Abstract
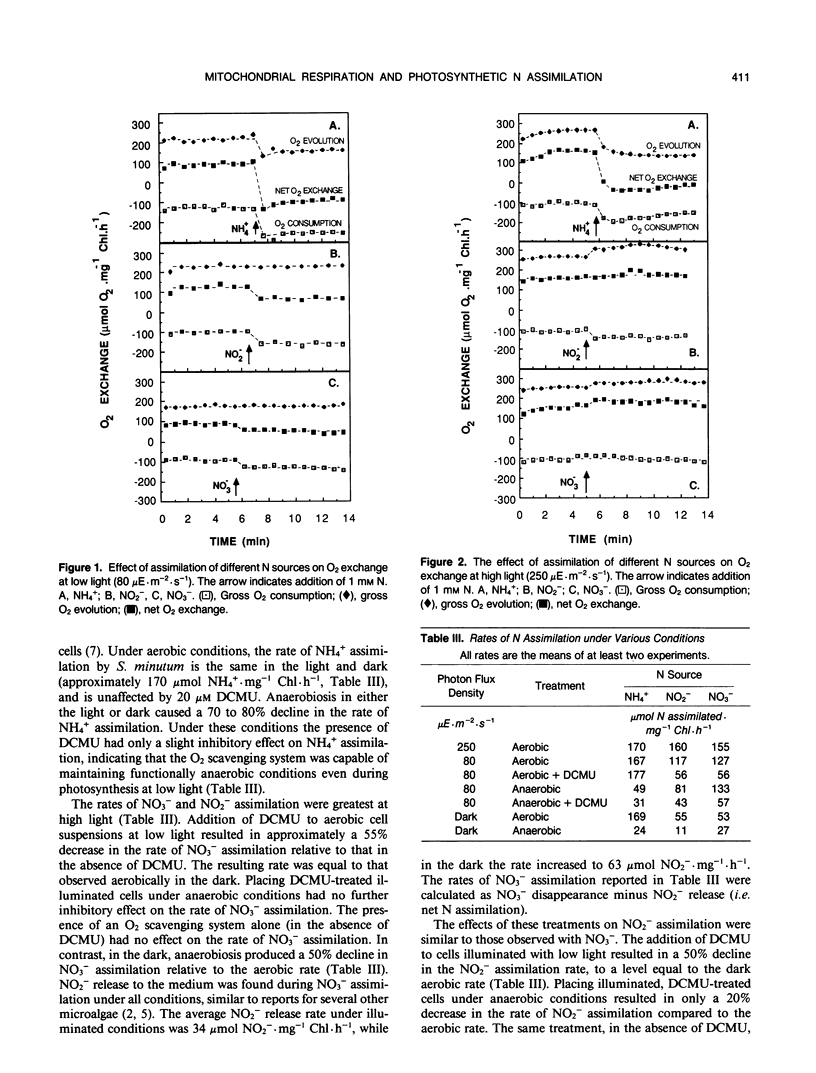
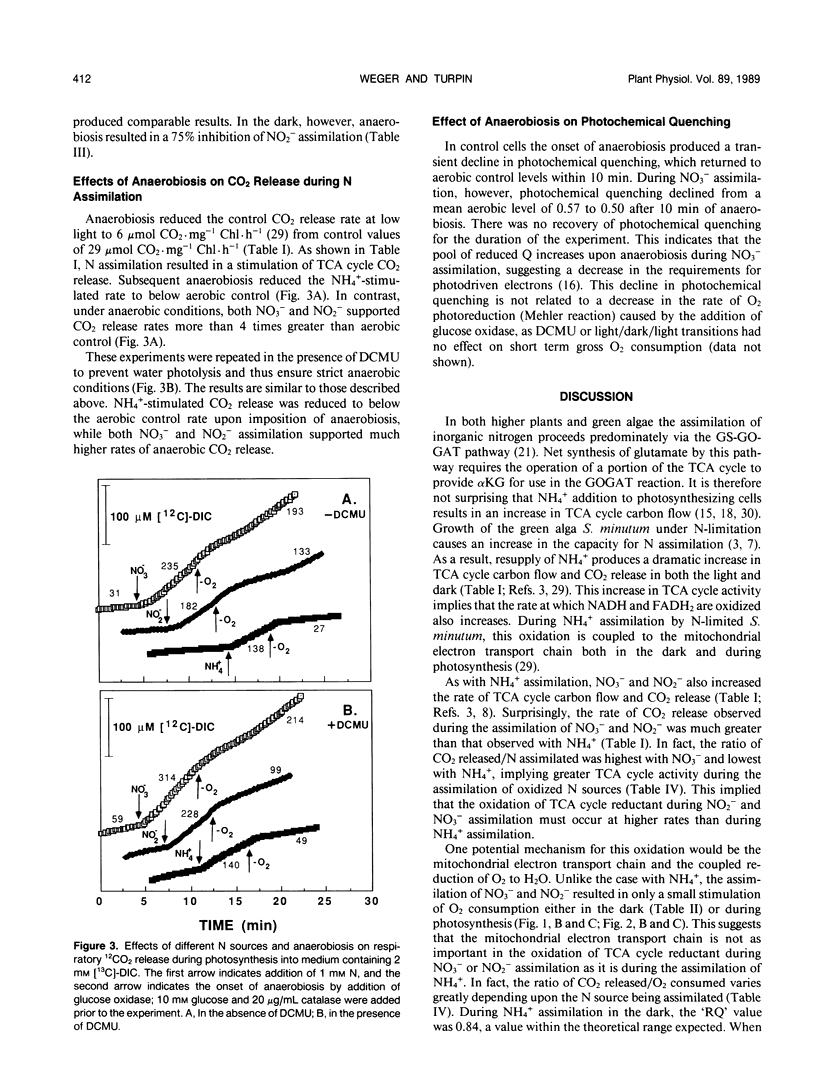
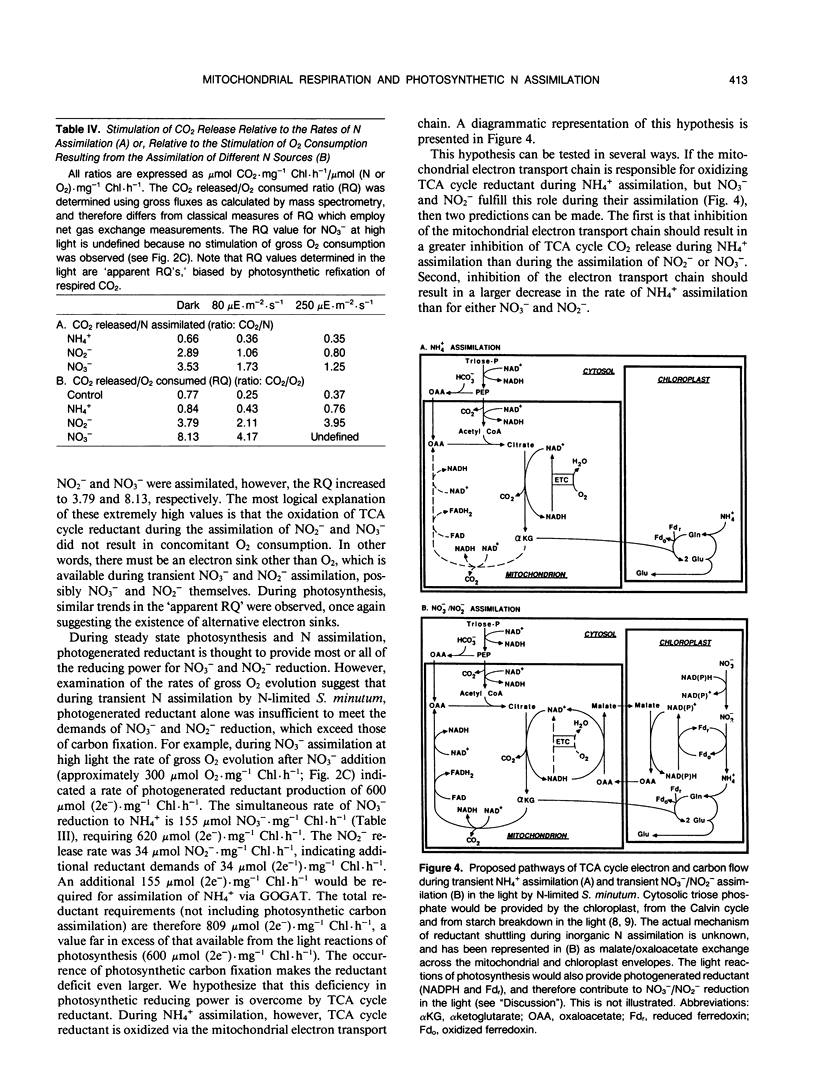
Mass spectrometric analysis shows that assimilation of inorganic nitrogen (NH4+, NO2−, NO3−) by N-limited cells of Selenastrum minutum (Naeg.) Collins results in a stimulation of tricarboxylic acid cycle (TCA cycle) CO2 release in both the light and dark. In a previous study we have shown that TCA cycle reductant generated during NH4+ assimilation is oxidized via the cytochrome electron transport chain, resulting in an increase in respiratory O2 consumption during photosynthesis (HG Weger, DG Birch, IR Elrifi, DH Turpin [1988] Plant Physiol 86: 688-692). NO3− and NO2− assimilation resulted in a larger stimulation of TCA cycle CO2 release than did NH4+, but a much smaller stimulation of mitochondrial O2 consumption. NH4+ assimilation was the same in the light and dark and insensitive to DCMU, but was 82% inhibited by anaerobiosis in both the light and dark. NO3− and NO2− assimilation rates were maximal in the light, but assimilation could proceed at substantial rates in the light in the presence of DCMU and in the dark. Unlike NH4+, NO3− and NO2− assimilation were relatively insensitive to anaerobiosis. These results indicated that operation of the mitochondrial electron transport chain was not required to maintain TCA cycle activity during NO3− and NO2− assimilation, suggesting an alternative sink for TCA cycle generated reductant. Evaluation of changes in gross O2 consumption during NO3− and NO2− assimilation suggest that TCA cycle reductant was exported to the chloroplast during photosynthesis and used to support NO3− and NO2− reduction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., House C. M. Polarographic study of oxaloacetate reduction by isolated pea chloroplasts. Plant Physiol. 