Abstract
Background
Food allergy (FA) has become a major public health concern affecting millions of children and adults worldwide. In Tunisia, published data on FA are scarce.
Methods
This study, was intended to fill the gap and estimate the frequency of allergy to different foods in the Sfax region, Tunisia, within self-reported FA. One hundred twenty-five (125) children (56% males, 1–17 years old), and 306 adults (17% males, 18–70 years old) were interviewed using a bilingual questionnaire.
Results
The number of self-reported food allergens in this sample was 105; allergens were clustered in 8 foods: fruits, seafood, eggs, milk and dairy, cereals, nuts, vegetables, and peanuts. Cutaneous reactions were the most frequent symptoms, in both children and adults. About 40% of children and 30% of adults had a family history of FA. About 81% of adults and 38% of children are allergic to at least 1 non-food allergen. The most prevalent food allergen was the fruit group in both adults and children, followed by seafood. Most food allergies were mutually exclusive and 90% of individuals have a single FA. The relationship between self-declared FA was modeled using a Bayesian network graphical model in order to estimate conditional probabilities of each FA when other FA is present.
Conclusions
Our findings suggest that the prevalence of self-reported FA in Tunisia depends on dietary habits and food availability since the most frequent allergens are from foods that are highly consumed by the Tunisian population.
Keywords: Food allergy, Prevalence, Probabilistic modeling, Self-reported survey, Tunisia
Introduction
Adverse reactions to food were described in different historical reports since about the third millennia BC in China, including a pharaoh who died of anaphylaxis after a bee sting., and later through writings of Hippocrates (460-377BCE) and Razi (865–925). It was only in the seventeenth century that medical literature began to recognize food allergy (FA) as a disease and described it as a hypersensitive reaction, having clinical symptoms like urticaria or anaphylaxis.1
In the 1990s, the US National Institute of Allergy and Infectious Diseases (NIAID) and the European Academy of Allergy and Clinical Immunology (EAACI) defined FA as “an adverse health effect arising from an immune response that occurs reproducibly on exposure to a given food”.2,3 Currently, FA is considered as having a complex mechanism that encompasses many clinical entities resulting from complex interactions between food intake, metabolism, immune system, genetic background, and socioeconomic factors.4 This breakdown result varies from immediate, IgE-mediated anaphylaxis, and chronic eosinophilic gastrointestinal disorders (EGID) to cell-mediated, and in certain cases, to life-threatening. Today, FA is being described as an emergency health care issue, whose prevalence is in constant significant increase.5 For instance, Clarke and et al6 reported that in Canada self-reported FA increased from 7.1 to 9.3% between 2010 and 2016. While FA based on physician diagnosis or history remained stable (5.9% versus 6.1%). This increase in self-reported FA is likely attributable to increasing awareness. However, many other studies from Europe, China, and Thailand7,8,9 have reported that the prevalence of FA among children has stabilized in the last decade.
The prevalence of FA in the African continent is not well characterized and shows large geographic variations due to heterogeneity in diet exposure effects, differences according to ethnicity (genetic background) and myriad other factors.10 The scarcity of data on FA prevalence can be attributed partly to the socio-cultural difficulties in well-planned surveys and accurate diagnosis.
Gaps in knowledge among healthcare providers regarding the prevention of FA is shown especially through the limited number of allergists in most developing nations. In fact, such symptoms of FA may overlap with those of malnutrition and other childhood diseases, preventing proper diagnosis. The under-recognition of allergy as a medical specialty, and the limited healthcare infrastructure, compromise the chance for definitive diagnosis of food hypersensitivity. The under-diagnosis of FA in Africa, resulting in insufficient available data, does not allow to perform systematic reviews and/or meta-analyses, due to inconsistent research methods and wide diversity of allergens that do not represent all African settings. Nevertheless, data on challenge-diagnosed FA in Africa show rates that are similar to those in Western countries.11 The reported prevalence of allergic disorders (including FA) in Africa range between 20% and 30% suggesting that allergy represents a morbid condition in the continent which matches HIV/AIDS, malaria, and tuberculosis.11 However, the calculation of certain prevalence statistics remains elusive because of the many manifestations of FA with different severities, diverse allergy definitions, and evaluation of specific study populations, focusing on specific foods and using different methodologies/protocols.
The prevalence of FA reported in the literature is highly variable from one country to another, depending on the age range of the considered sample and genetic and environmental factors. Comparable rates of wheezing between some high-income African urban centers and European countries were observed.10,12
A limited number of studies reported the prevalence of allergic disorders continent-wide. However, the African settings are underestimated, especially for Mediterranean countries. In fact, only 4 published data related countries were reported on food allergens.13, 14, 15, 16 All the data collected indicate a considerable heterogeneity in the incidence of FA across the Mediterranean region.
In this work, we provide original information on the patterns of reactivity to the main families of allergenic food by characterizing the prevalence of food allergies in adults and children in a sample from Sfax region (Tunisia). We also tried to develop predictive statistical tools that might help to support effective management of FA in Tunisia.
Methods
Study population
Tunisia is a north-African country bordered by the Mediterranean Sea to the north and east. According to the National Institute of Statistics, as of 2022, Tunisia has a population of 11 803 588 inhabitants. It has a lower middle-income economy with high unemployment, especially for youth and women. Sfax, where the survey took place, is the second biggest city in the country with 1 022 900 inhabitants in 2021.
The sample size was calculated to achieve a given accuracy in proportion estimation.16 The formula used is based on the expression of a confidence interval of a proportion; if we want a precision (width of the confidence interval) of w for a proportion of p, the sample size required is n = 4p(1-p)/w2. So if we wish a 3% accuracy (w = 0.03) for a proportion of 0.1 we need a sample of n = 400. n = 211 is needed if p = 0.05 with w = 0.03. Hence, a sample of 450–500 is sufficient to have a good accuracy (0.03–0.05) for proportion in the range we are interested in (5–20%).
