Summary
Carriers of BRCA1 germline pathogenic variants are at substantially higher risk of developing breast and ovarian cancer than the general population. Accurate identification of at-risk individuals is crucial for risk stratification and the implementation of targeted preventive and therapeutic interventions. Despite significant progress in variant classification efforts, a sizable portion of reported BRCA1 variants remain as variants of uncertain clinical significance (VUSs). Variants leading to premature protein termination and loss of essential functional domains are typically classified as pathogenic. However, the impact of frameshift variants that result in an extended incorrect terminus is not clear. Using validated functional assays, we conducted a systematic functional assessment of 17 previously reported BRCA1 extended incorrect terminus variants (EITs) and concluded that 16 constitute loss-of-function variants. This suggests that most EITs are likely to be pathogenic. However, one variant, c.5578dup, displayed a protein expression level, affinity to known binding partners, and activity in transcription and homologous recombination assays comparable to the wild-type BRCA1 protein. Twenty-three additional carriers of c.5578dup were identified at a US clinical diagnostic lab and assessed using a family history likelihood model providing, in combination with the functional data, a likely benign interpretation. These results, consistent with family history data in the current study and available data from ClinVar, indicate that most, but not all, BRCA1 variants leading to an extended incorrect terminus constitute loss-of-function variants and underscore the need for comprehensive assessment of individual variants.
We conducted a systematic assessment of all known BRCA1 frameshift variants that lead to extended incorrect C termini. We combined validated functional assays with family history data to provide an in-depth look at this class of BRCA1 variants and to assess their pathogenicity.
Main text
BRCA1 (breast cancer 1, early onset; OMIM: ∗113705) is a tumor-suppressor gene whose protein product is associated with several biological processes such as DNA damage repair, transcription, ubiquitylation, cell-cycle checkpoints, and centrosome duplication.1,2,3,4 Carriers of BRCA1 (NM_007294.4) germline pathogenic variants are predisposed to hereditary breast and ovarian cancer (HBOC) syndrome. Individuals harboring a pathogenic BRCA1 variant have cumulative risks of 72% and 44% by the age of 80 years for breast and ovarian cancers, respectively, making the identification of at-risk individuals essential for better risk reduction and therapeutic management.5 Despite recent progress in risk assessment and variant classification, a large portion of BRCA1 variants (∼37% in ClinVar) remain as variants of uncertain clinical significance (VUSs).6,7
The BRCA1 protein contains a RING-finger domain at its N-terminal region and two BRCT (BRCA1 C-terminal) domains in tandem at its C-terminal portion. The tandem BRCT (tBRCT) acts as a single structural unit.8,9 The importance of the structural integrity of the BRCT domain was initially exemplified by the known pathogenic nature of a nonsense variant p.(Tyr1853Ter), which results in a loss of the last 11 amino acid residues at the C-terminal end of the domain.10 Thus, any premature protein termination upstream to the Tyr1853 codon is inferred to be pathogenic. Despite extensive efforts to catalog and characterize BRCT VUSs, little is known about frameshift variants that encode extended (beyond the final amino acid residue 1863 of the reference sequence) incorrect termini.
Extended incorrect terminus variants (EITs) have been previously observed in different disease-associated genes. For example, a loss-of-function EIT was reported in the pseudohypoaldosteronism type 1 gene NR3C2, leading to a marked reduction in expression, which could explain its functional impact.11 On the other hand, it has been shown that a WT1 (Wilms tumor 1) EIT can lead to a gain-of-function phenotype, promoting WT cell proliferation.12 These data illustrate that EITs may not necessarily lead to loss of function. Importantly, there have been no comprehensive functional evaluation of EITs.
