Abstract
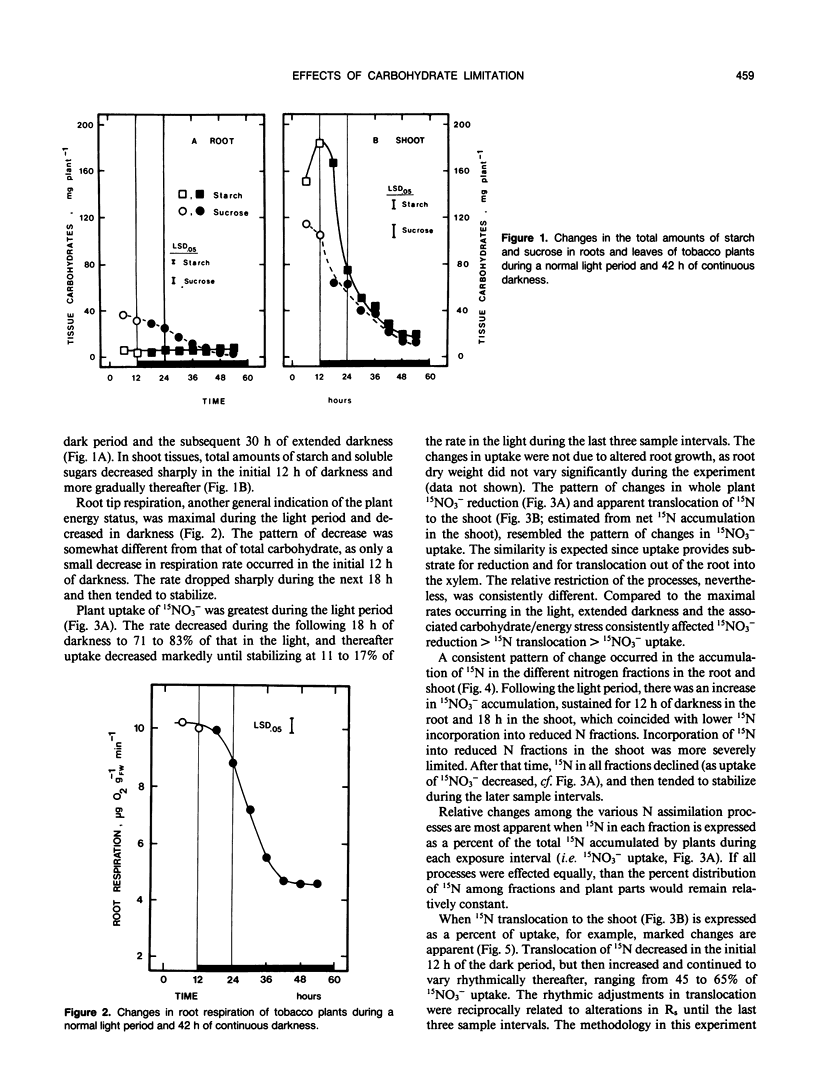
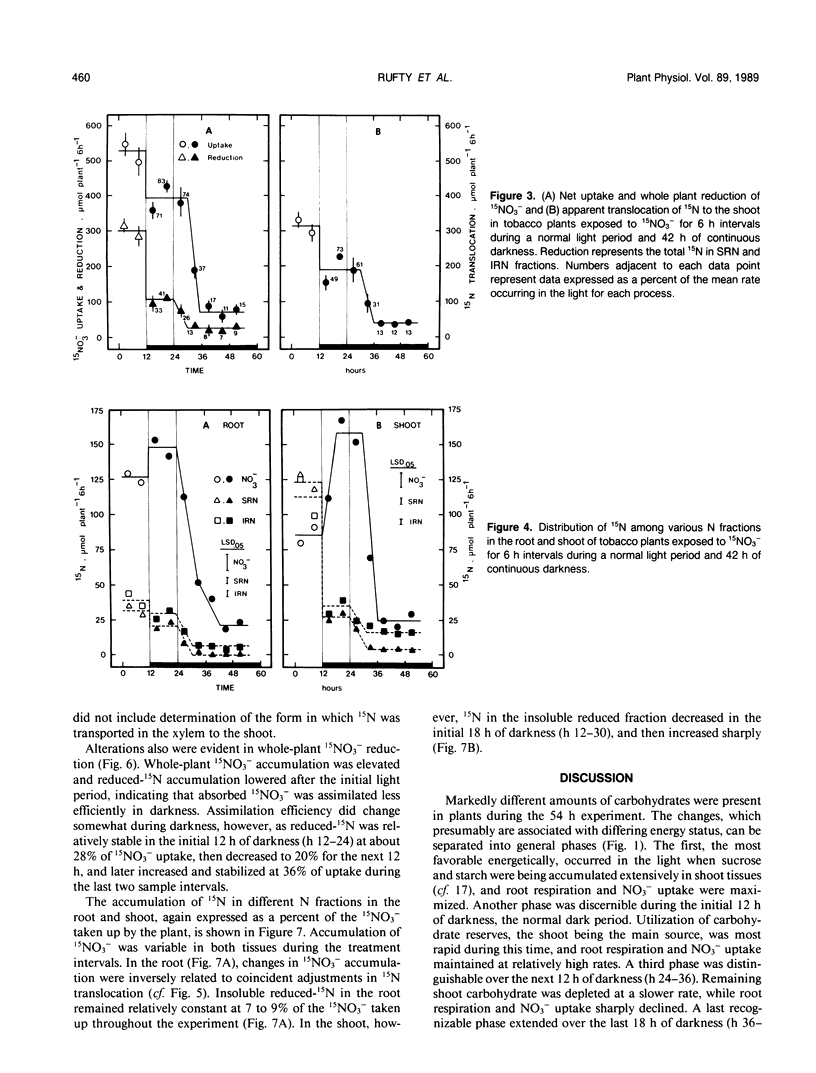
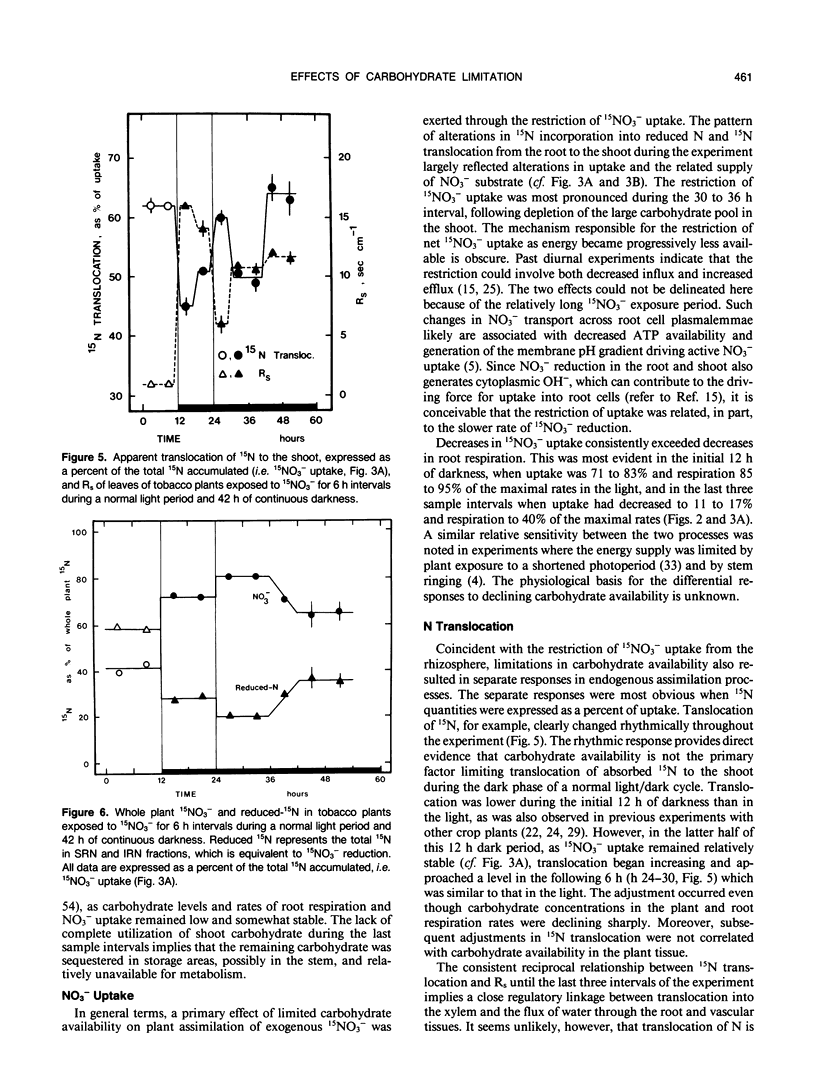
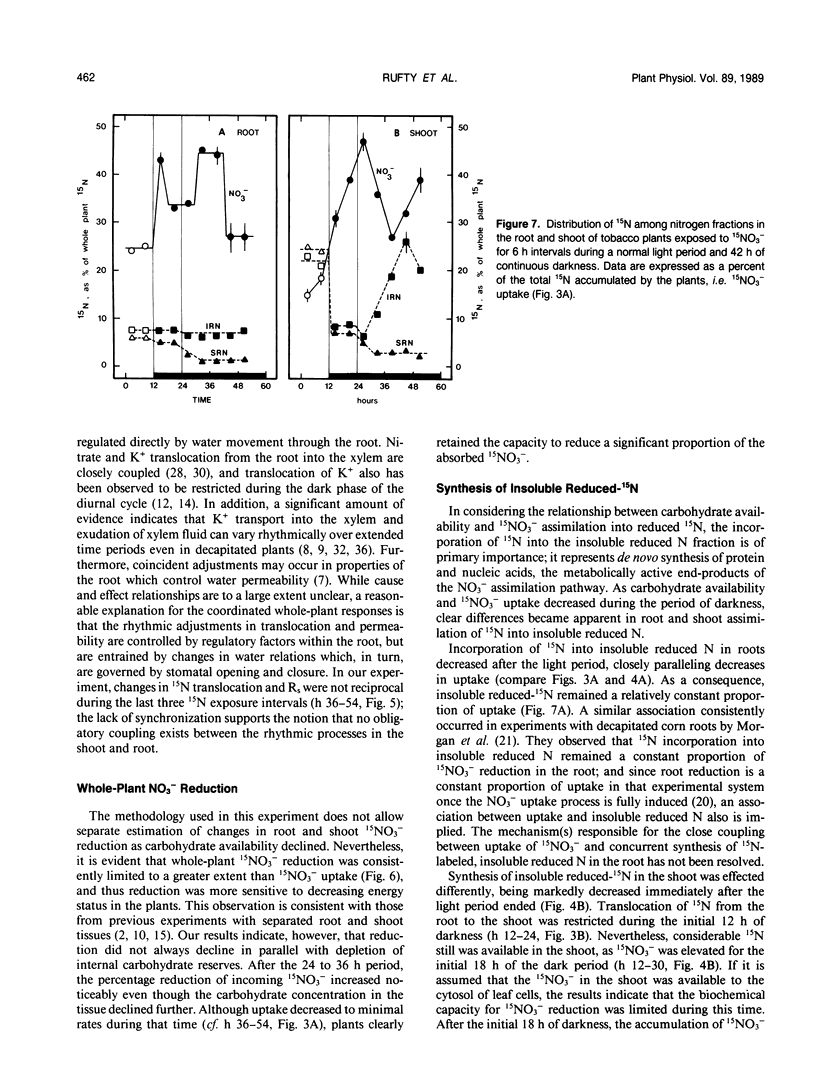
An experiment was conducted to investigate the relative changes in NO3− assimilatory processes which occurred in response to decreasing carbohydrate availability. Young tobacco plants (Nicotiana tabacum [L.], cv NC 2326) growing in solution culture were exposed to 1.0 millimolar 15NO3− for 6 hour intervals during a normal 12 hour light period and a subsequent period of darkness lasting 42 hours. Uptake of 15NO3− decreased to 71 to 83% of the uptake rate in the light during the initial 18 hours of darkness; uptake then decreased sharply over the next 12 hours of darkness to 11 to 17% of the light rate, coincident with depletion of tissue carbohydrate reserves and a marked decline in root respiration. Changes also occurred in endogenous 15NO3− assimilation processes, which were distinctly different than those in 15NO3− uptake. During the extended dark period, translocation of absorbed 15N out of the root to the shoot varied rhythmically. The adjustments were independent of 15NO3− uptake rate and carbohydrate status, but were reciprocally related to rhythmic adjustments in stomatal resistance and, presumably, water