Abstract
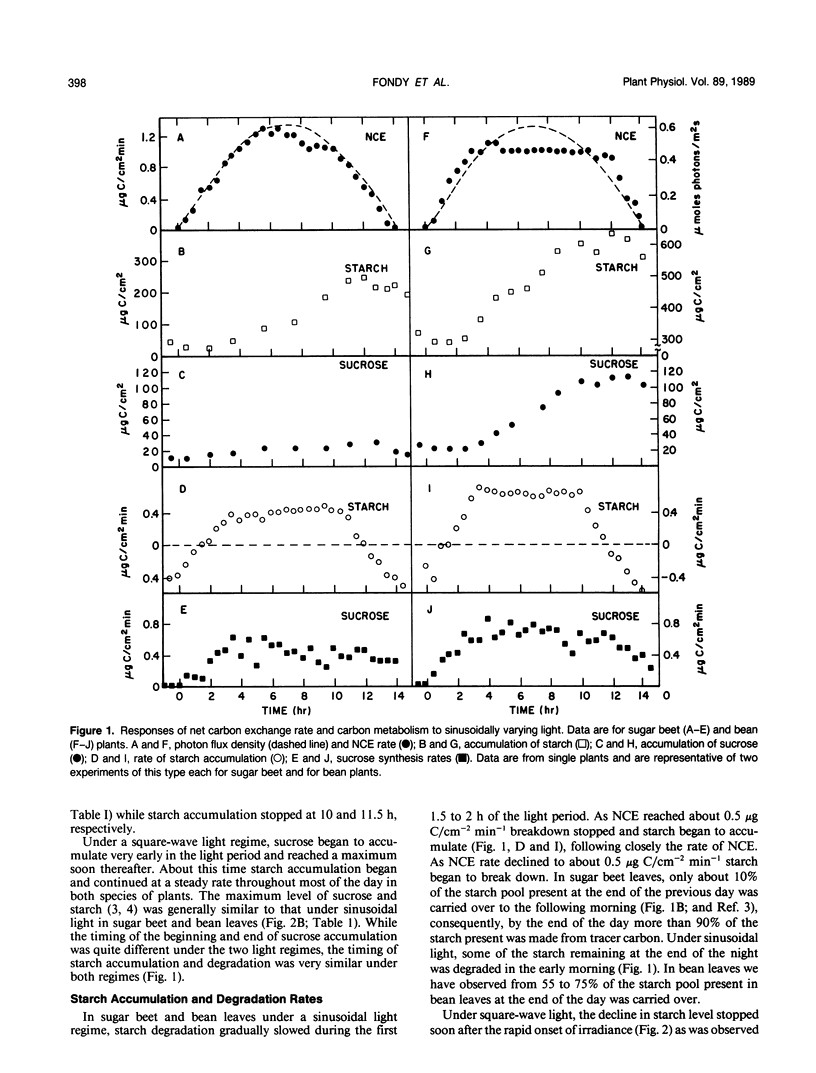
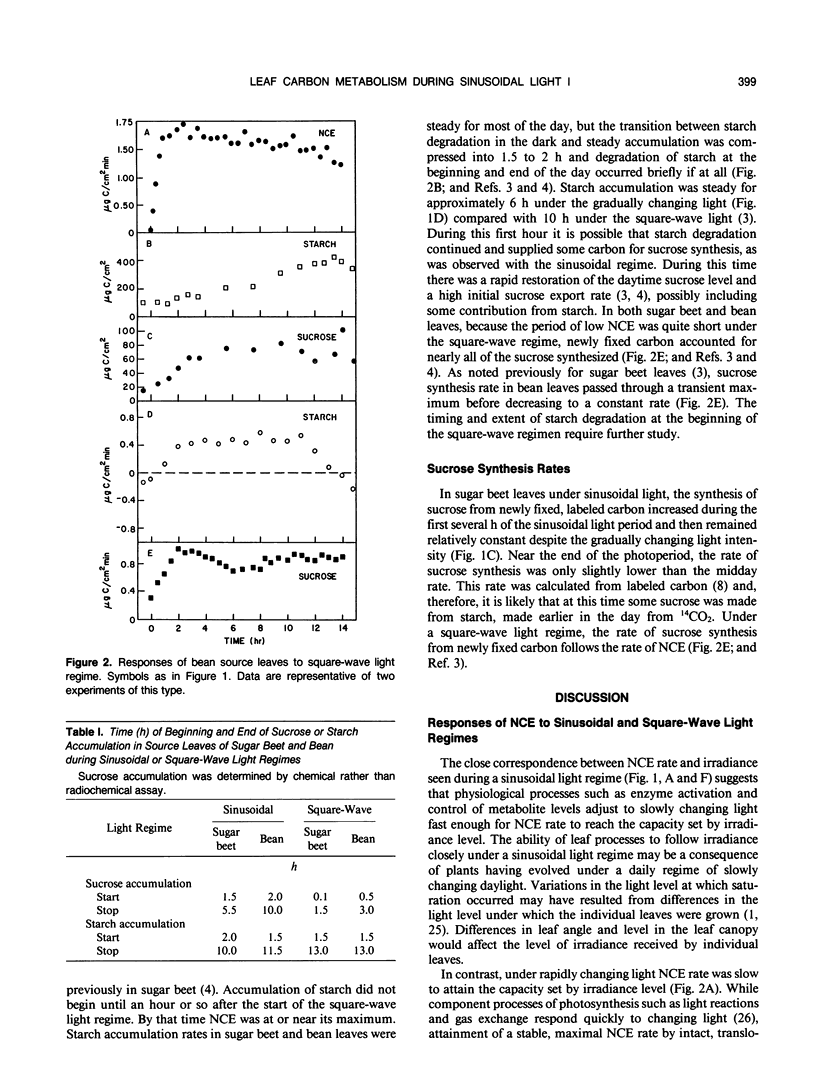
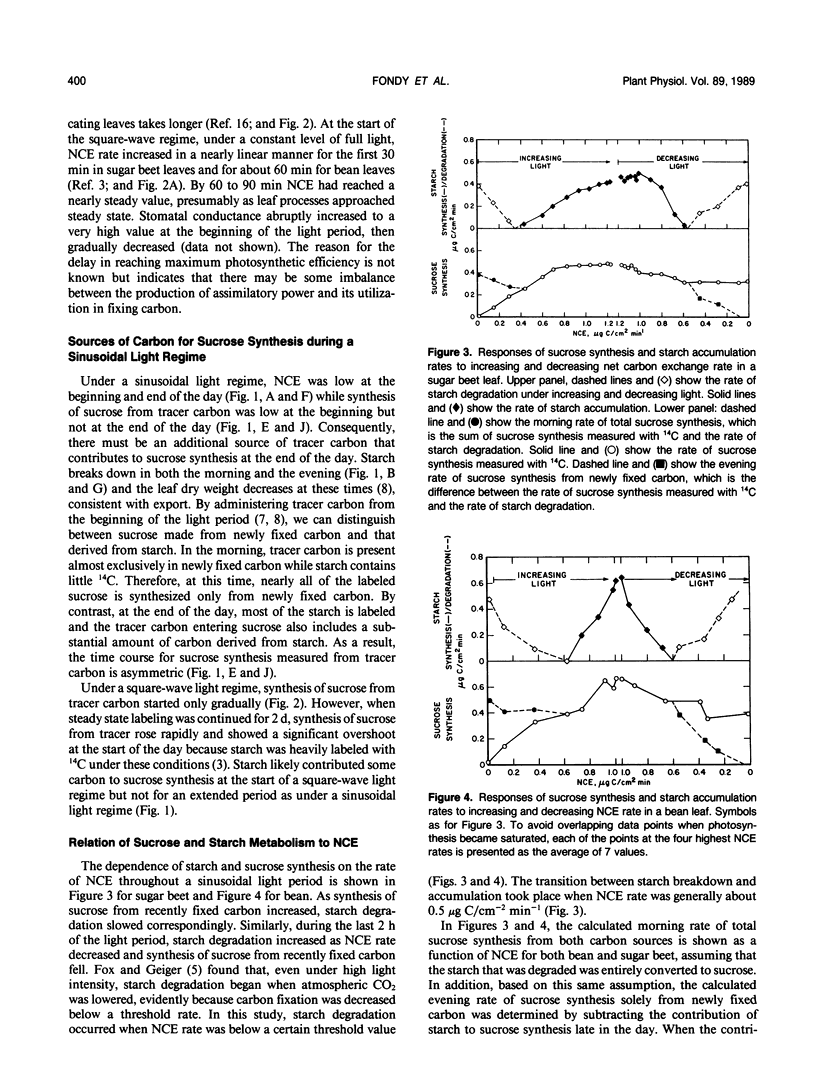
Rates of photosynthesis, sucrose synthesis, starch accumulation and degradation were measured in sugar beet (Beta vulgaris L.) and bean (Phaseolus vulgaris L.) plants under a square-wave light regime and under a sinusoidal regime that simulated the natural daylight period. Photosynthesis rate increased in a measured manner in direct proportion to the increasing light level. In contrast to this close correspondence between photosynthesis and light, a lag in photosynthesis rate was seen during the initial hour under square-wave illumination. The leaf appeared to be responding to limits set by carbon metabolism rather than by gas exchange or light reactions. Under the sinusoidal regime starch degradation occurred during the first and last 2 hours of the photoperiod, likely in response to photosynthesis rate rather than directly to light level. Starch broke down when photosynthesis was below a threshold rate and accumulated above this rate. Under square-wave illumination, accumulation of starch did not begin until irradiance was at full level for an hour or more and photosynthesis was at or near its maximum. Under a sinusoidal light regime, sucrose synthesis rate comprised carbon that was newly fixed throughout the day plus that from starch degradation at the beginning and end of the day. Synthesis of sucrose from recently fixed carbon increased with increasing net carbon fixation rate while its formation from degradation of starch decreased