Abstract
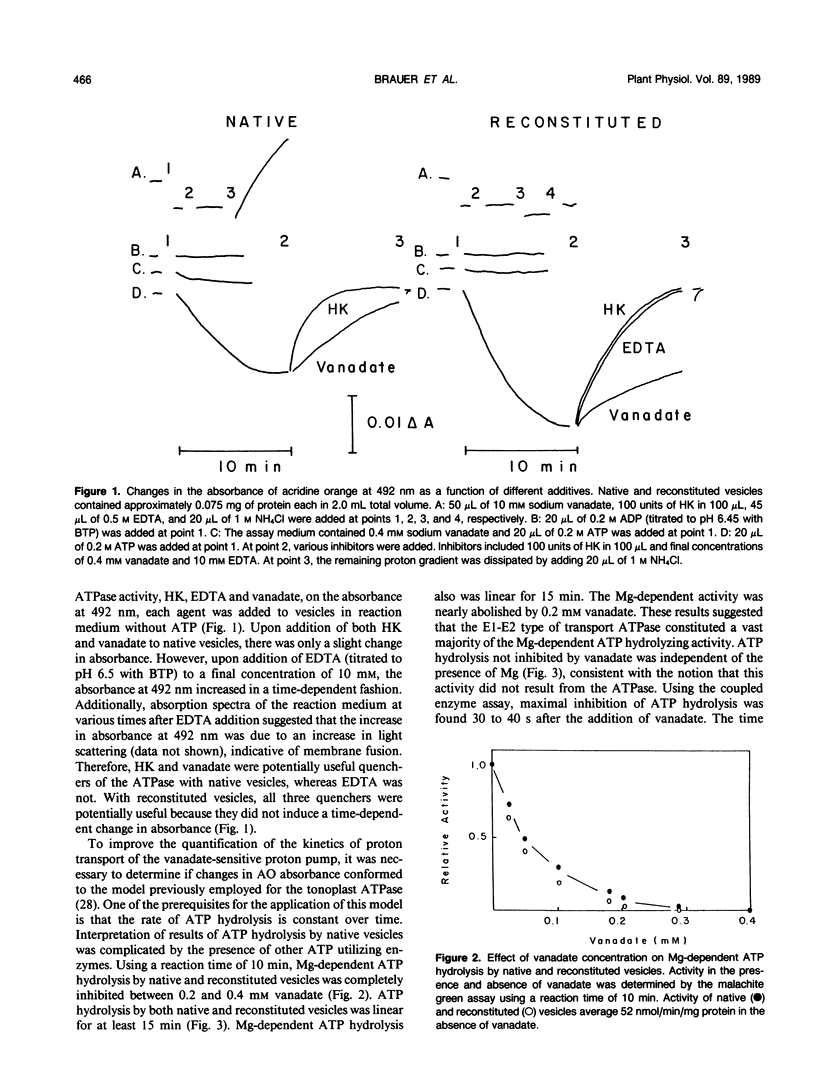
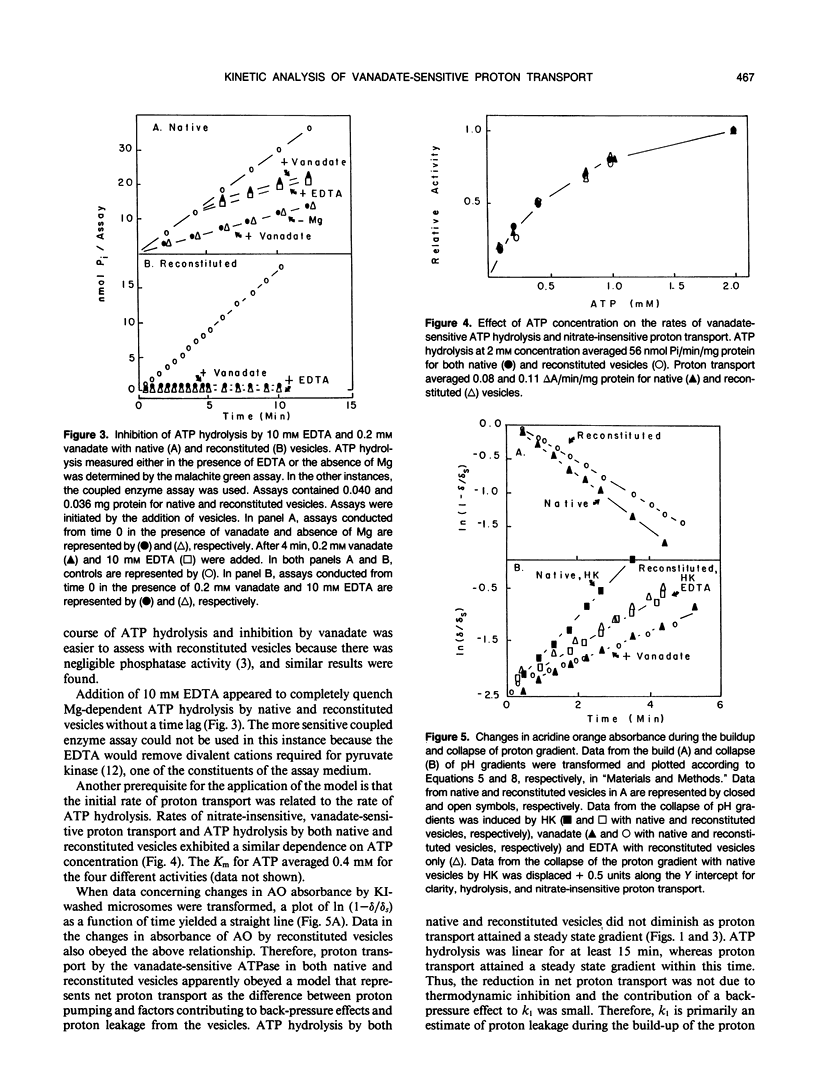
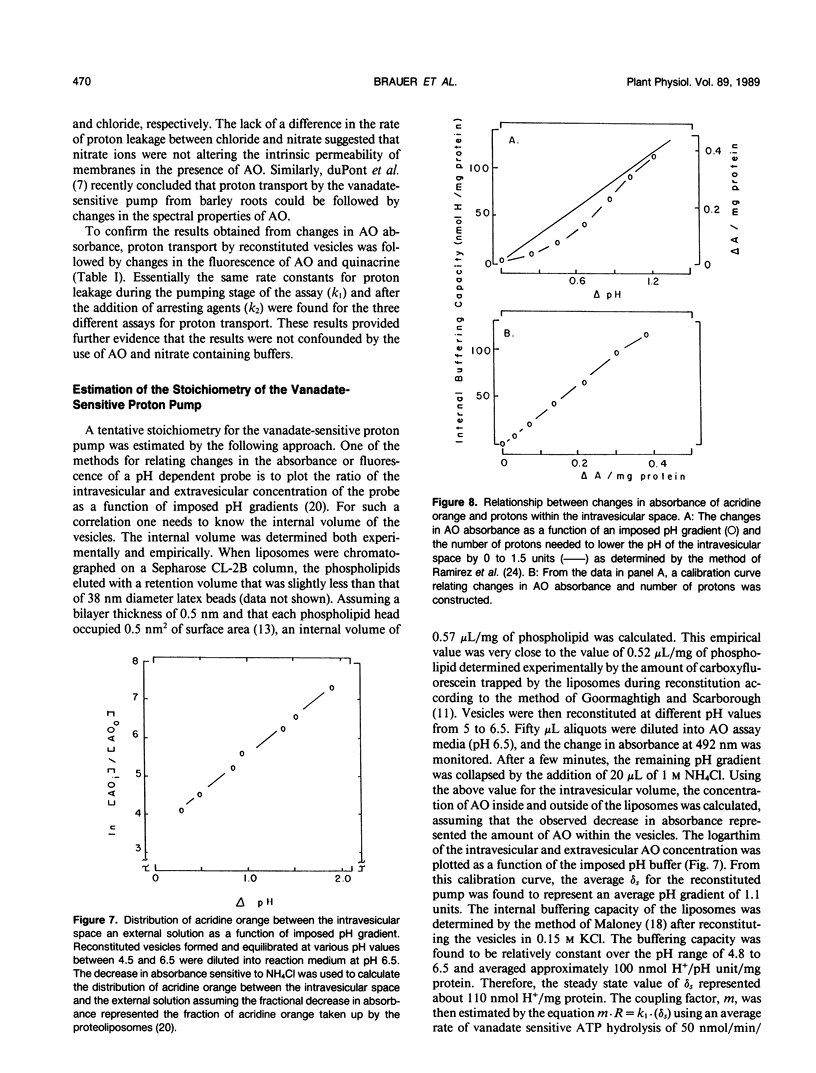
Proton transport by the nitrate-insensitive, vanadate-sensitive ATPase in Kl-washed microsomes and reconstituted vesicles from maize (Zea mays L.) roots was followed by changes in acridine orange absorbance in the presence of either KNO3 or KCl. Data from such studies obeyed a kinetic model in which net proton transport by the pump is the difference between the rate of proton transport by the action of the ATPase and the leak of protons from the vesicles in the direction opposite from the pump. After establishing the steady state proton gradient, the rate of return of transported protons was found to obey first-order kinetics when the activity of the ATPase was completely and rapidly stopped. The rate of return of these protons varied with the quencher used. When the substrate Mg:ATP was depleted by the addition of either EDTA or hexokinase, the rate at which the proton gradient collapsed was faster than when vanadate was used as the quencher. These trends were independent of the anion accompanying the K and the transport assay used.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amory A., Goffeau A., McIntosh D. B., Boyer P. Contribution of 18O technology to the mechanism of the H+-ATPase from yeast plasma membrane. Curr Top Cell Regul. 1984;24:471–483. doi: 10.1016/b978-0-12-152824-9.50047-2. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Brauer D., Hsu A. F., Tu S. I. Factors associated with the instability of nitrate-insensitive proton transport by maize root microsomes. Plant Physiol. 1988 Jul;87(3):598–602. doi: 10.1104/pp.87.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P. Intermediate reaction states of the red beet plasma membrane ATPase. Arch Biochem Biophys. 1986 Jul;248(1):106–115. doi: 10.1016/0003-9861(86)90406-6. [DOI] [PubMed] [Google Scholar]