Only six BRCA1 frameshift variants (c.68_69del, c.4243_4244insT, c.4936del, c.5266dup, c.5335del, and c.5553dup) have been functionally assessed to date (Table S1). Only one leads to an extended incorrect terminus (c.5553dup), while the other variants lead to premature protein termination. All were shown to display loss of function and are considered pathogenic by ClinVar (Table S1). We have previously assessed the impact of the c.5553dup (p.(Thr1852HisfsTer28); 5673insC, Breast Cancer Information Core, BIC designation), the only functionally assessed frameshift that leads to an extended incorrect terminus in BRCA1 by probing its impact on BRCA1 transcription activity. This frameshift variant produces a BRCA1 protein with the last 12 reference amino acid residues replaced with 27 incorrect ones at the C terminus and results in a striking reduction of BRCA1 activity.13
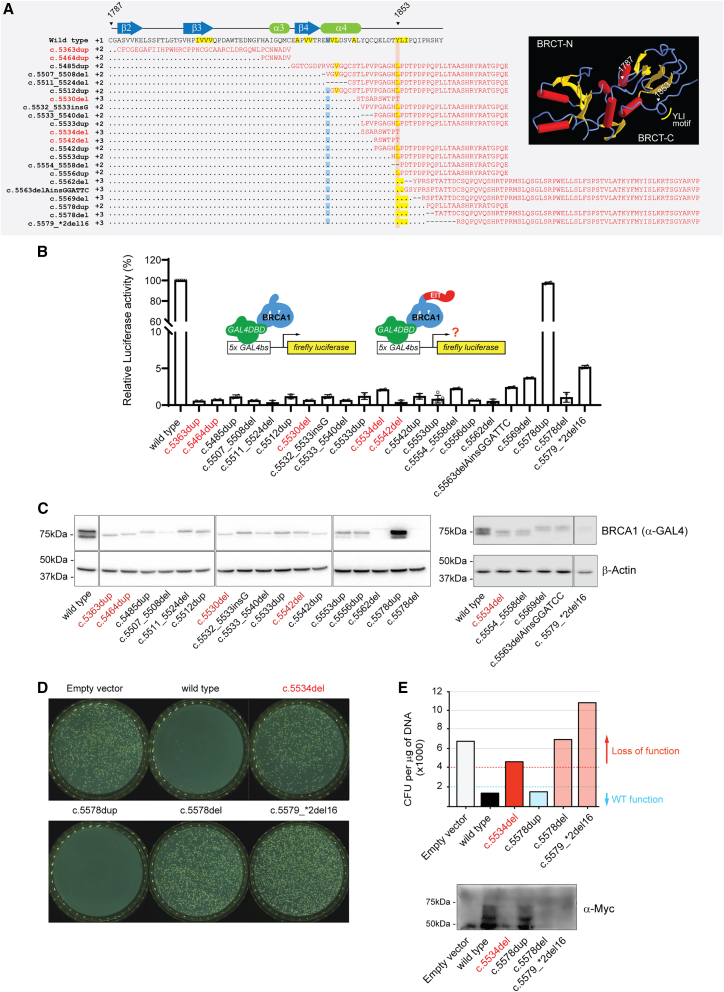
To systematically assess the functional impact of EITs in BRCA1, we evaluated a set of 22 frameshift variants including 17 EITs. Five frameshift variants that lead to premature protein termination (c.5363dup, c.5464dup, c.5530del, c.5534del, and c.5542del) were used as pathogenic controls (Table 1; see Figure S1 for an outline of the study design). The 17 EITs result in the addition of a variable number of incorrect residues at the two incorrect (+2 and +3) reading frames, extending into the noncoding region of the BRCA1 3′ UTR region (Figure 1A; Table 1).
Table 1.
BRCA1 frameshift variants: Annotation and summary of results
| Frameshift variant (nt) | Classa | HGVS variant (aa) | Indel (nt) | Number of incorrect amino acid residuesb | Number of lost correct amino acid residuesc | dbSNP | ClinVard and review statuse | Number of entries in ClinVar | Number of observations in BRCA LOVDf | Allele frequency (gnomad) | This study |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of observations (Ambry) | Number of families observed | TA VarCall fClassg | TA AMP/ACMG codeh | HR efficiencyi | HR AMP/ACMG codeh | |||||||||||
| c.5363dup | PPT | p.(Ala1789CysfsTer41) | G | 40 | 75 | – | – | – | 2 | – | – | – | 5 | PS3 | – | – |
| c.5464dup | PPT | p.(His1822ProfsTer8) | C | 7 | 42 | rs1567757816 | – | – | 2 | – | – | – | 5 | PS3 | – | – |
| c.5485dup | EIT | p.(Glu1829GlyfsTer51) | G | 50 | 35 | rs768401297 | P (3) | 2 | 6 | 0.000004 | – | – | 5 | PS3 | – | – |
| c.5507_5508del | EIT | p.(Glu1836ValfsTer43) | AG | 42 | 28 | – | – | – | 1 | – | – | – | 5 | PS3 | – | – |
| c.5511_5524del | EIT | p.(Trp1837CysfsTer38) | GGTGTTG GACAGTG |
37 | 27 | – | – | – | 1 | – | – | – | 5 | PS3 | – | – |
| c.5512dup | EIT | p.(Val1838GlyfsTer42) | G | 41 | 26 | – | – | – | 1 | – | – | – | 5 | PS3 | – | – |
| c.5530del | PPT | p.(Leu1844SerfsTer11) | C | 10 | 20 | – | P (n/r) | 1 | 3 | – | – | – | 5 | PS3 | – | – |
| c.5532_5533insG | EIT | p.(Tyr1845ValfsTer35) | G | 34 | 19 | rs397509293 | – | 1 | – | – | – | – | 5 | PS3 | – | – |
| c.5533_5540del | EIT | p.(Tyr1845ProfsTer32) | TACCAGTG | 31 | 19 | – | – | – | 1 | – | – | – | 5 | PS3 | – | – |
| c.5533dup | EIT | p.(Tyr1845LeufsTer35) | T | 34 | 19 | rs397509294 | P (3) | 4 | 2 | – | 1 | – | 5 | PS3 | – | – |
| c.5534del | PPT | p.(Tyr1845SerfsTer10) | A | 9 | 19 | rs1060505048 | P (3) | 3 | – | – | 1 | – | 5 | PS3 | Def. | PS3 |
| c.5542del | PPT | p.(Gln1848ArgfsTer7) | C | 6 | 16 | rs2152575457 | – | – | 1 | – | – | – | 5 | PS3 | – | – |
| c.5542dup | EIT | p.(Gln1848ProfsTer32) | C | 31 | 16 | – | – | – | – | – | – | – | 5 | PS3 | – | – |
| c.5553dup | EIT | p.(Thr1852HisfsTer28) | C | 27 | 12 | rs397509297 | P (3) | 1 | 2 | – | – | – | 5 | PS3 | – | – |
| c.5554_5558del | EIT | p.(Thr1852ProfsTer26) | ACCTA | 25 | 12 | rs1597796682 | P (1) | 1 | – | – | 1 | – | 5 | PS3 | – | – |
| c.5556dup | EIT | p.(Tyr1853LeufsTer27) | C | 26 | 11 | – | – | 1 | 2 | – | – | – | 5 | PS3 | – | – |
| c.5562del | EIT | p.(Ile1855TyrfsTer67) | G | 66 | 9 | rs886037795 | VUS (1) | 1 | – | – | – | – | 5 | PS3 | – | – |
| c.5563del AinsGGATTC |
EIT | p.(Ile1855GlyfsTer70) | delAins GGATTC |
68 | 9 | rs483353103 | conflict (1) | 2 | – | – | 1 | – | 5 | PS3 | – | – |
| c.5569del | EIT | p.(Gln1857ArgfsTer65) | C | 64 | 7 | rs886039675 | LP (2) | 4 | – | – | 2 | – | 5 | PS3 | – | – |
| c.5578dup | EIT | p.(His1860ProfsTer20) | C | 19 | 4 | rs397507254 | conflict (1) | 8 | 2 | – | 23 | 1 | 1 | BS3 | Prof. | BS3 |
| c.5578del | EIT | p.(His1860ThrfsTer62) | C | 61 | 4 | rs397507254 | LP (1) | 2 | – | – | – | 7 | 5 | PS3 | Def. | PS3 |
| c.5579_∗2del16 | EIT | p.(His1860ArgfsTer57) | ACAGCCA CTACTGAct |
56 | 4 | – | VUS (1) | 1 | – | – | 1 | – | 5 | PS3 | Def. | PS3 |
PPT (premature protein termination) variants used as (loss-of-function) pathogenic controls; EIT, extended incorrect terminus variants.