1979 Dec;64(6):1058–1063. doi: 10.1104/pp.64.6.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch D. G., Elrifi I. R., Turpin D. H. Nitrate and Ammonium Induced Photosynthetic Suppression in N-Limited Selenastrum minutum: II. Effects of NO(3) and NH(4) Addition to CO(2) Efflux in the Light. Plant Physiol. 1986 Nov;82(3):708–712. doi: 10.1104/pp.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. A., Graham D. The Effect of Light on the Tricarboxylic Acid Cycle in Green Leaves: II. Intermediary Metabolism and the Location of Control Points. Plant Physiol. 1974 Jun;53(6):886–892. doi: 10.1104/pp.53.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Holmes J. J., Weger H. G., Mayo W. P., Turpin D. H. RuBP Limitation of Photosynthetic Carbon Fixation during NH(3) Assimilation : Interactions between Photosynthesis, Respiration, and Ammonium Assimilation in N-Limited Green Algae. Plant Physiol. 1988 Jun;87(2):395–401. doi: 10.1104/pp.87.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. Nitrate and Ammonium Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1986 May;81(1):273–279. doi: 10.1104/pp.81.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. The Path of Carbon Flow during NO(3)-Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1987 Jan;83(1):97–104. doi: 10.1104/pp.83.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Neuburger M., Douce R. Role of Glutamate-oxaloacetate Transaminase and Malate Dehydrogenase in the Regeneration of NAD for Glycine Oxidation by Spinach leaf Mitochondria. Plant Physiol. 1981 Mar;67(3):467–469. doi: 10.1104/pp.67.