Description of questionnaire survey
A bilingual questionnaire in Arabic and French was designed, via Google Forms, including information on socio-demographic, FA, symptoms and co-occurring allergies (associated allergies), (Supplemental Appendix 1). Fourteen questions of 3 types have been used: multiple choice questions (choose between a set of many suggested responses), scale questions (yes/no) and open-response questions (free text). The questionnaire was prepared based on previous published questionnaires in use in different countries that have been already standardized.17,18
A panel of 8 foods that are either known as common cause of allergy reactions (Food and Agricultural Organization, FAO/World Health Organisation, WHO, 2018) or thought to be potentially allergic foods because of frequent consumption, was proposed. The list of foods is as follows: cow milk and dairy, cereals, vegetables, fruits, peanut, nuts, eggs, and seafood (fish and shellfish). Other allergies that are not mentioned in the list were also collected. The respondent was asked to name exactly the food that caused the allergic reaction. In case of self-reported allergy to a given food, detailed descriptions, including the age at the first clinical manifestations, duration of symptoms, medical diagnosis (yes/no) and treatments and particularly if an allergy test was done after recommendation of a medical doctor.
The questionnaire-based survey was performed from January 2018 to December 2019. Children and adults were recruited to take the survey voluntarily through direct interview, by onsite visits to randomly selected primary schools, high schools, and university institutions in the Sfax city, Tunisia. Children (under legal majority age) were asked to take the survey home and have parental authorization to fill it in. The survey was also carried out online via social-media platforms, while preserving anonymity.
The raw data were manually entered and curated by 4 independent researchers. This resulted in a set of multinomial variables, of various shapes and structures (numeric, complex strings), that were cleaned and recoded into a set of binary variables, according to the rules explained in Supplemental Appendix 2.
Data cleaning and study design
This cleaning/recoding process consisted of a set of R scripts that were applied on the following variables: allergenic foods, clinical symptoms, medication, diagnostic test, FA in the family, and co-occurring allergies. For instance, concerning allergenic foods, the starting variable was the response to the question: “Which food are you allergic to?” and if the answer was “Strawberry and sole”, the R script will attribute “strawberry” as a binary variable under the class “fruits” and “sole” under the class seafood. This recoding procedure transformed a set of initial multinomial variables called “Allergenic Foods” into 12 binary variables, each representing a “yes/no” allergic status to a food: fruits, seafood, cereals, milk and dairy, nuts, vegetables and legumes, eggs, peanuts, gluten, chocolate, commercial food products, and rare food allergies. In the final list of foods, chocolate, commercial food products and rare food allergies were removed, due to their low prevalence and the weak reliability of the response (intolerance to chocolate for example was thought by respondents to be an allergy, which is known to be extremely rare). Subjects who claim to have an allergy/intolerance to gluten were also removed because it was not clear if it was referring to a celiac disease or gluten intolerance.
The other variables were also recoded into new variables as follows:
The clinical symptoms were grouped into 4 categories according to the organ affected by the immune reaction:
-
➢
Oro-gastro-intestinal symptoms: tummy pain, feeling sick, vomiting, or diarrhea.
-
➢
Rhino-respiratory and ocular symptoms: Sneezing and an itchy, runny or blocked nose, itchy, red, watering eyes, wheezing, chest tightness, shortness of breath, and a cough.
-
➢
Dermatologic symptoms: a raised, itchy, red rash, swollen lips, tongue, eyes, or face.
-
➢
Other symptoms: headache, dizziness
The other variables were recoded as follows:
-
•
Diagnostic test: blood test (allergen-specific immunoglobulin E (IgE) test), skin prick test, other (eg, fibroscopy, biopsy), and doctor examination.
-
•
Medication (3 categories): dermatologic, antihistamine, others.
-
•
Presence of the FA in the family: first degree (child, parent), second degree (siblings and grandparents), and third degree (cousins, aunts and uncles).
Subjects with missing or inconsistent data of the following variables: allergic food, age, and clinical symptoms were removed. The inconsistency means a non-food allergy and/or a clinical symptom which is not specific to food allergy.
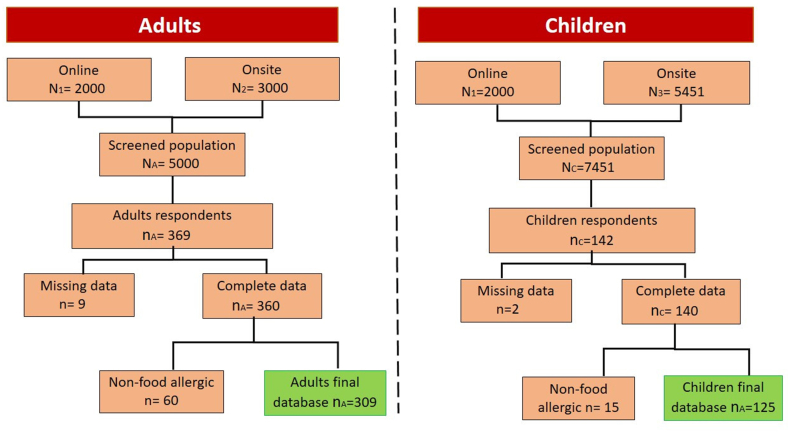
The flowchart showing the study methodology steps is illustrated in Fig. 1.
Fig. 1.
Flowchart of study methodology steps for adults and children.
Ethical consideration
Personal data protection National regulations were respected by anonymization procedures applied to all surveyed participants. All participants have agreed to take the survey without any persuasion and pressure and were understanding of what it is for and will be happening to the answers they provide. For the children, the parent of the participant provided informed consent and filled the questionnaire-survey. A description of the study was included for the respondent in the Google survey's forms. Privacy statement indications were also mentioned.
Statistical analysis
Data analyses were performed using R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria). The dataset was split into two different age groups: children (age under 18 years) and adults (age ≥ 18). All the tests and models were applied to the whole database and separately to the 2 subsets. In all statistical tests, the cutoff for significance was set to p < 0.05.
Descriptive and bivariate analysis
Frequencies of food allergies and other categorical variables were computed by a simple counting procedure, according to the number of informative cases (missing data were discarded). The Chi-square or Fisher's exact tests were carried out to test the association between categorical variables. The Phi-contingency coefficient of association was computed when needed.
Multivariate analyses
Logistic regression analysis was carried on, to study the linear relationships between the FAs in a multivariate context, where each FA was used as a dependent variable at a time and the remaining allergy variables, age and gender were used as independent (explanatory) variables. A stepwise backward selection based on the Akaike Information Criterion (AIC), which is an estimator of the relative quality of a statistical model,19 was used to identify the variables that are the most significantly associated to the dependent variable.