Number of incorrect amino acid residues downstream of reported mutation.
Number of correct amino acid residues lost downstream of reported mutation.
P, pathogenic; LP, likely pathogenic; conflict, conflicting interpretations; VUS, variant of uncertain significance.
ClinVar review status: 1 = criteria provided, single submitter; 2 = criteria provided multiple submitters; 3 = reviewed by expert panel; n/r, not reported; n/p, not provided.
LOVD, Leiden Open Variation database, obtained through BRCA Exchange.
TA VarCall functional classes: fClass 1 (nonpathogenic; PrDel ≤ 0.001), fClass 2 (likely not pathogenic; 0.001< PrDel ≤ 0.05), fClass 3 (uncertain; 0.05 > PrDel ≤ 0.95), fClass 4 (likely pathogenic; 0.95 < PrDel ≤ 0.99), and fClass 5 (pathogenic; PrDel > 0.99).
AMP/ACMG evidence strength codes: PS3, strong evidence for pathogenicity (odds of pathogenicity > 18.7); BS3, strong evidence for benignity (odds of pathogenicity <0.053); n/a, not applicable.
HR, homologous recombination; Prof., proficient; Def., deficient.
Figure 1.
Functional assessment of BRCA1 EITs
(A) Protein amino acid sequence alignment of human BRCA1 C-terminal wild-type sequence (aa 1787–1863; NP_009225.1) and frameshift variants assessed in this study. Vertical red highlight line indicates position of codon 1853. Residues in red represent incorrect protein sequences. Highlighted positions indicate hydrophobic residues (yellow) and tryptophan 1837 (blue) conserved in BRCT domains. Premature protein termination (PPT) variants are shown in red font. Inset, PDB image 1JNX of BRCA1 tandem BRCT highlighting features in the amino acid sequence.
(B) Transcription activity of EITs and PPT controls (c.5363dup, c.5464dup, c.5530del, c.5534del, and c.5542del) relative to wild-type BRCA1 in HEK293FT cells.
(C) BRCA1 protein levels in whole-cell extracts from transfected HEK293FT cells by immunoblotting using anti-GAL4 DNA-binding domain (DBD) and anti-β-actin.
(D) Small colony phenotype assay in AH109 yeast cells overexpressing a Myc-tagged GAL4 DBD fusion of wild-type BRCA1, c.5578dup, c.5578del, c.5579_∗2del16, or c.5534del (pathogenic control). Overexpression of wild-type BRCA1, but not pathogenic variants, leads to a small colony phenotype.
(E) Number of colony-forming units (CFUs) per mL observed in plates shown in (D). BRCA1 protein levels of constructs in whole-cell extracts from transformed AH109 cells by immunoblotting using anti-Myc.
First, we tested the functional impact of these variants using a validated transcription activation (TA) assay.14,15 The variants were generated by site-directed mutagenesis in a construct that ectopically expresses the BRCT region fused to the GAL4 DNA-binding domain (DBD) in human cells.16 Activity was measured using a luciferase reporter under the control of GAL4 binding sites (supplemental information).14 While benign ectopic BRCA1 variants drive expression of the reporter, pathogenic variants do not.
Consistent with previous data,14 a loss of more than seven amino acid residues at the C terminus of BRCA1 resulted in low TA activity (<5% of the wild-type activity) (Figure 1B; Tables 1 and S2). Interestingly, steady-state protein levels were also markedly low (<20% of the ectopically expressed wild-type BRCA1 C terminus) for 16 of the 17 EITs, which presented levels similar to those of premature termination variants (Figure 1C). These results provide functional assay evidence against pathogenicity (AMP/ACMG BS3 code)15 for the c.5578dup and for pathogenicity (AMP/ACMG PS3 code) for the other 16 variants (Table 1) and suggest that their loss of activity is due, at least in part, to protein instability.
