Abstract
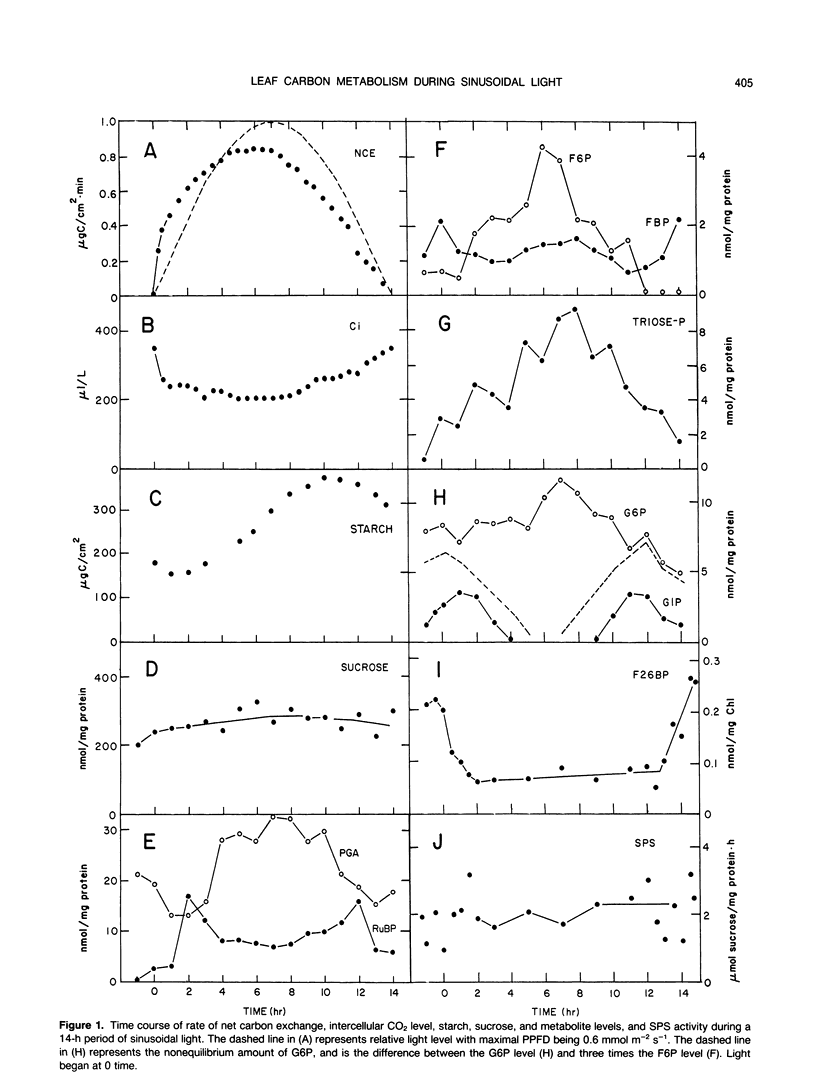
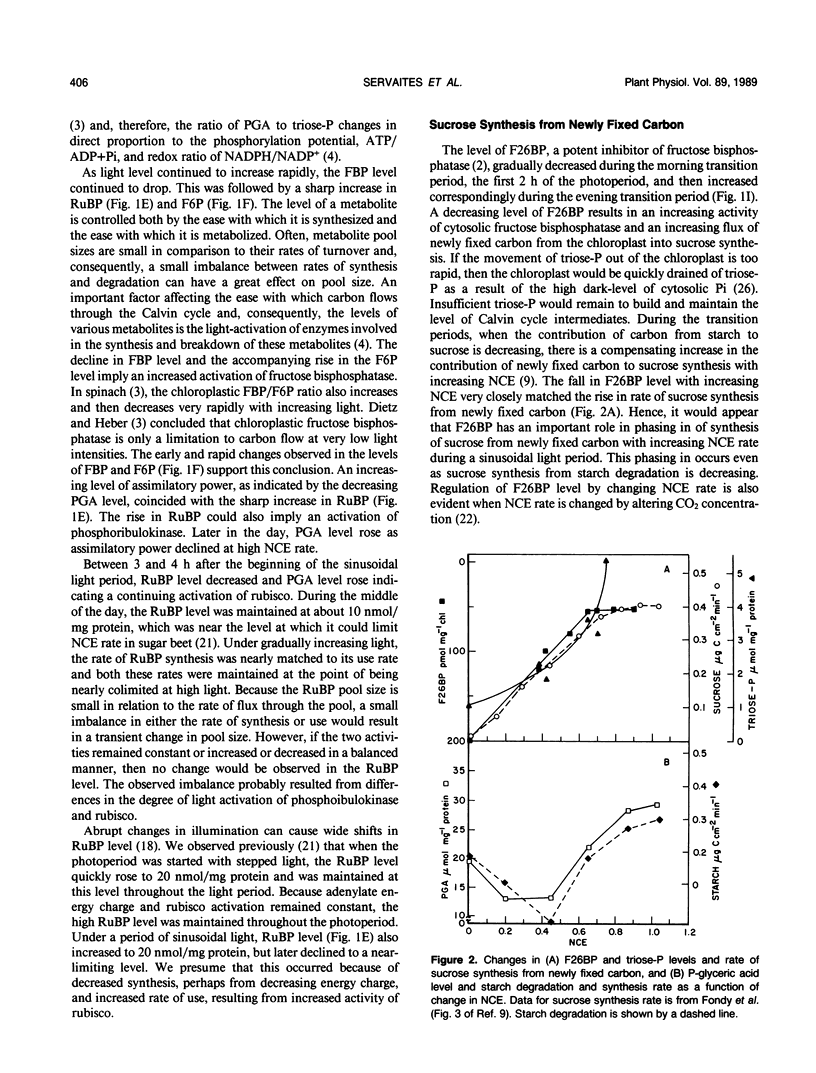
Photosynthesis rate, internal CO2 concentration, starch, sucrose, and metabolite levels were measured in leaves of sugar beet (Beta vulgaris L.) during a 14-h period of sinusoidal light, which simulated a natural light period. Photosynthesis rate closely followed increasing and decreasing light level. Chloroplast metabolite levels changed in a manner indicating differential activation of enzymes at different light levels. Starch levels declined during the first and last 2 hours of the photoperiod, but increased when photosynthesis rate was greater than 50% of maximal. Sucrose and sucrose phosphate synthase levels were constant during the photoperiod, which is consistent with a relatively steady rate of sucrose synthesis during the day as observed previously (BR Fondy et al. [1989] Plant Physiol 89: 396-402). When starch was being degraded, glucose 1-phosphate level was high and there was a large amount of glucose 6-phosphate above that in equilibrium with fructose 6-phosphate, while fructose 6-phosphate and triose-phosphate levels were very low. Likewise, the regulatory metabolite, fructose, 2,6-bisphosphate was high, indicating that little carbon could move to sucrose from starch by the triose-phosphate pathway. These data cast doubt upon the feasibility of significant carbon flow through the triose-phosphate pathway during starch degradation and support the need for an additional pathway for mobilizing starch carbon to sucrose.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert D. C., Huber S. C. Regulation of Spinach Leaf Sucrose Phosphate Synthase by Glucose-6-Phosphate, Inorganic Phosphate, and pH. Plant Physiol. 1983 Dec;73(4):989–994. doi: 10.1104/pp.73.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R. Diurnal Pattern of Translocation and Carbohydrate Metabolism in Source Leaves of Beta vulgaris L. Plant Physiol. 1982 Sep;70(3):671–676. doi: 10.1104/pp.70.