Probabilistic graphical modeling
Bayesian network modeling was carried on using a simplified database with 10 binary variables: food allergies,8 gender, and age. A Bayesian network (BN) is a graphical model (directed acyclic graph) used to represent the joint and conditional probability distributions of a set of variables related by complex non-linear relationships (Pearl, 1988). In the graph (structure) generated by a BN, nodes represent the variables and arcs describe the probabilistic dependencies between them. A BN is accompanied by conditional probability tables (parameters) according to the dependencies described by the graph.
The learning of the structure and parameters of the model was performed using the bnlearn package in R (bnlearn.com). The greedy search (GS) algorithm, also known as Hill-Climbing, was used for learning the structure of the BN with BIC score as an optimality criterion.
Results
Description of the study sample
For children
A total of 125 children (70 boys and 55 girls), their ages ranged from 1 to 17 years (median = 9, P25 = 6, P75 = 12 years), were surveyed and reported history of at least 1 allergic food reaction, among which 88 have been diagnosed by a doctor. In 63% of cases 1 of the 3 diagnosis tests was used; ie, skin prick test was used for 27.3% of the subjects, the same percentage for the IgE, 17% both tests were used, and 1.1% equally for biopsy + IgE, biopsy + IgE + skin prick and fibroscopy. However, 25% of the subjects have been diagnosed by a doctor without doing a diagnosis test.
Most clinical manifestations were skin reactions (60%), followed by gastrointestinal (14%), respiratory (13%,) and other reactions (13%). Regarding the history of familial allergy, 40.3% have reported having at least 1 family member allergic; among those 45.6% have the same allergy as their relatives. About 55.3% were under medication, mainly antihistamines (62.8% of cases).
For adults
A total of 306 adults, including 248 women and 58 men, with ages ranging from 18 to 70 years (median = 23, P25 = 19, P75 = 33 years) was kept for analysis. Among these subjects, 210 (56.9%) reported that they have FA confirmed by medical examination. Skin prick test was used for 22.5% of the subjects, 30% were diagnosed by an IgE test, and 8.8% by both tests. However, 34.8% of the subjects have been diagnosed by a doctor without doing a diagnosis test.
The most frequent clinical manifestations were skin reactions (77.9%), followed by gastrointestinal reactions (28.2%), respiratory reactions (22.8%) and other reactions (17.3%). About 30% reported a history of familial allergy, with 22.8% of them having the same allergy as their first-degree relatives (child or parent). About 46.2% were under medication, mainly antihistamines (37.2%).
Prevalence of allergic foods
For children
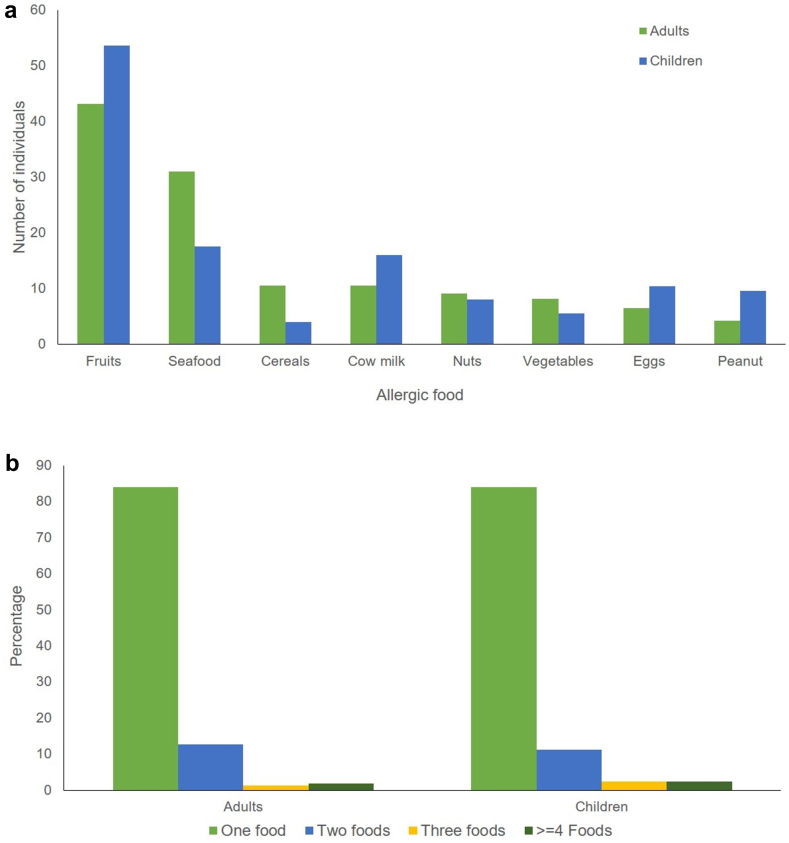
The frequencies of the 8 FA are summarized in Fig. 2 a. In children, fruits was the most prevalent allergen (53.6%), followed by seafood (17.6%), cow's milk (16%), and eggs (10.4%). Allergy to cereals was the least frequent allergy (4%).
Fig. 2.
a: Prevalence of food allergies in children and adults. b: Number of allergic foods causing allergy reaction in adults and Children.
For the group of subjects who are medically diagnosed, the prevalence of food allergens are as follows: 34% fruits (strawberry 51.2%, peach 26.8%, kiwi 4.8%, tomato 9.7%, other fruits 7.3%), 16% cow milk, 9.4% blue fish, 4.7% shellfish, 7.5% eggs, 8.5% nuts, 6.6% peanut, and 4.7% cereals.
For adults
The fruits and seafood were the most frequent FA in adults (43.1% and 31%, respectively). Strawberry was the most common allergic fruit (45%) followed by other local fruits (mainly apples, dates, melon, and watermelon). Blue fish (tuna and sardines) represent 67.4% of the reported allergies to fish, while allergies to eggs and peanuts were the least common allergies (6.5% and 4.2%, respectively). The frequencies are illustrated in Fig. 2a.