correspondingly. The complementary sources of carbon maintained a relatively steady rate of sucrose synthesis under the changing daytime irradiance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fondy B. R., Geiger D. R. Diurnal Pattern of Translocation and Carbohydrate Metabolism in Source Leaves of Beta vulgaris L. Plant Physiol. 1982 Sep;70(3):671–676. doi: 10.1104/pp.70.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R. Diurnal changes in allocation of newly fixed carbon in exporting sugar beet leaves. Plant Physiol. 1985 Aug;78(4):753–757. doi: 10.1104/pp.78.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R. Effect of Rapid Changes in Sink-Source Ratio on Export and Distribution of Products of Photosynthesis in Leaves of Beta vulgaris L. and Phaseolus vulgaris L. Plant Physiol. 1980 Nov;66(5):945–949. doi: 10.1104/pp.66.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. C., Geiger D. R. Effects of decreased net carbon exchange on carbohydrate metabolism in sugar beet source leaves. Plant Physiol. 1984 Nov;76(3):763–768. doi: 10.1104/pp.76.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Fondy B. R. A method for continuous measurement of export from a leaf. Plant Physiol. 1979 Sep;64(3):361–365. doi: 10.1104/pp.64.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Fondy B. R., Tucci M. A. A Method for Calculating Sucrose Synthesis Rates throughout a Light Period in Sugar Beet Leaves. Plant Physiol. 1988 Jul;87(3):776–780. doi: 10.1104/pp.87.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Swanson C. A. Evaluation of Selected Parameters in a Sugar Beet Translocation System. Plant Physiol. 