- Calahorra M., Ramírez J., Clemente S. M., Peña A. Electrochemical potential and ion transport in vesicles of yeast plasma membrane. Biochim Biophys Acta. 1987 May 29;899(2):229–238. doi: 10.1016/0005-2736(87)90404-4. [DOI] [PubMed] [Google Scholar]
- De Michelis M. I., Spanswick R. M. H-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots. Plant Physiol. 1986 Jun;81(2):542–547. doi: 10.1104/pp.81.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Tanaka C. K., Hurkman W. J. separation and Immunological Characterization of Membrane Fractions from Barley Roots. Plant Physiol. 1988 Mar;86(3):717–724. doi: 10.1104/pp.86.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Carroll E. J., Leonard R. T. A sensitive diffusion plate assay for screening inhibitors of protease activity in plant cell fractions. Plant Physiol. 1986 Jul;81(3):869–874. doi: 10.1104/pp.81.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini J. L., Briskin D. P. Proton Transport in Plasma Membrane and Tonoplast Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : A Comparative Study of Ion Effects on DeltapH and DeltaPsi. Plant Physiol. 1987 Jul;84(3):613–618. doi: 10.1104/pp.84.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini J. L., Gildensoph L. H., Briskin D. P. Selective production of sealed plasma membrane vesicles from red beet (Beta vulgaris L.) storage tissue. Arch Biochem Biophys. 1987 May 1;254(2):621–630. doi: 10.1016/0003-9861(87)90145-7. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Scarborough G. A. Density-based separation of liposomes by glycerol gradient centrifugation. Anal Biochem. 