Among the 210 subjects that were medically diagnosed, 35.4% are allergic to fruits (strawberry 58.1%, peach 5.4%, kiwi 5.4%, tomato 13.5%, pineapple 4%, 9.6% to cow milk, 17.7% to blue fish, 19.5% to shellfish, 6.2% to egg, 5.2% to nuts, 5.7% to peanut, and 4.3% to cereals.
Prevalence of individual allergic profiles
For children
Out of the 125 children, 105 (84%) had an allergy to only 1 food, while 14 (11.2%) had allergic reactions to 2 foods. Allergies to 3 foods or more are very rare; we found 3 children (2.4%) with 3 allergies and only 1 having 6 allergies. It is worth noting that these multi-allergy profiles always contain 1 or more of the 4 foods: nuts, peanuts, eggs, and milk, which are the most prevalent allergies in children (Fig. 2b).
For adults
Out of the 306 adults, 257 (84%) had an allergy to only 1 food, while 39 (12.7%) had allergic reactions to 2 foods. Allergies to 3 foods or more are very infrequent; we found 4 adults (1.3%) with 3 allergies and only 1 having 7 allergies. Note that in adults most multi-allergy profiles contain at least fruits or seafood (Fig. 2b).
Age at onset of the first clinical manifestation of FA
For children
The age at onset of FA was considered as the age of the occurrence of the first clinical symptoms and is known only for 103 patients. Most of the allergic reactions (61.4%) began between the first and fifth years of life, among them fruits have the highest prevalence (43.3% during the first ten years of life).
For adults
Allergies to fruits seem to occur at younger ages with half of the subjects declaring that they have had their clinical manifestation when they were younger than 14 years. However, subjects suffering from allergy to cereals, reported that the first reactions occurred at older ages (ranging from 18 to 45 years).
Co-occurring allergies
For children
Forty-four out of 116 respondents to the considered question reported co-occurring allergy reactions (37.9%). The most prevalent were allergies to pollen (56.8%), mites (40.9%) and pets (22.7%).
For adults
There are 179 out of 221 respondents to the considered question, who reported co-occurring allergy reactions (81%). Among these, the most prevalent were pollen (39.1%), mites (43.6%), and pets (16.7%).
Bivariate association between food allergies
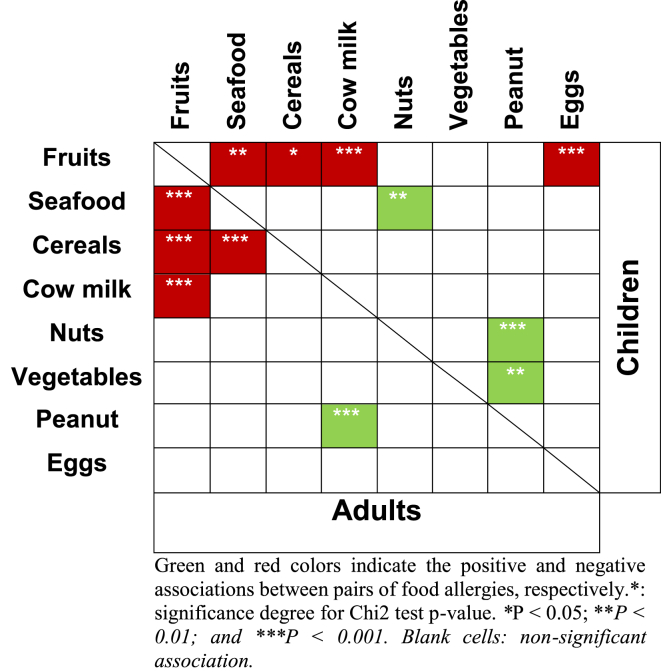
Table 1 illustrates the significant associations between pairs of FA, in adults and children.
Table 1.
Association between pairs of food allergies, in adults and children
For children
It can be observed that on one hand, a highly significant negative association exists between fruits and milk (p = 0.000006, phi = −0.42) and fruits and eggs (p = 0.00014, phi = −0.37), but on the other hand, a positive association exists between nuts and peanuts (p = 0.004, phi = 0.30) (Table 1). This suggests that when people have an allergy to fruits, they are very unlikely to have an allergy to milk (or eggs) and vice versa, while if they have an allergy to nuts they are likely to have an allergy for peanuts and vice versa.
In addition, a significant but weak association is noticed between allergy to fruits and seafood (p = 0.012; phi = −0.23), between nuts and seafood (p = 0.017; phi = 0.25), and between fruits and cereals (p = 0.046, phi = −0.21). Hence, if one summarizes the general trend, one would say that if people have an allergy to fruits they are less likely to have also an allergy to milk, eggs, seafood, and cereals than others who do not have an allergy to fruits.
For adults
We found a highly significant negative association between fruits and seafood (p = 5 × 10−16, phi = −0.48) and at a lower degree between fruits and cereals (p = 0.0001, phi = −0.37), fruits, and milk (p = 0.0017, phi = −0.18) (already reported for children) and between seafood and cereals (p = 0.002; phi = −0.18). To these is added a positive association between peanuts and milk (p = 0.003; phi = 0.19).
Accordingly, if one summarizes the general trend for adults, one would say that if people have an allergy to fruits they are less likely to have also an allergy to seafood, cereals, and milk than someone who does not have an allergy to fruits.
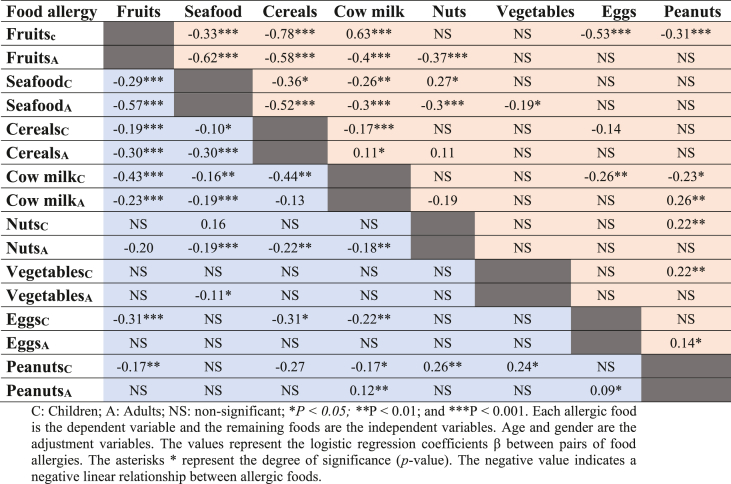
Logistic regression
The multivariate analyses confirm that most food allergies are mutually exclusive; that is if people have an allergy to one food, they are unlikely to have at the same time another allergy to a different food (Table 2). To this general rule, there are, however, few exceptions that should be noted. First, there are strong positive associations between nuts and peanuts, and between vegetables and peanuts in children, that vanish in adults. Contrarily, other strong positive associations are found in adults that do not exist in children, namely between milk and peanuts, and between eggs and peanuts.