To estimate the likelihood of pathogenicity (i.e., increased risk of breast and ovarian cancer, typically relative risk >4) of each variant given the results from the functional assays, we used the VarCall algorithm, a Bayesian two-component (i.e., damaging and not damaging) mixture model developed to integrate functional assay results to classify BRCA1 variants, in a joint analysis with published data from 347 variants.14,17 VarCall calculates the probability of pathogenicity of a variant given its functional data output; therefore, it can determine their pathogenicity irrespective of the underlying genetic change (i.e., missense, frameshift, or nonsense) (supplemental information). All 16 loss-of-function variants were categorized as functional class (fClass) 5 (pathogenic; Probability of being Deleleterious, PrDel > 0.99), while the c.5578dup variant was fClass 1 (nonpathogenic; PrDel ≤ 0.001) (Tables 1, S3, and S4; Figures 2 and S2).
Figure 2.
VarCall estimated variant-specific effects
High-resolution graph depicting boxplots summarizing the marginal posterior distributions of the variant-specific effect parameters generated by VarCall for all BRCA1 EITs and PPTs (blue boxes) tested in the current study. To facilitate visualization, this is a zoomed-in version of the complete graph (shown in Figure S2).
Interestingly, the two distinct variants located at nucleotide 5578 (c.5578dup and c.5578del) display opposite functional results. Both variants, while sharing the same wild-type residues up to the point of the single-nucleotide alteration, encode different extended incorrect termini (Figure 1A). The c.5578dup variant consistently presented >80% activity of the ectopically expressed wild-type BRCA1 C terminus, while the activity of c.5578del was severely compromised (Figure 1B). This was reflected on protein levels, with c.5578del resulting in undetectable levels and c.5578dup presenting levels comparable to the ectopically expressed wild-type BRCA1 C terminus (Figure 1C).
To further investigate the structure-function relationship of the c.5578dup variant, we took advantage of two additional EITs in our study (c.5578del and c.5579_∗2del16) that, like the c.5578dup, retained the Tyr1853-Leu1854-Iso1855 hydrophobic cluster, thought to be required for the stability of the BRCT. In addition, these variants lead to the loss of the same number of correct amino acid residues as the c.5578dup but with distinct incorrect termini (Figure 1).
The marked difference in protein steady-state levels between the c.5578dup on one hand and the c.5578del and c.5579_∗2del16 variants on the other raised the hypothesis that protein instability could explain their loss of function. We had previously observed that the expression of some moderately destabilizing human BRCA1 variants at 30°C can mitigate their protein instability, resulting in increased protein levels and, in some cases, restoration of protein activity.18,19 Thus, we assessed the impact of all three variants in a yeast-based assay (performed at 30°C). Yeast small colony phenotype assays were conducted in Saccharomyces cerevisiae AH109 cells expressing GAL4 DBD fusions of BRCT variants (supplemental information). The overexpression of wild-type or benign human BRCA1 variants, but not pathogenic variants, in yeast leads to a small colony phenotype in a BRCT-dependent manner.20,21,22 Even at 30°C, the c.5578del and c. c.5579_∗2del16 variants failed to show activity and led to the growth of average-size colonies, comparable to the c.5534del pathogenic control (Figures 1D and 1E), indicating that c.5578del and c. c.5579_∗2del16 lead to a severely destabilized protein.
The c.5578dup variant retained activity in both mammalian TA and in the yeast small colony phenotype assays, strongly suggesting that it is a benign variant. However, a limitation of our model is that it is based on the overexpression of the C-terminal segment of BRCA1; therefore, the impact of these variants in mRNA and protein stability in a full-length context is not assessed. This prompted us to further interrogate the functional impact of c.5578dup in the context of full-length BRCA1.
We then determined the impact of these variants in the full-length BRCA1 protein on homologous recombination (HR), the well-established function of BRCA1,23 using our previously validated DR-GFP reporter assay.24 In this model, cells silenced for endogenous BRCA1 are assessed for HR activity driven by the ectopic expression of full-length human BRCA1 variants. The premature protein termination (PPT) variant c.5534del was included as a pathogenic control. The c.5578dup variant displayed HR activity levels that were similar to the ectopic wild-type BRCA1, while the other two variants had a markedly reduced activity (Figure 3A). In addition, while c.5578dup was expressed at a level comparable to the ectopic wild-type protein, c.5534del, c.5578del, and c.5579_∗2del16 all showed strongly reduced protein levels (Figure 3B), consistent with the observation made with the fusion protein described earlier (Figure 1C). When the proteins were immunoprecipitated, c.5578dup showed similar levels of association with known BRCA1-BRCT binding partners BRIP125 and ABRAXAS26 to that of the ectopic wild-type protein, while the amounts of these two binding partners brought down by c.5534del, c.5578del, and c.5579_∗2del16 were reduced (Figures 3B and S3). However, while c.5534del completely failed to bind BRIP1 and ABRAXAS, c.5578del and c.5579_∗2del16 clearly were able to do so, even though their protein levels were lower than that of c.5534del. BRCA1 interacts with PALB2, another tumor-suppressor protein, to initiate HR.27,28,29 Finally, c.5534del, c.5578del, and c.5579_∗2del16 all brought down reduced amounts of PALB2, which interacts with a coiled-coil (CC) motif (aa 1397–1424) in BRCA1 far upstream of the tBRCT domains (aa 1646–1736 and 1760–1855),27 and the reduction appeared to be largely proportional to the reduction of the EIT’s protein amount (Figures 3B and S3). Structurally, the final three residues of BRCA1 are in a highly flexible region with few interactions with the core of the second BRCT domain.30 While c.5578dup retains the high predicted disorder propensity of the wild-type C terminus, both c.5578del and c.5579_∗2del16 exhibit a stretch of 20 residues with low predicted disorder propensity,31 raising the possibility that these longer extensions may fold with, or alter, the structure of the second BRCT domain. These data, consistent with the TA results, indicate that the BRCA1 variant c.5578dup is stable and HR repair proficient (AMP/ACMG BS3 code). On the other hand, c.5578del and c.5579_∗2del16 appear to be unstable but retain substantial BRIP binding and partial ABRAXAS binding capacities. It is likely that the reduced protein level and diminished ABRAXAS binding capacity of c.5578del and c.5579_∗2del16 combine to render these two variants functionally deficient (AMP/ACMG PS3 code).