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R. Diurnal changes in allocation of newly fixed carbon in exporting sugar beet leaves. Plant Physiol. 1985 Aug;78(4):753–757. doi: 10.1104/pp.78.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R., Servaites J. C. Photosynthesis, Carbohydrate Metabolism, and Export in Beta vulgaris L. and Phaseolus vulgaris L. during Square and Sinusoidal Light Regimes. Plant Physiol. 1989 Feb;89(2):396–402. doi: 10.1104/pp.89.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Fondy B. R., Tucci M. A. A Method for Calculating Sucrose Synthesis Rates throughout a Light Period in Sugar Beet Leaves. Plant Physiol. 1988 Jul;87(3):776–780. doi: 10.1104/pp.87.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Stitt M., Heldt H. W. Subcellular Metabolite Levels in Spinach Leaves : Regulation of Sucrose Synthesis during Diurnal Alterations in Photosynthetic Partitioning. Plant Physiol. 1987 Feb;83(2):399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt-Torres W., Kerr P. S., Usuda H., Huber S. C. Diurnal changes in maize leaf photosynthesis : I. Carbon exchange rate, assimilate export rate, and enzyme activities. Plant Physiol. 1987 Feb;83(2):283–288. doi: 10.1104/pp.83.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Measurement of the intermediates of the photosynthetic carbon reduction cycle, using enzymatic methods. Methods Enzymol. 1972;24:261–268. doi: 10.1016/0076-6879(72)24073-3. [DOI] [PubMed] [Google Scholar]
- Levi C., Preiss J. Amylopectin degradation in pea chloroplast extracts. Plant Physiol. 1978 Feb;61(2):218–220. doi: 10.1104/pp.61.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott K. A., Jensen R. G., O'leary J. W., Berry J. A. Photosynthesis and Ribulose 1,5-Bisphosphate Concentrations in Intact Leaves of Xanthium strumarium L. Plant Physiol. 1984 Dec;76(4):968–971. doi: 10.1104/pp.76.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer G., Heber U. Glucose transport into spinach chloroplasts. Plant Physiol. 