Abstract
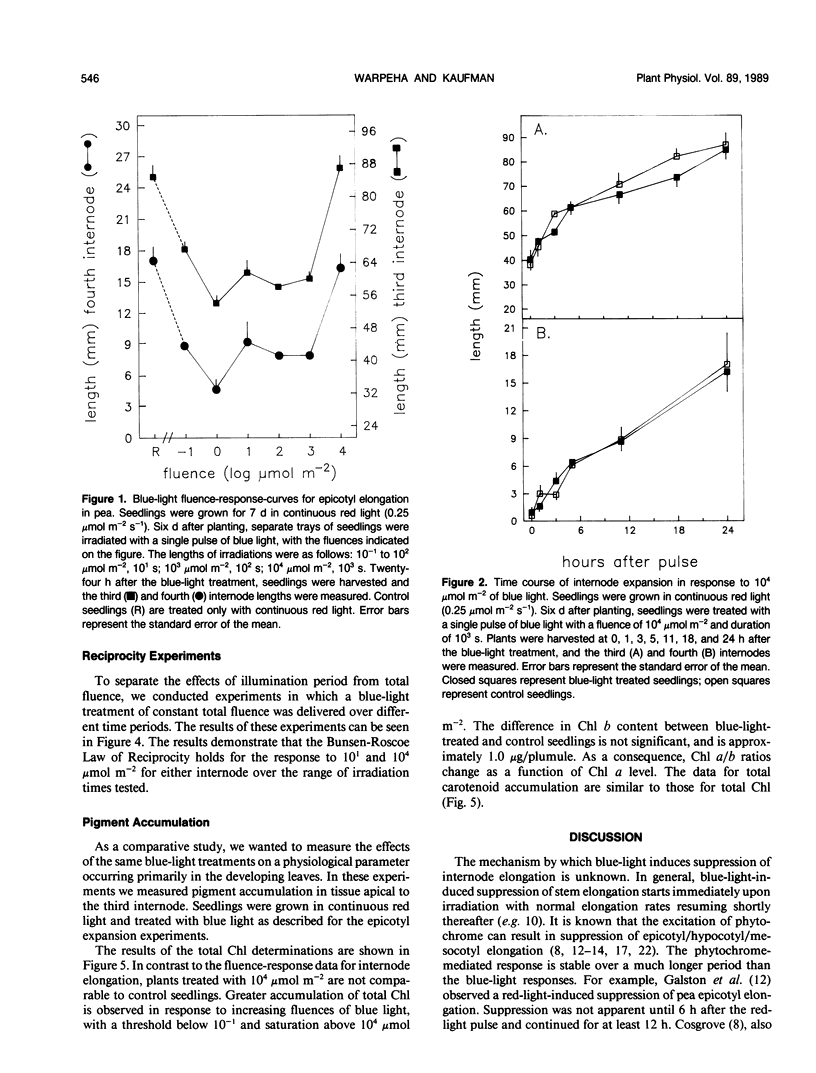
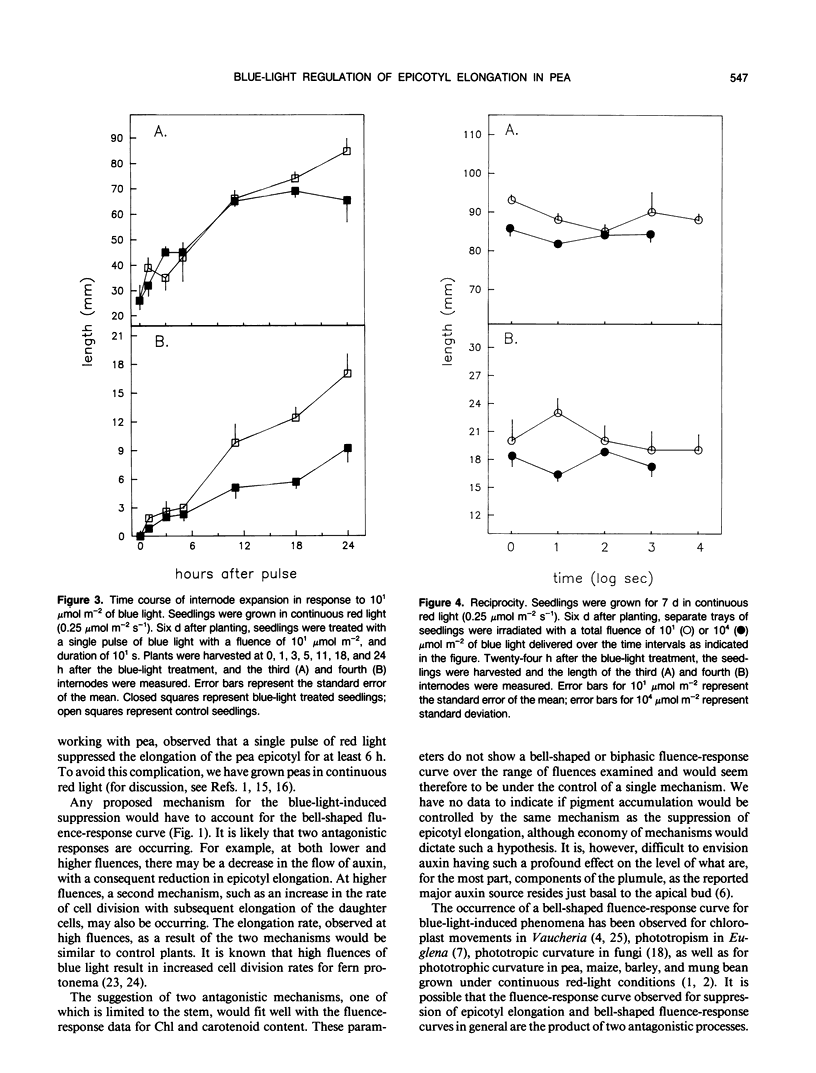
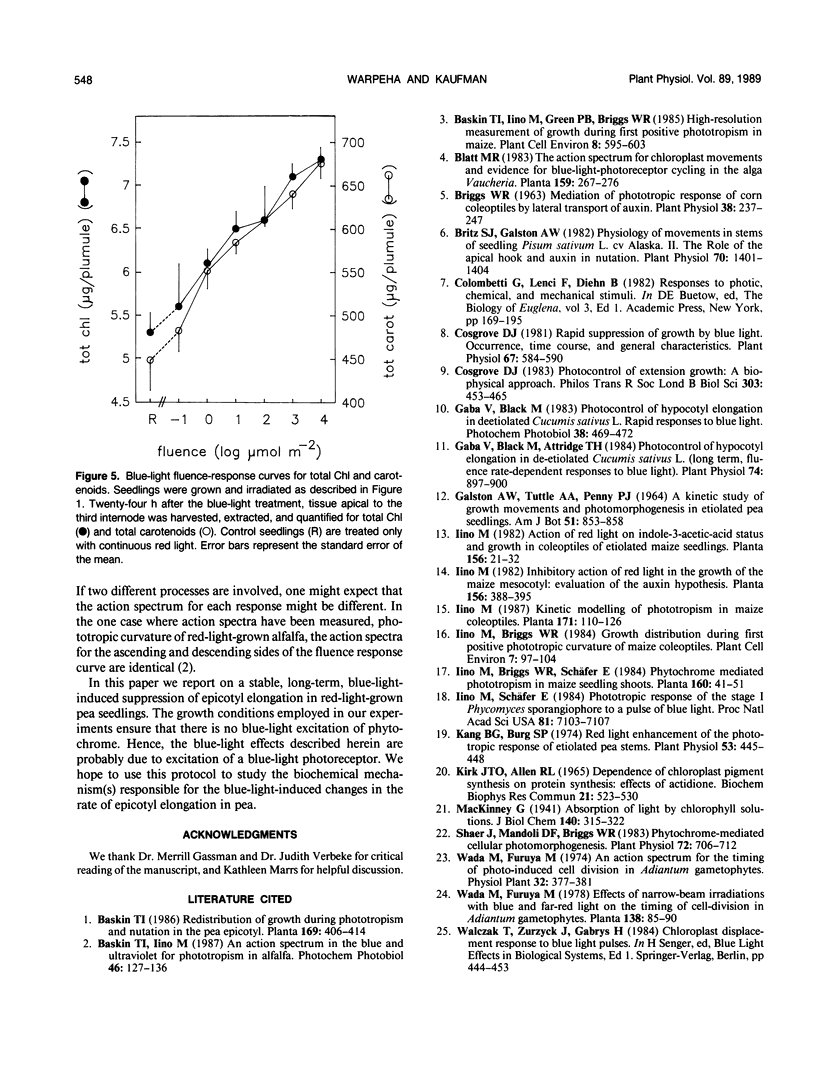
Blue light is known to induce suppression of stem elongation. To avoid the complication of blue-light-induced transformation of phytochrome we have adapted the procedure of measuring blue-light-induced suppression of stem elongation in Pisum sativum L. var Alaska grown under continuous red light. The resulting fluence-response curve for suppression of epicotyl elongation measured twenty-four hours after a blue-light treatment is bell-shaped, with the peak of suppression between 100 and 101 micromoles per square meter, and no suppression at 104 micromoles per square meter. Suppression is first observed 5 and 11 hours after the blue-light treatment for the fourth and third internodes, respectively. No significant differences in elongation rates were noted for the 104 micromoles per square meter treated seedlings throughout the 24 hour period. Reciprocity holds for both third and fourth internodes in response to 101 and 104 micromoles per square meter of blue light over the range of irradiation times tested (100 to 104 seconds, 101 micromoles per square meter; 100 to 103 seconds, 104 micromoles per square meter). In contrast to the bell-shaped fluence-response obtained for epicotyl elongation, measurements of chlorophyll and carotenoid accumulation indicate increasing accumulation with increasing fluence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs W. R. Mediation of Phototropic Responses of Corn Coleoptiles by Lateral Transport of Auxin. Plant Physiol. 