Figure 3.
Homologous recombination (HR) assay and family history (FHx) analysis
(A) Relative HR activities of the BRCA1 variants studied. Variants were introduced into full-length BRCA1 with 3XMyc tag at the N terminus and their activities assessed in U2OS/DR-GFP cells depleted of the endogenous BRCA1. Activity values were normalized against that of the WT BRCA1. Error bars represent standard deviations from 3 independent experiments.
(B) Expression levels of the variants studied and their binding capacities to known BRCA1 interacting partners in 293T cells. Cells were transfected with an empty vector (EV) or 3XMyc-tagged BRCA1 constructs. Whole-cell extracts (WCEs) were subjected to direct western blotting or immunoprecipitation (IP) with anti-Myc antibody followed by western blotting analysis as indicated.
(C) FHx likelihood model assessment of aggregate FHx for 23 carriers of c.5578dup (blue vertical line) plotted relative to the distribution of simulated control groups for benign (green curve) and pathogenic (red curve) variants. Dashed and solid vertical lines represent the 95th and 99th percentiles for the control curves. One count refers to one simulated dataset with the same number of probands as the test dataset. Each curve in the graph, benign and pathogenic, is comprised of 100,000 simulated datasets with the same number of probands as the test dataset, and count refers to the number of those simulated datasets. LLR, log likelihood ratio.
The BRCA1 c.5578del variant has so far been found in seven individual/families undergoing clinical screening for hereditary breast cancer in Sweden. Two of them have an extensive family history of breast and ovarian cancer. The index of family 1 had bilateral breast cancer and three primary tumors at ages 47, 49, and 65: two of them were triple-negative (estrogen receptor-negative, progesterone receptor-negative, HER2-negative) breast cancer, and the third was a luminal B tumor, consistent with a BRCA1-linked tumor.32,33,34 Her sister had triple-negative breast cancer at age 60 but was found not to carry the variant. A paternal aunt and cousin diagnosed with ovarian cancer at ages 60 and 64, respectively, have not been tested. Family 2 includes two sisters with serous ovarian cancer diagnosed at ages 44 and 62, both carriers of the variant. Their father had a history of esophageal cancer diagnosed at age 64 and was also a carrier. A paternal cousin was diagnosed with ovarian cancer at age 60 and was not a carrier. Nevertheless, the clinical context of BRCA1 c.5578del seems to be similar to other protein-truncating BRCA1 variants, despite the incomplete segregation and phenocopy in the two families. The remaining five families are as yet not informative.
The c.5578dup variant was found in a 64-year-old female with epithelial ovarian cancer diagnosed at age 63. She is of Filipino ancestry and has eight sisters, all unaffected, and 1 of 3 of her brothers was diagnosed with prostate cancer at age 78. She also had a maternal uncle with a possible diagnosis of prostate cancer but no other family history of cancer (family 8). Twenty-three additional carriers of c.5578dup were identified at a US clinical diagnostic lab. These probands, who were largely of Filipino descent, underwent multigene panel testing for hereditary cancer. Applying a family history likelihood model (supplemental information) resulted in a log likelihood ratio of −4.16, providing strong evidence of benignity (Figure 3C). The family history (FHx) modeling results, in combination with the functional analysis herein, resulted in a likely benign interpretation from the participating clinical diagnostic laboratory (ACMG/AMP BS3 and BS2). Due to the rarity and low number of cases with the other EIT alterations, we were unable to use FHx modeling to assess the pathogenicity of the other EIT BRCA1 variants.
In summary, we provide a comprehensive functional evaluation of all BRCA1 EITs reported to date. We used validated functional assays following AMP/ACMG guidelines, which provide calibrated evidence criteria for clinical classification.35 This analysis resulted in PS3 codes, equivalent to strong evidence of pathogenicity (odds of pathogenicity > 18.7) being applied to 16 EITs (Table 1). For three EITs, we obtained an additional PS3 score from an independent functional assay. We also assign PS3 codes to three PPT variants that have not yet been reviewed by the expert panel in ClinVar.
The mechanisms by which coding pathogenic variants display decreased activity include unstable mRNA targeted for nonsense-mediated decay (NMD), splicing alterations, misfolded or unstable proteins, or structural changes that disrupt protein-protein interactions.36 Only mutations upstream of codon 5418 (50 amino acid residues from the final intron/exon junction) are expected to trigger NMD; therefore, most of the variants tested in the current study (except c.5363dup) are not expected to be degraded. Likewise, none of the 22 variants are predicted to affect splicing by the SpliceAI deep-learning tool, even when using a relatively permissive delta score threshold (i.e., delta score > 0.2)37,38 (Table S5). However, assays used in our study were cDNA based and independent of the endogenous BRCA1 and cannot directly assess the contribution of these mechanisms to reduced activity. Pathogenicity can also be achieved through dominant-negative activity, but somatic mutation data and mouse model experiments suggest that this is not a common event in BRCA1-linked cancers.39 Our studies indicate that protein instability leading to reduced steady-state levels combined with structural changes affecting protein-protein interactions contribute to the mechanisms leading to loss of activity.