movement through the root system. Whole plant reduction of 15NO3− always was limited more than uptake. The assimilation of 15N into insoluble reduced-N in roots remained a constant proportion of uptake throughout, while assimilation in the shoot declined markedly in the first 18 hours of darkness before stabilizing at a low level. The plants clearly retained a capacity for 15NO3− reduction and synthesis of insoluble reduced-15N even when 15NO3− uptake was severely restricted and minimal carbohydrate reserves remained in the tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C. Dependency of Nitrate Reduction on Soluble Carbohydrates in Primary Leaves of Barley under Aerobic Conditions. Plant Physiol. 1984 Jul;75(3):623–628. doi: 10.1104/pp.75.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Huffaker R. C. In Vivo Nitrate Reduction in Roots and Shoots of Barley (Hordeum vulgare L.) Seedlings in Light and Darkness. Plant Physiol. 1982 Oct;70(4):1009–1013. doi: 10.1104/pp.70.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscus E. L. Diurnal changes in volume and solute transport coefficients of phaseolus roots. Plant Physiol. 1986 Mar;80(3):752–759. doi: 10.1104/pp.80.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossenbacher K. A. DIURNAL FLUCTUATION IN ROOT PRESSURE. Plant Physiol. 1938 Oct;13(4):669–676. doi: 10.1104/pp.13.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan R. M. AUTONOMIC DIURNAL CYCLES IN THE WATER RELATIONS OF NONEXUDING DETOPPED ROOT SYSTEMS. Plant Physiol. 1949 Jul;24(3):441–454. doi: 10.1104/pp.24.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B., Biddulph O. The Diurnal Variation in the Translocation of Minerals across Bean Roots. Plant Physiol. 1953 Jul;28(3):356–370. doi: 10.1104/pp.28.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. S., Rufty T. W., Huber S. C. Changes in Nonstructural Carbohydrates in Different Parts of Soybean (Glycine max [L.] Merr.) Plants during a Light/Dark Cycle and in Extended Darkness. Plant Physiol. 1985 Jul;78(3):576–581. doi: 10.1104/pp.78.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. S., Rufty T. W., Huber S. C. Endogenous Rhythms in Photosynthesis, Sucrose Phosphate Synthase Activity, and Stomatal Resistance in Leaves of Soybean (Glycine max [L.] Merr.). Plant Physiol. 1985 Feb;77(2):275–280. doi: 10.1104/pp.77.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace G. M., Mackown C. T., Volk R. J. Minimizing Nitrate Reduction during Kjeldahl Digestion of Plant Tissue Extracts and Stem Exudates : APPLICATION TO N STUDIES. Plant Physiol. 