1965 Sep;40(5):942–947. doi: 10.1104/pp.40.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Stitt M., Heldt H. W. Subcellular Metabolite Levels in Spinach Leaves : Regulation of Sucrose Synthesis during Diurnal Alterations in Photosynthetic Partitioning. Plant Physiol. 1987 Feb;83(2):399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt-Torres W., Kerr P. S., Usuda H., Huber S. C. Diurnal changes in maize leaf photosynthesis : I. Carbon exchange rate, assimilate export rate, and enzyme activities. Plant Physiol. 1987 Feb;83(2):283–288. doi: 10.1104/pp.83.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J. Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol. 1979 Jul;64(1):79–82. doi: 10.1104/pp.64.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Geiger D. R., Tucci M. A., Fondy B. R. Leaf Carbon Metabolism and Metabolite Levels during a Period of Sinusoidal Light. Plant Physiol. 1989 Feb;89(2):403–408. doi: 10.1104/pp.89.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Limitation of Photosynthesis by Carbon Metabolism : I. Evidence for Excess Electron Transport Capacity in Leaves Carrying Out Photosynthesis in Saturating Light and CO(2). Plant Physiol. 1986 Aug;81(4):1115–1122. doi: 10.1104/pp.81.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Sivak M. N., Prinsley R. T., Cheesbrough J. K. Simultaneous measurement of oscillations in oxygen evolution and chlorophyll a fluorescence in leaf pieces. Plant Physiol. 1983 Nov;73(3):542–549. doi: 10.1104/pp.73.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]