Notwithstanding the strength of the evidence provided, the functional evidence criteria are not meant to be stand-alone evidence, and at least one other evidence type is required to reach a pathogenic classification for clinical purposes. Additional evidence is very limited for rare variants, and the information provided here will be instrumental for a final clinical classification. For one EIT (c.5578dup), which is currently listed as conflicting classification in ClinVar, we provide unambiguous evidence for clinical classification as benign. Both functional assays provide the strongest AMP/ACMG functional evidence for benignity, BS3 (odds of pathogenicity < 0.053), and the FHx contributes to classification with a BS2 code.
Collectively, our analysis supports the notion that most frameshift changes in BRCA1 that produce extended incorrect termini are likely to lead to loss of function, even when they retain intact conserved motifs in the BRCT domains (e.g., Trp1837 and the Tyr1853-Leu1854-Iso1855 hydrophobic cluster40,41), at least partially due to protein instability. There is strong evidence that these variants are pathogenic. Conversely, the c.5578dup variant is likely to produce a functional protein and to represent a likely benign variant. Interestingly, other EITs that share amino acid segments coded by the same incorrect frame (+2) or the same start of the incorrect peptide at a different incorrect frame (+3) with the c.5578dup show severe loss of function. This study highlights the need for individual assessment of complex variants to correctly identify individuals at risk. The data presented here build on the characterization of BRCA1 variants and identify variants located at the C-terminal region of the protein that are likely associated with an elevated risk of breast and ovarian cancer.
Data and code availability
The published article includes all the functional datasets generated or analyzed during this study. The clinical datasets supporting the current study are available as aggregate data upon request but, in accordance with HIPAA compliance, have not been deposited.
Acknowledgments
This study was supported by awards from the Sarasota Innovation Fund/Moffitt Foundation (A.N.A.M.), NIH R35 CA253187 (F.J.C.), the Breast Cancer Research Foundation (F.J.C.), NIH R01CA138804 (B.X.), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (M.A.C.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (M.A.C.), and Fundação do Câncer (Programa de Oncobiologia) (M.A.C.). T.C.N. is the recipient of the Ann and Sol Schreiber Mentored Investigator Award from the Ovarian Cancer Research Alliance. The Molecular Genomics Core is supported in part by an NCI Cancer Center Support Grant (P30-CA076292).
Author contributions
Conceptualization, T.C.N., M.A.C., and A.N.A.M.; data curation, T.C.N., T.K.F., J.M.O.R., A.v.W., D.E., R.K., S.A., and E.I.; resources, T.C.N., T.K.F., M.E.R., J.M.O.R., J.W., M.J.V., A.A., D.G., A.v.W., D.E., R.K., S.A., E.I., F.J.C., A.B., B.X., M.A.C., and A.N.A.M.; formal analysis, T.C.N., T.K.F., M.E.R., J.M.O.R., E.I., B.X., M.A.C., and A.N.A.M.; funding acquisition, F.J.C., A.B., B.X., M.A.C., and A.N.A.M.; investigation, T.C.N., T.K.F., M.E.R., J.M.O.R., J.W., M.J.V., A.v.W., D.E., R.K., S.A., E.I., F.J.C., A.B., B.X., M.A.C., and A.N.A.M.; visualization, T.C.N., T.K.F., J.M.O.R., E.I., B.X., M.A.C., and A.N.A.M.; supervision, M.E.R., A.B., B.X., M.A.C., and A.N.A.M.; writing – original draft, T.C.N., M.A.C., and A.N.A.M.; writing – review & editing, all authors.
Declaration of interests
M.E.R., J.M.O.R., J.W., and M.J.V. are full-time, salaried employees of Ambry Genetics.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xhgg.2023.100240.
Contributor Information
Marcelo A. Carvalho, Email: marcelo.carvalho@ifrj.edu.br.
Alvaro N.A. Monteiro, Email: alvaro.monteiro@moffitt.org.
