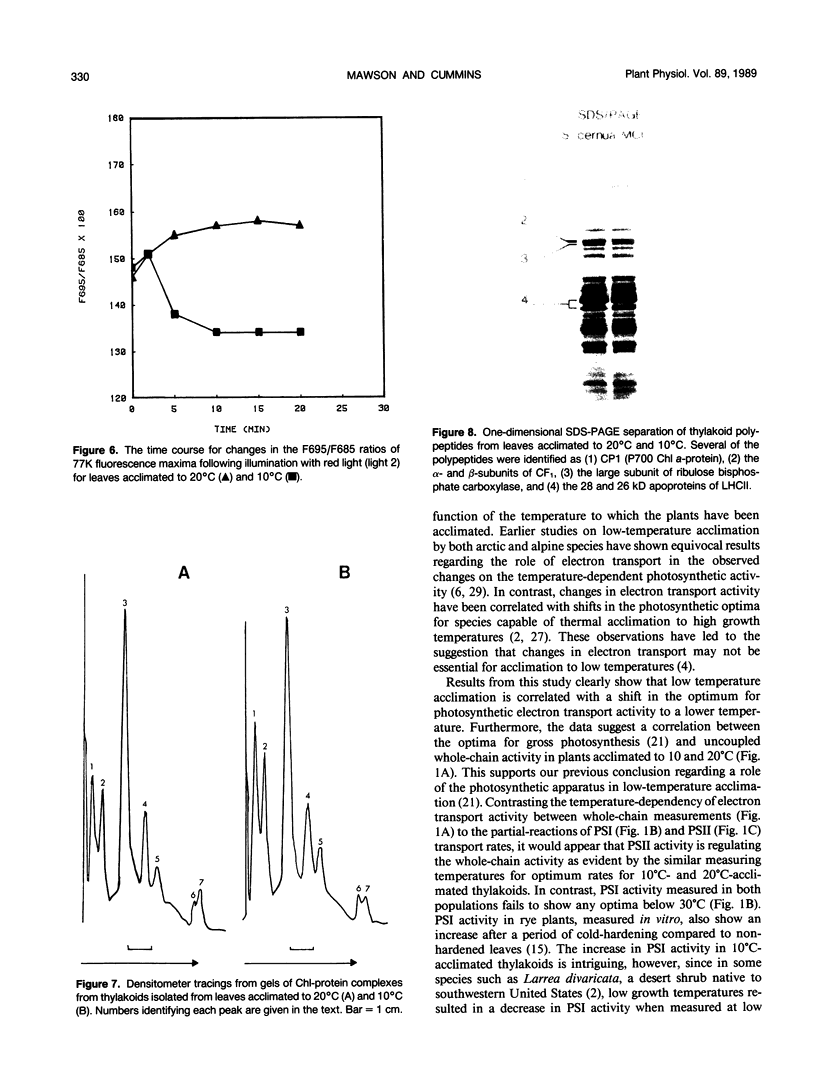
Abstract
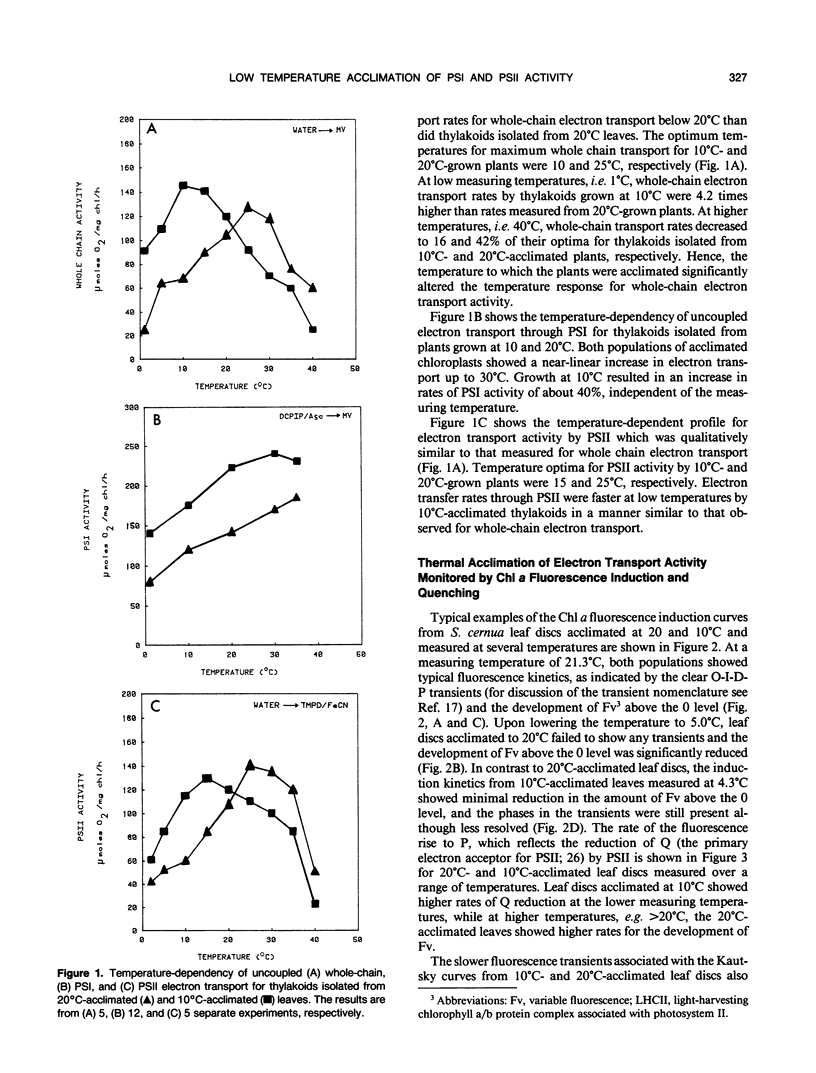
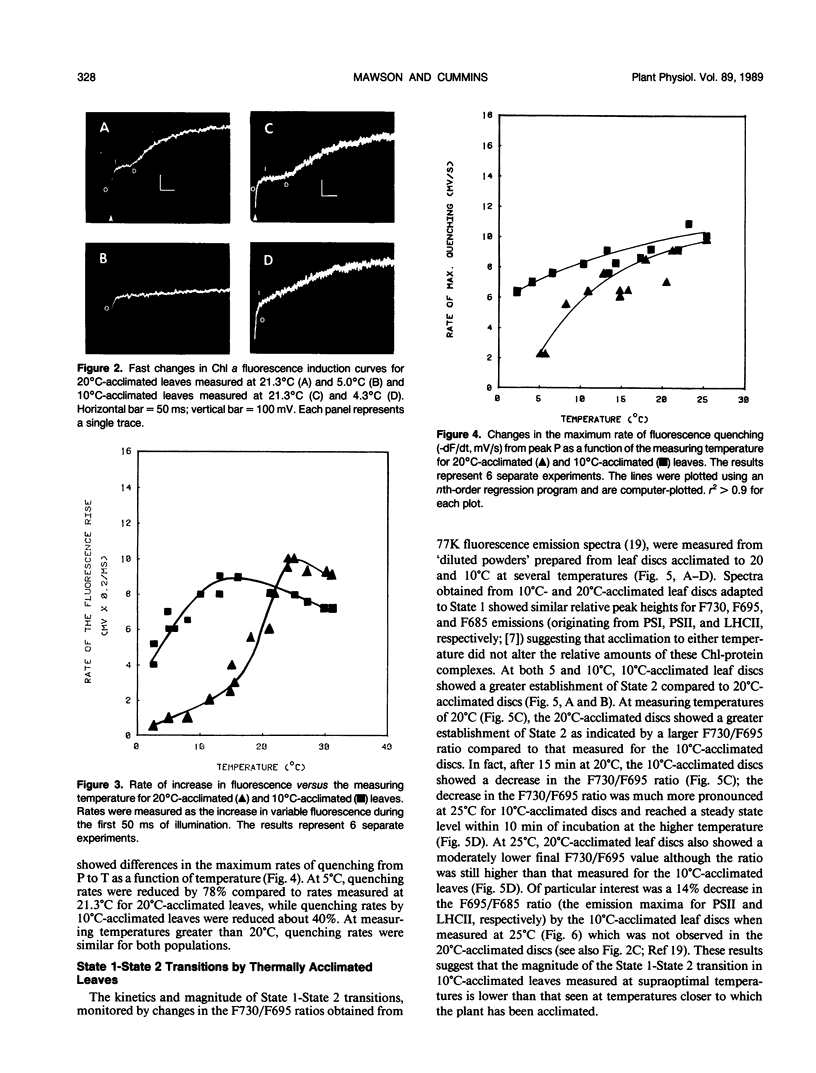
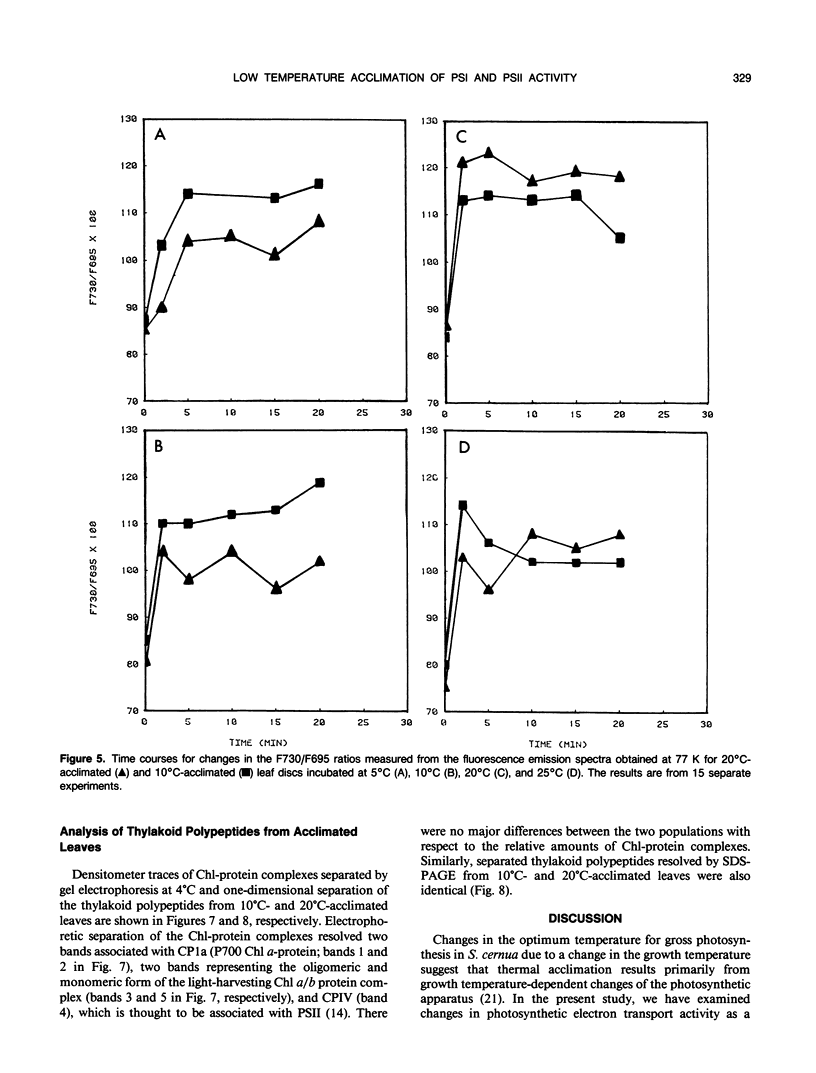
Thermal acclimation by Saxifraga cernua to low temperatures results in a change in the optimum temperature for gross photosynthetic activity and may directly involve the photosynthetic apparatus. In order to test this hypothesis photosynthetic electron transport activity of S. cernua thylakoids acclimated to growth temperatures of 20°C and 10°C was measured in vitro. Both populations exhibited optimum temperatures for whole chain and PSII electron transport activity at temperatures close to those at which the plants were grown. Chlorophyll a fluorescence transients from 10°C-acclimated leaves showed higher rates in the rise and subsequent quenching of variable fluorescence at low measuring temperatures; 20°C-acclimated leaves showed higher rates of fluorescence rise at higher measuring temperatures. At these higher temperatures, fluorescence quenching rates were similar in both populations. The kinetics of State 1-State 2 transitions in 20°C- and 10°C-acclimated leaf discs were measured as changes in the magnitude of the fluorescence