Table 2.
Association assessment using logistic regression between the different food allergies in children (C) and adults (A).
Graphical modeling
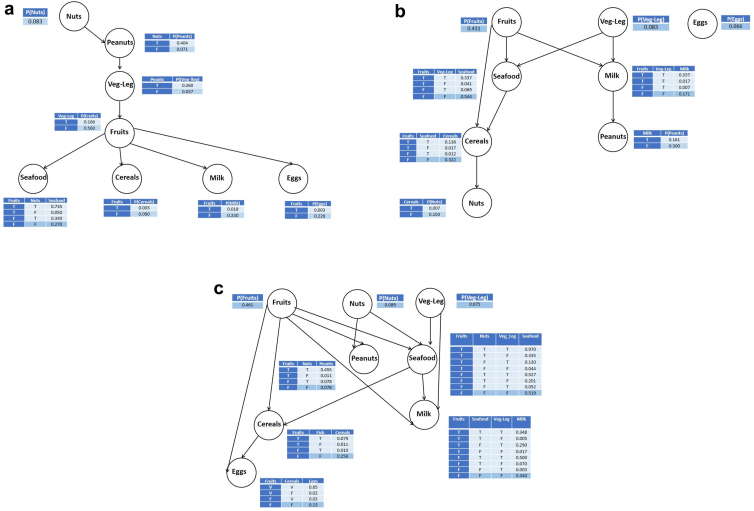
The probabilistic graphical modeling using Bayesian networks allows to identify and visualize complex relationships that cannot be captured by bivariate and multivariate statistical analyses. Fig. 3a and b show that we have 2 different patterns of allergy in children and adults with the common point that allergy to fruits comes as the node that has the highest number of connections to other allergies: 3 and 4 child nodes for adults and children, respectively.
Fig. 3.
a: Bayesian Network for children. Veg-Leg: Vegetables-Legumes, P: probability, T: True, F: False. b: Bayesian Network for adults. Veg-Leg: Vegetables-Legumes, P: probability, T: True, F: False, c: Bayesian Network for all individuals. Veg-Leg: Vegetables-Legumes, P: probability, T: True, F: False.
As far as children are concerned (Fig. 3a), we find out that nuts come as a cue node (no parents) and is the parent of peanuts and seafood, confirming the positive association previously reported between nuts and peanuts. Fruits group is connected negatively, as a parent, to seafood, cereals, milk, and eggs; that means that if fruits is true, the probability of having the other allergies is low and vice versa.
For adults (Fig. 3b), the egg is disconnected from other foods, and fruits comes as a cue node with 3 child nodes: seafood, cereals, and milk, with the same trend as in children. It is worth noting that in adults, nuts and peanuts are not directly connected as in children, in agreement with the results of logistic regression.
In the whole sample, the Bayesian network structure is more complex (Fig. 3c), with 2 allergies having 3 parents (seafood and milk) and 3 having 2 parents (peanuts, cereals. and eggs) while fruits, nuts, and vegetables are cue nodes. The conditional probability tables allow to compute a smart score.
Discussion
This cross-sectional study explores the prevalence of self-reported food allergies among adults and children in Tunisia (sample from Sfax region) through a questionnaire-based survey. The collected data allowed to estimate the prevalence of individual food allergies among allergic subjects in Tunisia and also provided insights into the food-induced symptoms, medication, diagnostic test, family allergy, and co-occurring allergies.
Our findings suggest that the number of self-reported food allergens in this sample was relatively high (105 individual allergenic foods). In this list, we found the “Big eight” food allergens (FAO/WHO, 2018), as the most common and responsible for 90% of food allergic reactions in the United States and also the 14 allergens recognized as the most common and potent causes of food allergies and intolerances across Europe (https://farrp.unl.edu/ref-sit-eu Accessed November 20, 2022). However, the most common food allergens reported in the present study were fruits in adults, as well as in children, which is not reported in the lists above.
Given the differences that may exist between the feeding pattern, dietary habit, and food availability across the world,20 it is expected to find specificities depending on the geographical location.21 This divergence indicates that the allergy is likely to appear for the most consumed foods in a country or region. As a matter of fact, bioclimatic areas, eg, tropical countries, or the Mediterranean region, seem to share comparable allergen patterns.22, 23, 24 Tunisia belongs to the Mediterranean area and thus has similar allergens to other Mediterranean cities or countries. For instance, in Turkey (Antalya), the fruits (orange, banana, peach, apple, and strawberry) are the most common allergens.25 Likewise, allergy to Rosaceae fruits were reported as the most prevalent in Spain, Greece, and Italy.26 In our population of children and adults, strawberry followed by other local fruits, peach, tomato, apples, dates, and melon, were the most frequent (Supplemental Appendix 3). This finding is in agreement with the allergies to Rosaceae fruits in the northern part of the Mediterranean Sea.27
Besides, the high frequency of fruit allergies is also consistent with allergy trends in Europe, where allergies to plants/trees such as apples, hazelnuts, and peach are the most frequent,17 being the most consumed since childhood. For instance, in France, Prunoideae, fruits of the latex group, and Apiaceae are the first allergenic foods in adults.28 In this study, allergy to fruits of the latex-pollen group, which represents 83% of fruits allergies, manifests itself even after simple contact with the fruit in question.