Supplemental information
References
- 1.Yoshino Y., Fang Z., Qi H., Kobayashi A., Chiba N. Dysregulation of the centrosome induced by BRCA1 deficiency contributes to tissue-specific carcinogenesis. Cancer Sci. 2021;112:1679–1687. doi: 10.1111/cas.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao W., Wiese C., Kwon Y., Hromas R., Sung P. The BRCA Tumor Suppressor Network in Chromosome Damage Repair by Homologous Recombination. Annu. Rev. Biochem. 2019;88:221–245. doi: 10.1146/annurev-biochem-013118-111058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X., Li R. BRCA1-Dependent Transcriptional Regulation: Implication in Tissue-Specific Tumor Suppression. Cancers. 2018;10 doi: 10.3390/cancers10120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witus S.R., Stewart M.D., Klevit R.E. The BRCA1/BARD1 ubiquitin ligase and its substrates. Biochem. J. 2021;478:3467–3483. doi: 10.1042/BCJ20200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuchenbaecker K.B., Hopper J.L., Barnes D.R., Phillips K.A., Mooij T.M., Roos-Blom M.J., Jervis S., van Leeuwen F.E., Milne R.L., Andrieu N., et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 6.Parsons M.T., Tudini E., Li H., Hahnen E., Wappenschmidt B., Feliubadaló L., Aalfs C.M., Agata S., Aittomäki K., Alducci E., et al. Large scale multifactorial likelihood quantitative analysis of BRCA1 and BRCA2 variants: An ENIGMA resource to support clinical variant classification. Hum. Mutat. 2019;40:1557–1578. doi: 10.1002/humu.23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteiro A.N., Bouwman P., Kousholt A.N., Eccles D.M., Millot G.A., Masson J.Y., Schmidt M.K., Sharan S.K., Scully R., Wiesmüller L., et al. Variants of uncertain clinical significance in hereditary breast and ovarian cancer genes: best practices in functional analysis for clinical annotation. J. Med. Genet. 2020;57:509–518. doi: 10.1136/jmedgenet-2019-106368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Periasamy J., Kurdekar V., Jasti S., Nijaguna M.B., Boggaram S., Hurakadli M.A., Raina D., Kurup L.M., Chintha C., Manjunath K., et al. Targeting Phosphopeptide Recognition by the Human BRCA1 Tandem BRCT Domain to Interrupt BRCA1-Dependent Signaling. Cell Chem. Biol. 2018;25:677–690.e12. doi: 10.1016/j.chembiol.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams R.S., Green R., Glover J.N. Crystal structure of the BRCT repeat region from the breast cancer- associated protein BRCA1. Nat. Struct. Biol. 2001;8:838–842. doi: 10.1038/nsb1001-838. [DOI] [PubMed] [Google Scholar]
- 10.Friedman L.S., Ostermeyer E.A., Szabo C.I., Dowd P., Lynch E.D., Rowell S.E., King M.C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat. Genet. 1994;8:399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- 11.Kawashima Sonoyama Y., Tajima T., Fujimoto M., Hasegawa A., Miyahara N., Nishimura R., Hashida Y., Hayashi A., Hanaki K., Kanzaki S. A novel frameshift mutation in NR3C2 leads to decreased expression of mineralocorticoid receptor: a family with renal pseudohypoaldosteronism type 1. Endocr. J. 2017;64:83–90. doi: 10.1507/endocrj.EJ16-0280. [DOI] [PubMed] [Google Scholar]
- 12.Busch M., Schwindt H., Brandt A., Beier M., Görldt N., Romaniuk P., Toska E., Roberts S., Royer H.D., Royer-Pokora B. Classification of a frameshift/extended and a stop mutation in WT1 as gain-of-function mutations that activate cell cycle genes and promote Wilms tumour cell proliferation. Hum. Mol. Genet. 2014;23:3958–3974. doi: 10.1093/hmg/ddu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho M., Pino M.A., Karchin R., Beddor J., Godinho-Netto M., Mesquita R.D., Rodarte R.S., Vaz D.C., Monteiro V.A., Manoukian S., et al. Analysis of a set of missense, frameshift, and in-frame deletion variants of BRCA1. Mutat. Res. 2009;660:1–11. doi: 10.1016/j.mrfmmm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes V.C., Golubeva V.A., Di Pietro G., Shields C., Amankwah K., Nepomuceno T.C., de Gregoriis G., Abreu R.B.V., Harro C., Gomes T.T., et al. Impact of amino acid substitutions at secondary structures in the BRCT domains of the tumor suppressor BRCA1: Implications for clinical annotation. J. Biol. Chem. 2019;294:5980–5992. doi: 10.1074/jbc.RA118.005274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyra P.C.M., Jr., Nepomuceno T.C., de Souza M.L.M., Machado G.F., Veloso M.F., Henriques T.B., Dos Santos D.Z., Ribeiro I.G., Ribeiro R.S., Jr., Rangel L.B.A., et al. Integration of functional assay data results provides strong evidence for classification of hundreds of BRCA1 variants of uncertain significance. Genet. Med. 2021;23:306–315. doi: 10.1038/s41436-020-00991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes F., Cayanan C., Barillà D., Monteiro A.N. Functional assay for BRCA1: mutagenesis of the COOH-terminal region reveals critical residues for transcription activation. Cancer Res. 2000;60:2411–2418. [PMC free article] [PubMed] [Google Scholar]
- 17.Iversen E.S., Couch F.J., Goldgar D.E., Tavtigian S.V., Monteiro A.N.A. A Computational Method to Classify Variants of Uncertain Significance Using Functional Assay Data with Application to BRCA1. Cancer Epidemiol. Biomark. Prev. 2011;20:1078–1088. doi: 10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho M.A., Billack B., Chan E., Worley T., Cayanan C., Monteiro A.N.A. Mutations in the BRCT Domain Confer Temperature Sensitivity to BRCA1 in Transcription Activation. Cancer Biol. Ther. 2002;1:502–508. doi: 10.4161/cbt.1.5.165. [DOI] [PubMed] [Google Scholar]