1982 Jan;69(1):32–36. doi: 10.1104/pp.69.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A. J., Canvin D. T., Sherrard J. H., Hageman R. H. Assimilation of [N]Nitrate and [N]Nitrite in Leaves of Five Plant Species under Light and Dark Conditions. Plant Physiol. 1983 Feb;71(2):291–294. doi: 10.1104/pp.71.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Jackson W. A., Raper C. D. Nitrate Reduction in Roots as Affected by the Presence of Potassium and by Flux of Nitrate through the Roots. Plant Physiol. 1981 Sep;68(3):605–609. doi: 10.1104/pp.68.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Volk R. J., Mackown C. T. Endogenous NO(3) in the Root as a Source of Substrate for Reduction in the Light. Plant Physiol. 1987 Aug;84(4):1421–1426. doi: 10.1104/pp.84.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk R. J., Pearson C. J., Jackson W. A. Reduction of plant tissue nitrate to nitric oxide for mass spectrometric 15N analysis. Anal Biochem. 1979 Aug;97(1):131–135. doi: 10.1016/0003-2697(79)90336-1. [DOI] [PubMed] [Google Scholar]
- Wallace A., Soufi S. M., Hemaidan N. Day-night Differences in the Accumulation and Translocation of Ions by Tobacco Plants. Plant Physiol. 1966 Jan;41(1):102–104. doi: 10.1104/pp.41.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]