In contrast, the FA patterns in this study are relatively different from those reported in other North African countries. In fact, while in our study allergy to foods of vegetal origin were more prevalent than those of animal origin in both children and adults, the inverse trend was reported in Morocco where eggs and cow's milk were the most prevalent.29 Moreover, fruits and seafood were the most reported food allergen in our study, whereas the most common food allergens are fruit and wheat in Algeria,30 banana and Bakers-Yeast in Libya,31 and milk and banana in Egypt.32
Seafood is one of the most common foods responsible for allergic reactions worldwide, reactions which are potentially life-threatening. The prevalence of seafood allergy varies considerably across countries; however, it appears predominant in regions where seafood is a staple food.22 For instance, it provokes the most common allergic reactions in the Mediterranean countries of Spain and Portugal33 and also in Asia where the seafood is the main ingredient in their dietary habits.34 Similarly, we found that seafood is the second most frequent allergens among children and adults, as the fish and shellfish are widely consumed in Tunisia and particularly in a coast city like Sfax. Interestingly, blue fish (sardine and tuna), that are the most consumed fish in the country, due to their high production and low cost, induced the highest frequency of allergy. Indeed, a study carried out among children of school age in 2014 (unpublished) showed a high rate of sensitization to fish, which reaches 10–12%.
Remarkably, peanuts are the least common allergen, since it is not so much consumed at an early age or even later age, although peanut allergy has increased in prevalence in the last decade. Whereas, local foods, eg, local fruits and fish, were the most frequent allergenic foods, which supports the hypothesis that the environment, or in other words the “exposure”, is a determining factor in allergy. This stems from the fact that blue fish and local fruits are widely consumed from a young age by Tunisians, which leads to sensitization to the allergens of these foods.
Our results showed that animal-derived foods were more reported than foods of plant origin in both children and adults (36.7% vs 29.4%): and (17.3% vs 5.3%), respectively. The most frequently reported animal foods were eggs (10.1% and 2.9%), milk (5.3% and 4.2%), and blue fish (9.8% and 9.4%) for both children and adults, respectively. The rates of reported adverse reactions to these foods were significantly different between children and adults (p < 0.001). Indeed, a statistically significant difference in FA prevalence was found between children and adults in cereals (p = 0.009), seafood (p = 0.005), peanut (p = 0.041) and, fruits (p = 0.03).
In our study, the multi-allergy profiles in children always contain at least 1 of the most prevalent food allergies; however, only 2.4% reported allergy to 3 or more foods. This contrasts with the rates in the United States, where 40–70% of children were allergic to multiple foods.35
Interestingly, adult women reported more frequently allergies to vegetables and legumes than adult men (p = 0.038). This is consistent with the study of Pali-Schöll et al that showed a higher prevalence of food allergies in adult women.36 This could be explained by differences in the gut microbiome, a genetic predisposition linked to gender and very likely to hormone-dependent estrogen factors involved in the pathophysiology of FA.
Regarding symptoms, skin reactions and oral allergy reactions were the most prevalent in children (66%) and in adults (∼80%), while gastrointestinal and respiratory reactions were the least frequently reported by subjects. Urticarial wheals, itching, redness, and oral symptoms occurred more often. The majority of comparable studies confirmed that skin reactions were the most common presentation of food allergies, and the less commonly reported were rhino-respiratory manifestations.1,37, 38, 39
In this study, 40% of adults and 30% of children have a family member who is also allergic. This member has the same allergy in 45.6% and 22.8% of the cases, respectively. Hence, having an allergic family member could be predictive of FA. Indeed, according to Koplin et al (2013) having at least 1 family member with a history of allergic disease, increased the risk to have a FA in child, although, having 2 or more allergic members was more strongly predictive of FA.40 However, Koplin et al and Keet et al reported that the family history is a major risk for FA, except for peanuts.40,41
When the immune system identifies the proteins in 1 substance (eg, pollen) and the proteins in another (eg, raw vegetables or fruit) as being similar, it is called cross-allergy or allergy cross-reactivity.3 The immune system may respond similarly to either when people come into contact with the allergenic protein, regardless of whether this protein in question is one to which they are actually allergic. In some situations, this reaction can result in allergy symptoms (American Academy of Allergy, Asthma & Immunology).42 Indeed, in this study, 9 (2, 94%) subjects are allergic to fruits of the latex-pollen group, have claimed that even the smell or their presence in a place where this fruit is present triggers the allergic reaction.
In our study, the association analysis showed that when adult individuals are allergic to peanuts, they are probably allergic to a vegetable. This result can be explained by the fact that similar allergenic plant proteins are present in families and super-families of plants and peanut.43 Besides, we found that when children are allergic to a nut, they are likely allergic to peanuts. The cross-reactivity between peanuts and tree nuts or seeds has been previously described by De Leon et al as a result of a cross-reactive B cell epitopes present in several peanuts and tree nut allergens.44 Nonetheless, it does not appear that the allergenic cross-reactivity of peanuts and tree nuts could be predicted by their plant taxonomic classification.44 Yet, this cross-reactivity is still prevalent and according to McWilliam et al, 20–30% of those with a peanuts allergy are also allergic to 1 or more types of tree nuts.45 Regarding the cross-reactivity between fruits and cereals found in children and adults, it could be explained by the fact that both contain pollen or proteins homologous to those in specific pollens which was the source of sensitization.2
The major limitation of this study is that FA was self-reported. Self-reporting may lead to some bias resulting in overestimating FA prevalence by three- or four-fold the actual prevalence.46 Conversely, underestimation of the number of diagnosed food allergies can also occur, because some people might not seek medical help if they experience mild food allergies or are not aware of their condition due to inadequate exposure to the type of food in question. Furthermore, this study was cross-sectional, precluding conclusions about causation. However, our study design was strengthened by the use of cluster random sampling in academic institutions of the Sfax region, potentially limiting the risk of selection bias and allowing for the recruitment of a balanced study sample in terms of gender and age.
Conclusions
Prior to this study, most research on food allergies in the Tunisia population was collected from specific and non-generalizable settings11 and there has not been any large-scale study on that population. This study brings new insights into the prevalence of self-reported food allergies and sources of allergens among adults and children. For these reasons we think that our study provides valuable information for clinicians and policymakers, particularly in terms of updating food allergen labeling regulations in processed food products.
A relatively large sample size with good coverage of adults and children in the Sfax region (Tunisia) is the originality of this work, especially considering the current limited information available on food allergies in this population. This work needs to be sustained to cover other regions of Tunisia; based on a mobile application that alerts on FA reactions, we are currently setting up a big data platform to collect online information from informed and consenting users. This will open the door to a better prevention and mitigation strategies of food allergies, with increased efficiency and reduced cost to the national health care system.