- 19.Worley T., Vallon-Christersson J., Billack B., Borg A., Monteiro A.N.A. A naturally occurring allele of BRCA1 coding for a temperature-sensitive mutant protein. Cancer Biol. Ther. 2002;1:497–501. doi: 10.4161/cbt.1.5.164. [DOI] [PubMed] [Google Scholar]
- 20.Humphrey J.S., Salim A., Erdos M.R., Collins F.S., Brody L.C., Klausner R.D. Human BRCA1 inhibits growth in yeast: potential use in diagnostic testing. Proc. Natl. Acad. Sci. USA. 1997;94:5820–5825. doi: 10.1073/pnas.94.11.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyne R.S., McDonald H.B., Edgemon K., Brody L.C. Functional Characterization of BRCA1 Sequence Variants Using a Yeast Small Colony Phenotype Assay. Cancer Biol. Ther. 2004;3:453–457. doi: 10.4161/cbt.3.5.809. [DOI] [PubMed] [Google Scholar]
- 22.Thouvenot P., Ben Yamin B., Fourrière L., Lescure A., Boudier T., Del Nery E., Chauchereau A., Goldgar D.E., Houdayer C., Stoppa-Lyonnet D., et al. Functional Assessment of Genetic Variants with Outcomes Adapted to Clinical Decision-Making. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moynahan M.E., Chiu J.W., Koller B.H., Jasin M. Brca1 controls homology-directed DNA repair. Mol. Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 24.Anantha R.W., Simhadri S., Foo T.K., Miao S., Liu J., Shen Z., Ganesan S., Xia B. Functional and mutational landscapes of BRCA1 for homology-directed repair and therapy resistance. Elife. 2017;6 doi: 10.7554/eLife.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantor S.B., Bell D.W., Ganesan S., Kass E.M., Drapkin R., Grossman S., Wahrer D.C., Sgroi D.C., Lane W.S., Haber D.A., Livingston D.M. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001;105:149–160. doi: 10.1016/s0092-8674(01)00304-x. [DOI] [PubMed] [Google Scholar]
- 26.Wang B., Matsuoka S., Ballif B.A., Zhang D., Smogorzewska A., Gygi S.P., Elledge S.J. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science. 2007;316:1194–1198. doi: 10.1126/science.1139476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foo T.K., Xia B. BRCA1-Dependent and Independent Recruitment of PALB2-BRCA2-RAD51 in the DNA Damage Response and Cancer. Cancer Res. 2022;82:3191–3197. doi: 10.1158/0008-5472.Can-22-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F., Ma J., Wu J., Ye L., Cai H., Xia B., Yu X. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr. Biol. 2009;19:524–529. doi: 10.1016/j.cub.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sy S.M.H., Huen M.S.Y., Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc. Natl. Acad. Sci. USA. 2009;106:7155–7160. doi: 10.1073/pnas.0811159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaiser O.J., Ball L.J., Schmieder P., Leitner D., Strauss H., Wahl M., Kühne R., Oschkinat H., Heinemann U. Solution structure, backbone dynamics, and association behavior of the C-terminal BRCT domain from the breast cancer-associated protein BRCA1. Biochemistry. 2004;43:15983–15995. doi: 10.1021/bi049550q. [DOI] [PubMed] [Google Scholar]
- 31.Mészáros B., Erdos G., Dosztányi Z. IUPred2A: context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 2018;46:W329–W337. doi: 10.1093/nar/gky384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 33.Stevens K.N., Vachon C.M., Couch F.J. Genetic susceptibility to triple-negative breast cancer. Cancer Res. 2013;73:2025–2030. doi: 10.1158/0008-5472.CAN-12-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metcalfe K., Lubinski J., Lynch H.T., Ghadirian P., Foulkes W.D., Kim-Sing C., Neuhausen S., Tung N., Rosen B., Gronwald J., et al. Family history of cancer and cancer risks in women with BRCA1 or BRCA2 mutations. J. Natl. Cancer Inst. 2010;102:1874–1878. doi: 10.1093/jnci/djq443. [DOI] [PubMed] [Google Scholar]
- 35.Brnich S.E., Abou Tayoun A.N., Couch F.J., Cutting G.R., Greenblatt M.S., Heinen C.D., Kanavy D.M., Luo X., McNulty S.M., Starita L.M., et al. Recommendations for application of the functional evidence PS3/BS3 criterion using the ACMG/AMP sequence variant interpretation framework. Genome Med. 2019;12:3. doi: 10.1186/s13073-13019-10690-13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabo C.I., Worley T., Monteiro A.N.A. Understanding germ-line mutations in BRCA1. Cancer Biol. Ther. 2004;3:515–520. doi: 10.4161/cbt.3.6.841. [DOI] [PubMed] [Google Scholar]
- 37.Jaganathan K., Kyriazopoulou Panagiotopoulou S., McRae J.F., Darbandi S.F., Knowles D., Li Y.I., Kosmicki J.A., Arbelaez J., Cui W., Schwartz G.B., et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell. 2019;176:535–548.e24. doi: 10.1016/j.cell.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Riepe T.V., Khan M., Roosing S., Cremers F.P.M., t Hoen P.A.C. Benchmarking deep learning splice prediction tools using functional splice assays. Hum. Mutat. 2021;42:799–810. doi: 10.1002/humu.24212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hohenstein P., Fodde R. Of mice and (wo)men: genotype-phenotype correlations in BRCA1. Hum.Mol.Genet 12 Spec No. Hum. Mol. Genet. 2003;12 Spec No 2:R271–R277. doi: 10.1093/hmg/ddg258. [DOI] [PubMed] [Google Scholar]
- 40.Callebaut I., Mornon J.P. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. doi: 10.1016/s0014-5793(96)01312-9. [DOI] [PubMed] [Google Scholar]
- 41.Bork P., Hofmann K., Bucher P., Neuwald A.F., Altschul S.F., Koonin E.V. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. Faseb. J. 1997;11:68–76. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all the functional datasets generated or analyzed during this study. The clinical datasets supporting the current study are available as aggregate data upon request but, in accordance with HIPAA compliance, have not been deposited.