1963 May;38(3):237–247. doi: 10.1104/pp.38.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz S. J., Galston A. W. Physiology of Movements in Stems of Seedling Pisum sativum L. cv Alaska : II. The Role of the Apical Hook and of Auxin in Nutation. Plant Physiol. 1982 Nov;70(5):1401–1404. doi: 10.1104/pp.70.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J. Rapid Suppression of Growth by Blue Light: OCCURRENCE, TIME COURSE, AND GENERAL CHARACTERISTICS. Plant Physiol. 1981 Mar;67(3):584–590. doi: 10.1104/pp.67.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaba V., Black M., Attridge T. H. Photocontrol of Hypocotyl Elongation in De-Etiolated Cucumis sativus L. : Long Term, Fluence Rate-Dependent Responses to Blue Light. Plant Physiol. 1984 Apr;74(4):897–900. doi: 10.1104/pp.74.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Schäfer E. Phototropic response of the stage I Phycomyces sporangiophore to a pulse of blue light. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7103–7107. doi: 10.1073/pnas.81.22.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Burg S. P. Red light enhancement of the phototropic response of etiolated pea stems. Plant Physiol. 1974 Mar;53(3):445–448. doi: 10.1104/pp.53.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Schaer J. A., Mandoli D. F., Briggs W. R. Phytochrome-mediated cellular photomorphogenesis. Plant Physiol. 1983 Jul;72(3):706–712. doi: 10.1104/pp.72.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]