emission maxima measured at 77K. Leaves acclimated at 10°C showed a larger F730/F695 ratio at low temperatures, while at higher temperatures, 20°C-acclimated leaves showed a higher F730/F695 ratio after the establishment of State 2. High incubation temperatures also resulted in a decrease in the F695/F685 ratio for 10°C-acclimated leaves, suggesting a reduction in the excitation transfer from the light-harvesting complex of photosystem II to photosystem II reaction centers. The relative amounts of chlorophyll-protein complexes and thylakoid polypeptides separated electro-phoretically were similar for both 20°C- and 10°C-acclimated leaves. Thus, photosynthetic acclimation to low temperatures by S. cernua is correlated with an increase in photosynthetic electron transport activity but does not appear to be accompanied by major structural changes or different relative amounts in thylakoid protein composition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armond P. A., Schreiber U., Björkman O. Photosynthetic Acclimation to Temperature in the Desert Shrub, Larrea divaricata: II. Light-harvesting Efficiency and Electron Transport. Plant Physiol. 1978 Mar;61(3):411–415. doi: 10.1104/pp.61.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M., Huner N. P., Kyle D. J. Fluorescence Properties Indicate that Photosystem II Reaction Centers and Light-Harvesting Complex Are Modified by Low Temperature Growth in Winter Rye. Plant Physiol. 1984 Oct;76(2):381–385. doi: 10.1104/pp.76.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner N. P., Krol M., Williams J. P., Maissan E., Low P. S., Roberts D., Thompson J. E. Low Temperature Development Induces a Specific Decrease in trans-Delta-Hexadecenoic Acid Content which Influences LHCII Organization. Plant Physiol. 1987 May;84(1):12–18. doi: 10.1104/pp.84.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawson B. T., Colman B., Cummins W. R. Abscisic Acid and photosynthesis in isolated leaf mesophyll cell. Plant Physiol. 1981 Feb;67(2):233–236. doi: 10.1104/pp.67.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawson B. T., Cummins W. R. The kinetics of in vivo state transitions in mesophyll and guard cell chloroplasts monitored by 77 k fluorescence emission spectra. Plant Physiol. 1986 Dec;82(4):873–879. doi: 10.1104/pp.82.