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Larsen P. O., Cornwell K. L., Gee S. L., Bassham J. A. Amino Acid Synthesis in Photosynthesizing Spinach Cells : EFFECTS OF AMMONIA ON POOL SIZES AND RATES OF LABELING FROM CO(2). Plant Physiol. 1981 Aug;68(2):292–299. doi: 10.1104/pp.68.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ruiz A., Verbelen J. P., Roldan J. M., Diez J. Nitrate reductase of green algae is located in the pyrenoid. Plant Physiol. 1985 Dec;79(4):1006–1010. doi: 10.1104/pp.79.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier G., Thibault P. O(2) uptake in the light in chlamydomonas: evidence for persistent mitochondrial respiration. Plant Physiol. 1985 Sep;79(1):225–230. doi: 10.1104/pp.79.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Lilley R. M., Heldt H. W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982 Oct;70(4):971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin D. H., Weger H. G. Steady-State Chlorophyll a Fluorescence Transients during Ammonium Assimilation by the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1988 Sep;88(1):97–101. doi: 10.1104/pp.88.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger H. G., Birch D. G., Elrifi I. R., Turpin D. H. Ammonium Assimilation Requires Mitochondrial Respiration in the Light : A Study with the Green Alga Selenastrum minutum. Plant Physiol. 1988 Mar;86(3):688–692. doi: 10.1104/pp.86.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Jokinen M., Canvin D. T. Reduction of Nitrate via a Dicarboxylate Shuttle in a Reconstituted System of Supernatant and Mitochondria from Spinach Leaves. Plant Physiol. 1980 Mar;65(3):433–436. doi: 10.1104/pp.65.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]