Abbreviations
AIC, Akaike Information Criterion; BC, Before Jesus Christ; BIC, Bayesian Information Criterion; BN, Bayesian network; EGID, Eosinophilic Gastrointestinal Disorders; F, False; FA, Food Allergy; FAO, Food and Agricultural Organization; GS, Greedy Search; T, True; US, United States; USA, United States of America; WHO, World Health Organization.
Funding
This work was funded by the Ministry of Higher Education and Scientific Research as a part of the national federated project PARADIS (Grant N°: PRF2017D3P1). The funder had no role in study design, data collection, analysis, and interpretation of data.
Availability of data and materials
All the data, materials and the supplemental material are included in this paper.
Authors’ contributions
SB and RA: investigation, data curation, formal analysis, writing.
FB: formal analysis.
SB, RA, FEB, HG, IA, NBA, BA, NA: survey and data collection.
RBM and NT: data curation.
MT, MK, MJ: funding acquisition.
NK and AR: conceptualization, methodology, validation, formal analysis, investigation, writing, supervision, project administration, funding acquisition.
All authors read and approved the final manuscript.
Ethics statement
All participants have agreed to take the survey without any persuasion and pressure and were understanding of what it is for and will be happening to the answers they provide. For the children, the parents of the participant provided informed consent and filled the questionnaire-survey. A description of the study was included for the respondent in the Google survey's forms. Privacy statement indications were also mentioned.
Consent for publication
All authors have agreed with this publication in the World Allergy Organization Journal.
Declaration of competing interest
The authors have no conflict of interest to declare.
Acknowledgements
The authors are grateful to the Regional Commission of Education of Sfax (Ministry of Education) and the University of Sfax (Ministry of Higher Education and Scientific Research) for facilitating data collection in schools and university institutions. We warmly thank the directors of the sampled schools and university institutions for their collaboration and help in the survey.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2023.100813.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sampson H.A. Food allergy: past, present and future. Allergol Int. 2016 Oct;65(4):363–369. doi: 10.1016/j.alit.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Boyce J.A., Assa’ad A., Burks A.W., et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Am Acad Dermatol. 2011;64(1):175–192. doi: 10.1016/j.jaad.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Matricardi P.M., Kleine-Tebbe J., Hoffmann H.J., et al. EAACI molecular allergology user's guide. Pediatr Allergy Immunol. 2016 May;27:1–250. doi: 10.1111/pai.12563. [DOI] [PubMed] [Google Scholar]
- 4.Yu W., Freeland D.M.H., Nadeau K.C. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16(12):751–765. doi: 10.1038/nri.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren C.M., Jiang J., Gupta R.S. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep. 2020 Feb;20(2):6. doi: 10.1007/s11882-020-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke A.E., Elliott S.J., St Pierre Y., Soller L., La Vieille S., Ben-Shoshan M. Temporal trends in prevalence of food allergy in Canada. J Allergy Clin Immunol Pract. 2020 Apr;8(4):1428–1430.e5. doi: 10.1016/j.jaip.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Nwaru B.I., Hickstein L., Panesar S.S., et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69(1):62–75. doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 8.Ma Z., Chen L., Xian R., Fang H., Wang J., Hu Y. Time trends of childhood food allergy in China: three cross-sectional surveys in 1999, 2009, and 2019. Pediatr Allergy Immunol. 2021;32(5):1073–1079. doi: 10.1111/pai.13490. [DOI] [PubMed] [Google Scholar]
- 9.Rangkakulnuwat P., Lao-Araya M. The prevalence and temporal trends of food allergy among preschool children in Northern Thailand between 2010 and 2019. World Allergy Organization J. 2021 Oct 1;14(10) doi: 10.1016/j.waojou.2021.100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kung S.J., Steenhoff A.P., Gray C. Food allergy in Africa: myth or reality? Clin Rev Allergy Immunol. 2014;46(3):241–249. doi: 10.1007/s12016-012-8341-z. [DOI] [PubMed] [Google Scholar]
- 11.Hossny E., Ebisawa M., El-Gamal Y., et al. Challenges of managing food allergy in the developing world. World Allergy Organization J. 2019;12(11) doi: 10.1016/j.waojou.2019.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Gamal Y.M., Hossny E.M., El-Sayed Z.A., Reda S.M. Allergy and immunology in Africa: challenges and unmet needs. J Allergy Clin Immunol. 2017;140(5):1240–1243. doi: 10.1016/j.jaci.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Ouahidi I., El Hamsas A.E.Y., Aarab L. Modulation of egg white protein allergenicity under physical and chemical treatments. Food Agric Immunol. 2011;22(1):57–68. [Google Scholar]
- 14.Ghadi A., Dutau G., Rancé F. Étude des sensibilisations chez l’enfant atopique à Marrakech. Étude prospective chez 160 enfants entre 2002 et 2005. Rev Fr Allergol Immunol Clin. 2007;47(6):409–415. [Google Scholar]
- 15.Bouhsain S., Kamouni Y., Dami A., et al. Annales de Biologie Clinique. 2008. Biological profile of type I allergies at mohamed V hospital (Rabat-Morocco) pp. 643–646. [DOI] [PubMed] [Google Scholar]
- 16.Masmoudi A., Maalej A., Marrekchi S., Hlima N.B., Turki H., Hachicha M. Profil épidémiologique, clinique et allergologique de la dermatite atopique dans le sud tunisien. Tunis Med. 2007;85(8):679–683. [PubMed] [Google Scholar]
- 17.Lyons S.A., Burney P.G.J., Ballmer-Weber B.K., et al. Food allergy in adults: substantial variation in prevalence and causative foods across Europe. J Allergy Clin Immunol Pract. 2019 Jul;7(6):1920–1928.e11. doi: 10.1016/j.jaip.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 18.Wu T.C., Tsai T.C., Huang C.F., et al. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Intern Med J. 2012;42(12):1310–1315. doi: 10.1111/j.1445-5994.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 19.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1974 Dec;19(6):716–723. [Google Scholar]
