems to be the binding of FC to a FC-binding protein (FCBP) in the PM. We solubilized 90% of the FCBP from oat (Avena sativa L. cv Victory) root PM in an active form with 1% octyl-glucoside. The FCBP was stabilized by the presence of protease inhibitors. The FCBP was purified by affinity chromatography using FC-linked adipic acid dihydrazide agarose (FC-AADA). Upon elution with 8 molar urea, two major protein bands on sodium dodecyl sulfate-polyacrylamide gel electrophoresis with molecular weights of 29,700 and 31,000 were obtained. Successive chromatography on DEAE Bio-Gel A, hexyl agarose, and FC-AADA resulted in the same two bands when the FC-AADA was eluted with sodium dodecyl sulfate. A direct correlation was made between 3H-FC-binding activity and the presence of the two protein bands. The stoichiometry of the 29,700 and 31,000 molecular weight bands was 1:2. This suggests that the FCBP occurs in the native form as a heterotrimer with an apparent molecular weight of approximately 92,000.
Full text
PDF




Images in this article
Selected References
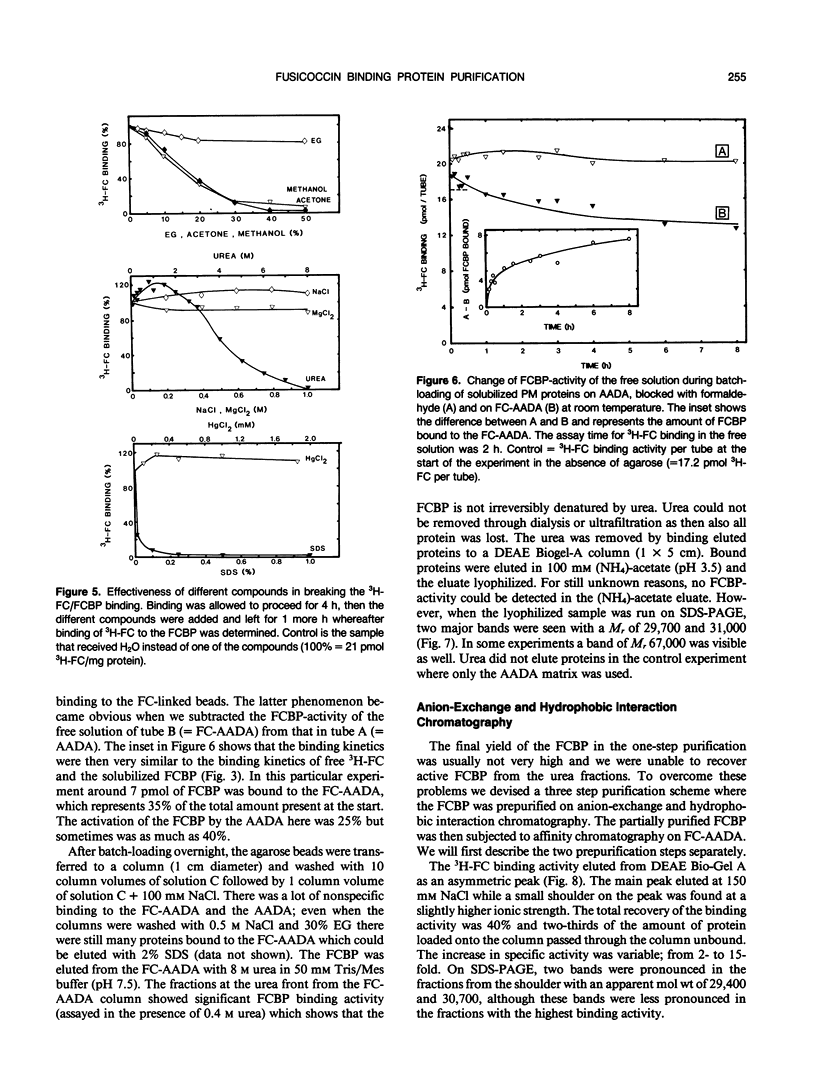
These references are in PubMed. This may not be the complete list of references from this article.
- Bidwai A. P., Takemoto J. Y. Bacterial phytotoxin, syringomycin, induces a protein kinase-mediated phosphorylation of red beet plasma membrane polypeptides. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6755–6759. doi: 10.1073/pnas.84.19.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Forbush B., 3rd Characterization of right-side-out membrane vesicles rich in (Na,K)-ATPase and isolated from dog kidney outer medulla. J Biol Chem. 1982 Nov 10;257(21):12678–12684. [PubMed] [Google Scholar]
- Karlin A., DiPaola M., Kao P. N., Lobel P. Functional sites and transient states of the nicotinic acetylcholine receptor. Soc Gen Physiol Ser. 1987;41:43–65. [PubMed] [Google Scholar]
- Lamed R., Levin Y., Wilchek M. Covalent coupling of nucleotides to agarose for affinity chromatography. Biochim Biophys Acta. 1973 Apr 28;304(2):231–235. doi: 10.1016/0304-4165(73)90239-0. [DOI] [PubMed] [Google Scholar]
- Löbler M., Klämbt D. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). I. Purification by immunological methods and characterization. J Biol Chem. 1985 Aug 15;260(17):9848–9853. [PubMed] [Google Scholar]
- Marrè M. T., Romani G., Bellando M., Marrè E. Stimulation of Weak Acid Uptake and Increase in Cell Sap pH as Evidence for Fusicoccin- and K-Induced Cytosol Alkalinization. Plant Physiol. 1986 Sep;82(1):316–323. doi: 10.1104/pp.82.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom R. P., Deboer A. H., Lomax T. L., Cleland R. E. Latency of Plasma Membrane H-ATPase in Vesicles Isolated by Aqueous Phase Partitioning : Increased substrate Accessibility or Enzyme Activation. Plant Physiol. 1987 Nov;85(3):693–698. doi: 10.1104/pp.85.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura S., Sotobayashi T., Futai M., Fukui T. Purification and properties of an auxin-binding protein from maize shoot membranes. J Biochem. 1986 May;99(5):1513–1524. doi: 10.1093/oxfordjournals.jbchem.a135621. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Partial characterization of fusicoccin binding to receptor sites on oat root membranes. Plant Physiol. 1980 Sep;66(3):353–359. doi: 10.1104/pp.66.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]