Abstract
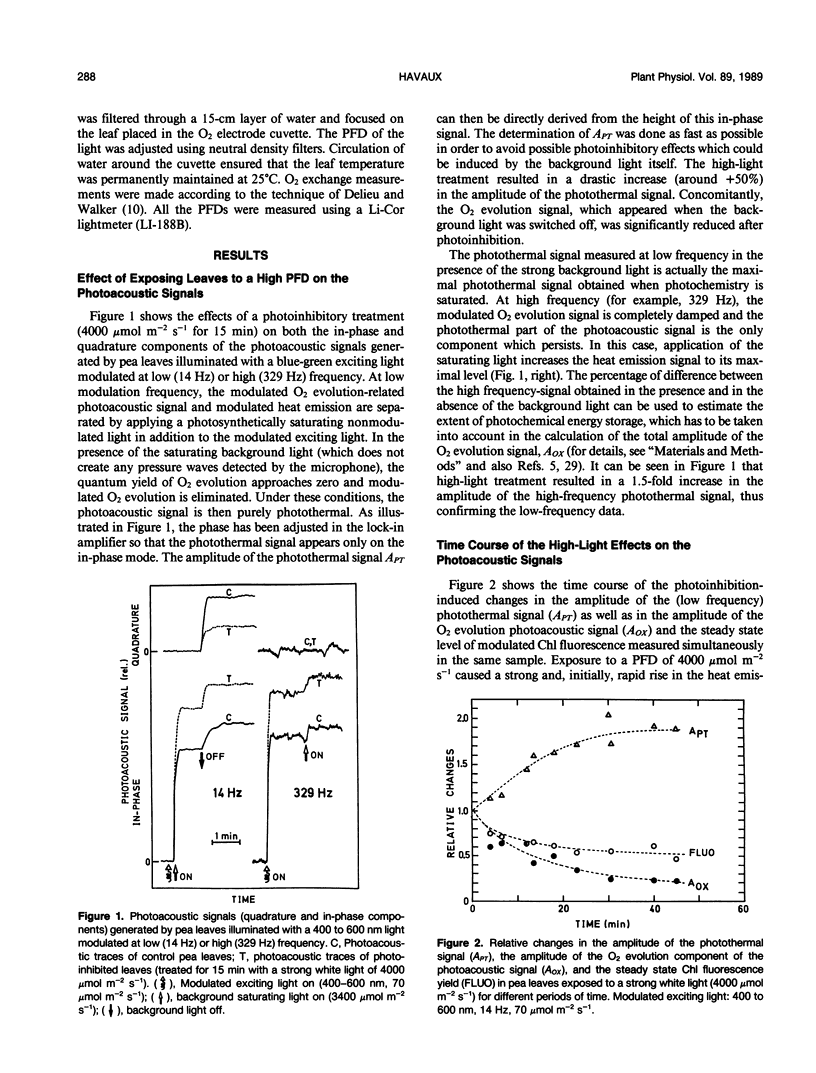
The photoacoustic technique was used to monitor thermal deexcitation of the photosynthetic pigments in intact pea leaves (Pisum sativum L.) submitted to photoinhibitory treatments. When the leaves were exposed to photon flux densities above 1000 micromoles per square meter per second, the amplitude of the photothermal component of the in vivo photoacoustic signal strongly increased. This high-light-induced stimulation of nonradiative energy dissipation (heat emission) was accompanied by an inverse change in the O2 evolution activity and in the steady state emission of 685 nanometer chlorophyll fluorescence. The time course of these effects was shown to be very rapid, with a t1/2 of around 15 minutes. When high-light-treated leaves were readapted to the dark, the heat emission changes were reversed, following somewhat slower kinetics. A reversible increase in the rate of light energy dissipation via radiationless transitions could be a photoprotective mechanism eliminating excess excitation energy from the photosynthetic reaction centers. Interestingly, this process does not operate at temperatures below about 12°C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briantais J. M., Vernotte C., Picaud M., Krause G. H. Chlorophyll fluorescence as a probe for the determination of the photo-induced proton gradient in isolated chloroplasts. Biochim Biophys Acta. 1980 Jun 10;591(1):198–202. doi: 10.1016/0005-2728(80)90233-9. [DOI] [PubMed] [Google Scholar]
- Delieu T. J., Walker D. A. Simultaneous measurement of oxygen evolution and chlorophyll fluorescence from leaf pieces. Plant Physiol. 1983 Nov;73(3):534–541. doi: 10.1104/pp.73.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M., Canaani O., Malkin S. Photosynthetic responses of leaves to water stress, expressed by photoacoustics and related methods : I. Probing the photoacoustic method as an indicator for water stress in vivo. Plant Physiol. 1986 Nov;82(3):827–833. doi: 10.1104/pp.82.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]