- 20.Loh W., Tang M.L.K. The epidemiology of food allergy in the global context. Int J Environ Res Publ Health. 2018;15(9) doi: 10.3390/ijerph15092043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampath V., Abrams E.M., Adlou B., et al. Food allergy across the globe. J Allergy Clin Immunol. 2021;148(6):1347–1364. doi: 10.1016/j.jaci.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Ruethers T., Taki A.C., Johnston E.B., et al. Seafood allergy: a comprehensive review of fish and shellfish allergens. Mol Immunol. 2018;100:28–57. doi: 10.1016/j.molimm.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Cariñanos P., Delgado-Capel M., Maradiaga-Marín M.F., Benítez G. Considerations on the allergy-risks related to the consumption of fruits from urban trees in Mediterranean cities. Urban For Urban Green. 2019;45 [Google Scholar]
- 24.Sánchez J., Sánchez A. Epidemiologic studies about food allergy and food sensitization in tropical countries. Results and limitations. Allergol Immunopathol. 2019;47(4):401–408. doi: 10.1016/j.aller.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Yalcin A.D., Hüseyin Polat H. Adults food allergies in mediterranean region of Turkey. J Allergy Ther. 2013;3:3. [Google Scholar]
- 26.Nucera E., Mezzacappa S., Aruanno A., et al. Hypersensitivity to major panallergens in a population of 120 patients. Adv Dermatology and Allergology/Post?py Dermatologii i Alergologii. 2015;32(4):255–261. doi: 10.5114/pdia.2015.53321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández-Rivas M. The place of lipid transfer proteins (LTP) in the cross-reactivity of plant foods. Rev Fr Allergol. 2009;49(5):433–436. [Google Scholar]
- 28.Just J., Deschildre A., Beaudouin E. Elsevier; Paris, France: 2017. Allergies Alimentaires: Nouveaux Concepts, Affections Actuelles, Perspectives Thérapeutiques. [Google Scholar]
- 29.Azdad O., Mejrhit N., Chda A., et al. The protective effect of milk consumption on milk allergy in children and adults in Fez-Meknes region of Morocco. NFS. 2019 Jul 8;49(4):639–653. [Google Scholar]
- 30.Yakhlef M., Souiki L. Contribution to the estimation of the prevalence of food allergy in schoolchildren in the city of Guelma (Algeria) Rev Fr Allergol. 2021;61(8):573–578. [Google Scholar]
- 31.Elfowiris A., Sharif M., Bianco S. Investigation into causes of allergic diseases using quantitative measurement of allergen-specific ige in serum in Al-bayda, Libya. Al-Mukhtar J Sci. 2018;33(4):332–339. [Google Scholar]
- 32.Mohamed M., Zakaraya D., Abd-El Wahab H., Ashou Z. Prevalence of confirmed immunoglobulin E-mediated food allergy among adult Egyptian patients. Egypt J Immunol. 2021;28(1):23–32. [PubMed] [Google Scholar]
- 33.Bragança M., Bartolomé B., Coimbra A., Carneiro-Leão L., Amaral L. Fish allergy: unusual patterns of parvalbumin allergenicity. Ann Allergy Asthma Immunol. 2022;128(5):607–609. doi: 10.1016/j.anai.2022.01.039. [DOI] [PubMed] [Google Scholar]
- 34.Cyy Wai, Leung N.Y.H., Leung A.S.Y., Wong G.W.K., Leung T.F. Seafood allergy in Asia: geographical specificity and beyond. Front Allergy. 2021 Jul 8;2 doi: 10.3389/falgy.2021.676903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta R.S., Springston E.E., Warrier M.R., et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011 Jul 1;128(1):e9–e17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 36.Pali-Schöll I., Jensen-Jarolim E. Gender aspects in food allergy. Curr Opin Allergy Clin Immunol. 2019 Jun;19(3):249–255. doi: 10.1097/ACI.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 37.Lee S.C., Kim S.R., Park K.H., Lee J.H., Park J.W. Clinical features and culprit food allergens of Korean adult food allergy patients: a cross-sectional single-institute study. Allergy Asthma Immunol Res. 2019;11(5):723. doi: 10.4168/aair.2019.11.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irani C., Maalouly G. Prevalence of self-reported food allergy in Lebanon: a middle-eastern taste. Int Sch Res Notices. 2015:1–5. doi: 10.1155/2015/639796. 2015 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi Y., Ju S., Chang H. Food allergy knowledge, perception of food allergy labeling, and level of dietary practice: a comparison between children with and without food allergy experience. Nutr Res Prac. 2015;9(1):92. doi: 10.4162/nrp.2015.9.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koplin J., Allen K., Gurrin L., et al. The impact of family history of allergy on risk of food allergy: a population-based study of infants. IJERPH. 2013 Oct 25;10(11):5364–5377. doi: 10.3390/ijerph10115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keet C., Pistiner M., Plesa M., et al. Age and eczema severity, but not family history, are major risk factors for peanut allergy in infancy. J Allergy Clin Immunol. 2021 Mar;147(3):984–991.e5. doi: 10.1016/j.jaci.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.https://www.aaaai.org/
- 43.Breiteneder H., Radauer C. A classification of plant food allergens. J Allergy Clin Immunol. 2004 May;113(5):821–830. doi: 10.1016/j.jaci.2004.01.779. [DOI] [PubMed] [Google Scholar]
- 44.De Leon M.P., Glaspole I.N., Drew A.C., Rolland J.M., O'Hehir R.E., Suphioglu C. Immunological analysis of allergenic cross-reactivity between peanut and tree nuts: allergenic cross-reactivity between nuts. Clin Exp Allergy. 2003 Sep;33(9):1273–1280. doi: 10.1046/j.1365-2222.2003.01761.x. [DOI] [PubMed] [Google Scholar]
- 45.McWilliam V., Koplin J., Lodge C., Tang M., Dharmage S., Allen K. The prevalence of tree nut allergy: a systematic review. Curr Allergy Asthma Rep. 2015 Sep;15(9):54. doi: 10.1007/s11882-015-0555-8. [DOI] [PubMed] [Google Scholar]
- 46.Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016 May 4;9:211–217. doi: 10.2147/JMDH.S104807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data, materials and the supplemental material are included in this paper.