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawson B. T., Franklin A., Filion W. G., Cummins W. R. Comparative Studies of Fluorescence from Mesophyll and Guard Cell Chloroplasts in Saxifraga cernua: Analysis of Fluorescence Kinetics as a Function of Excitation Intensity. Plant Physiol. 1984 Mar;74(3):481–486. doi: 10.1104/pp.74.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B. A., Steinback K. E. Chilling Sensitivity in Oryza sativa: The Role of Protein Phosphorylation in Protection against Photoinhibition. Plant Physiol. 1986 Feb;80(2):420–423. doi: 10.1104/pp.80.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Relationships between the Transition of the Physical Phase of Membrane Lipids and Photosynthetic Parameters in Anacystis nidulans and Lettuce and Spinach Chloroplasts. Plant Physiol. 1975 Oct;56(4):508–517. doi: 10.1104/pp.56.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy R. W. Effects of Growth Temperature on the Thermal Stability of the Photosynthetic Apparatus of Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1977 May;59(5):873–878. doi: 10.1104/pp.59.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieszen L. L., Helgager J. A. Genetic and physiological adaptation in the Hill reaction of Deschampsia caespitosa. Nature. 1968 Sep 7;219(5158):1066–1067. doi: 10.1038/2191066a0. [DOI] [PubMed] [Google Scholar]