1986 Nov 15;159(1):122–131. doi: 10.1016/0003-2697(86)90316-7. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L., Petersen J. Chymotryptic cleavage of alpha-subunit in E1-forms of renal (Na+ + K+)-ATPase: effects on enzymatic properties, ligand binding and cation exchange. Biochim Biophys Acta. 1985 Dec 5;821(2):319–333. doi: 10.1016/0005-2736(85)90102-6. [DOI] [PubMed] [Google Scholar]
- Kurtenbach E., Verjovski-Almeida S. Labeling of a thiol residue in sarcoplasmic reticulum ATPase by pyrene maleimide. Solvent accessibility studied by fluorescence quenching. J Biol Chem. 1985 Aug 15;260(17):9636–9641. [PubMed] [Google Scholar]
- Lüdi H., Hasselbach W., Gaugler H. Tryptophan fluorescence of sarcoplasmic reticulum ATPase. A fluorescence quench study. Biochim Biophys Acta. 1985 Mar 28;814(1):120–124. doi: 10.1016/0005-2736(85)90426-2. [DOI] [PubMed] [Google Scholar]
- Maloney P. C. Membrane H+ conductance of Streptococcus lactis. J Bacteriol. 1979 Oct;140(1):197–205. doi: 10.1128/jb.140.1.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Brown C. L. Identification of structurally distinct catalytic intermediates of the H+-ATPase from yeast plasma membranes. J Biol Chem. 1987 May 15;262(14):6788–6794. [PubMed] [Google Scholar]
- Perlin D. S., San Francisco M. J., Slayman C. W., Rosen B. P. H+/ATP stoichiometry of proton pumps from Neurospora crassa and Escherichia coli. Arch Biochem Biophys. 1986 Jul;248(1):53–61. doi: 10.1016/0003-9861(86)90400-5. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Azzone G. F., Walz D. Effect of funiculosin and antimycin A on the redox-driven H+-pumps in mitochondria: on the nature of "leaks'. Eur J Biochem. 1981 Jul;117(2):389–394. doi: 10.1111/j.1432-1033.1981.tb06350.x. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Zoratti M., Azzone G. F., Stucki J. W., Walz D. Non-equilibrium thermodynamic assessment of redox-driven H+ pumps in mitochondria. Eur J Biochem. 1982 Oct;127(3):483–494. doi: 10.1111/j.1432-1033.1982.tb06897.x. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Okazaki H., Tu S., Hutchinson H. Proton movement in reconstituted purple membrane of halobacteria: effects of pH and ionic composition of the medium. Arch Biochem Biophys. 1983 Apr 15;222(2):464–472. doi: 10.1016/0003-9861(83)90545-3. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. Binding energy, conformational change, and the mechanism of transmembrane solute movements. Microbiol Rev. 1985 Sep;49(3):214–231. doi: 10.1128/mr.49.3.214-231.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S. I., Hutchinson H. Temperature dependence of light-induced proton movement in reconstituted purple membrane. Arch Biochem Biophys. 1984 Feb 1;228(2):609–616. doi: 10.1016/0003-9861(84)90029-8. [DOI] [PubMed] [Google Scholar]
- Tu S. I., Nagahashi G., Brouillette J. N. Proton pumping kinetics and origin of nitrate inhibition of tonoplast-type H+-ATPase. Arch Biochem Biophys. 1987 Aug 1;256(2):625–637. doi: 10.1016/0003-9861(87)90620-5. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Hellingwerf K. J., Arents J. C., Scholte B. J., Van Dam K. Mosaic nonequilibrium thermodynamics describes biological energy transduction. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3554–3558. doi: 10.1073/pnas.78.6.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff H. V., Scholte B. J., Hellingwerf K. J. Bacteriorhodopsin in liposomes. I. A description using irreversible thermodynamics. Biochim Biophys Acta. 1979 Sep 11;547(3):544–560. doi: 10.1016/0005-2728(79)90033-1. [DOI] [PubMed] [Google Scholar]