1977 Aug;60(2):286–289. doi: 10.1104/pp.60.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Tucci M. A., Geiger D. R. Glyphosate effects on carbon assimilation, ribulose bisphosphate carboxylase activity, and metabolite levels in sugar beet leaves. Plant Physiol. 1987 Oct;85(2):370–374. doi: 10.1104/pp.85.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C., Kremer D. F. Changes of Sucrose-Phosphate Synthase Activity in Barley Primary Leaves during Light/Dark Transitions. Plant Physiol. 1984 Dec;76(4):910–912. doi: 10.1104/pp.76.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Fructose 2,6-bisphosphate and plant carbohydrate metabolism. Plant Physiol. 1987 Jun;84(2):201–204. doi: 10.1104/pp.84.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Heldt H. W. Physiological rates of starch breakdown in isolated intact spinach chloroplasts. Plant Physiol. 1981 Sep;68(3):755–761. doi: 10.1104/pp.68.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Limitation of Photosynthesis by Carbon Metabolism : I. Evidence for Excess Electron Transport Capacity in Leaves Carrying Out Photosynthesis in Saturating Light and CO(2). Plant Physiol. 1986 Aug;81(4):1115–1122. doi: 10.1104/pp.81.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H., Kalt-Torres W., Kerr P. S., Huber S. C. Diurnal Changes in Maize Leaf Photosynthesis : II. Levels of Metabolic Intermediates of Sucrose Synthesis and the Regulatory Metabolite Fructose 2,6-Bisphosphate. Plant Physiol. 1987 Feb;83(2):289–293. doi: 10.1104/pp.83.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Sivak M. N., Prinsley R. T., Cheesbrough J. K. Simultaneous measurement of oscillations in oxygen evolution and chlorophyll a fluorescence in leaf pieces. Plant Physiol. 1983 Nov;73(3):542–549. doi: 10.1104/pp.73.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H. W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980 Jul;66(1):187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


