Abstract
Exposure to the legacy and emerging persistent organic pollutants (POPs) incessantly has become an important threat to individual health, which is closely related to neurodevelopment, endocrine and cardiovascular homeostasis. Exercise, on the other hand, has been consistently shown to improve physical fitness. Whereas associations between traditional air pollutants, exercise and lung function have been thoroughly reviewed, reviews on associations between persistent organic pollutants and exercise are scarce. Hence, a literature review focused on exercise, exposure to POPs, and health risk assessment was performed for studies published from 2004 to 2022. The purpose of this review is to provide an overview of exposure pathways and levels of POPs during exercise, as well as the impact of exercise on health concerns attributable to the redistribution, metabolism, and excretion of POPs in vivo. Therein lies a broader array of exercise benefits, including insulin sensitizing, mitochondrial DNA repair, lipid metabolism and intestinal microecological balance. Physical exercise is conducive to reduce POPs body burden and resistant to health hazards of POPs generally. Besides, individual lipid metabolism condition is a critical factor in evaluating potential link in exercise, POPs and health effects.
Keywords: Exercise, Persistent organic pollutants, Exposure, Health risk assessments
1. Introduction
Environmental pollutants have been one of the major risk factors that give rise to the 47.8% disability-adjusted life years globally [1]. Due to their persistence and versatile profile, these pollutants compromise the overall health, from a cardiovascular to neurodevelopmental and reproductive point of view. In this prospective, environmental pollutants are epigenetic hazards, which interact with a specific phenotype, changing directly or indirectly its inner molecular and cellular balances [[2], [3], [4], [5], [6]]. Among environmental pollutants, an essential role is played by persistent organic pollutants (POPs), whose application can cause adverse health effects in animal models and humans. Different types of POPs are widely used in agriculture, and show a very strong persistence, with bioaccumulation in the food web [7]. They can be transported over long distances through various environmental media (atmosphere, water, organisms, etc.) with long-term residues, bioaccumulation, semi-volatility and high toxicity, seriously harming the surrounding environment and human health. Humans exposed to POPs via inhalation or dermal absorption mainly besides daily diet. Then POPs would accumulate in adipose tissues or organs for decades [8]. As environmental endocrine disruptors (EEDs), POPs show potential obesity-inducing and epigenetic disrupting effects [9]. Low-dose POPs compete with endogenous hormones for receptor binding, or produce pass-through toxicity through epigenetic modification, such as DNA methylation, histone modification, and non-coding RNA mediation [[10], [11], [12], [13], [14]]. Thus, exposure to POPs has become increasing concern over the chronic metabolic diseases, such as obesity and diabetes [[15], [16], [17]].
Outdoor or indoor physical exercise such as household duties and leisure time activities link constant exposure to dust and air particles with an increased health risk of POPs. The semi-volatilization of POPs enables them to volatilize from soil, dust and water, exist in the air in the form of vapor or adsorb on the atmospheric particulate matters. Lipid mobilization during exercise provokes re-releasing POPs into the bloodstream, increasing internal exposure levels and health risks from exposure to POPs [[18], [19], [20]]. Everyone faces challenges from exogenous and acute endogenous exposure risks of POPs caused by pollution and lipid mobilization [21,22]. Current studies have shown that aerobic and resistance exercises decrease all-cause mortality through improving cognitive functions, mental health, body composition and reducing cardiovascular risk [23,24]. However, numerous epidemiological and toxicological studies have shown the negative association between environmental pollutants exposure during exercise and human health benefits, including cardiovascular disease, respiratory injuries, metabolic disorders and premature death [25,26]. Among them, POPs account for important factors of environmental health risks. But public-and restricted-access venues such as parks, plazas and gyms are often exposed to polluted air and street dust due to decoration materials and automobile exhaust [27]. There might be an equilibrium between health promotion of exercise and health hazard of pollutants exposure. Thus, the role of physical exercise in the exposure and health hazards of POPs needs to be further explored.
The current controversy over reducing POPs health burden relying on physical exercise highlights the necessity of the thorough and critical look at researches on relevant topics. Previous reviews focused on the air pollution, exercise and health. In addition to the common air pollutants such as ozone, nitrogen oxide, particulate matters, more attention should be paid to study the health risks from exposure to POPs during exercise. To verify the frequently POPs exposed, POPs body burden and health outcomes during exercise is of great importance. Herein, we provide a comprehensive review of the key findings, questions and challenges surrounding mechanisms and regulation of exercise, its potential impacts on internal exposure to POPs and its role in health protection.
2. Methods
2.1. Search strategy
Literature search strategies were developed using medical subject headings (MeSH). The following MeSH terms and all their synonyms were used: exercise, persistent organic pollutants (POPs), exposure, health risk assessments. The following databases were used: PubMed, Excerpt Medica Database (EMBASE) and Google Scholar databases. Those MeSH terms were first expanded with the Boolean operator ‘or’, then linked together using ‘and’. The search was restricted to publications in English.
2.2. Study selection
Published manuscripts that potentially contained results of Physical exercise and health risks of exposure to POPs were selected for critical appraisal of the full text. Exclusion criteria were papers with an absence of POPs exposure, exercise and health risk assessments information and literature reviews. Initially, titles and abstracts were screened against the eligibility criteria above. This was performed independently by two researchers (CL and HSL). If the article was clearly not eligible based on the title and/or abstract alone, it was discarded. Otherwise, the full-text was reviewed by both researchers independently and the eligibility criteria were again applied. Disagreements were resolved by consensus.
3. Result
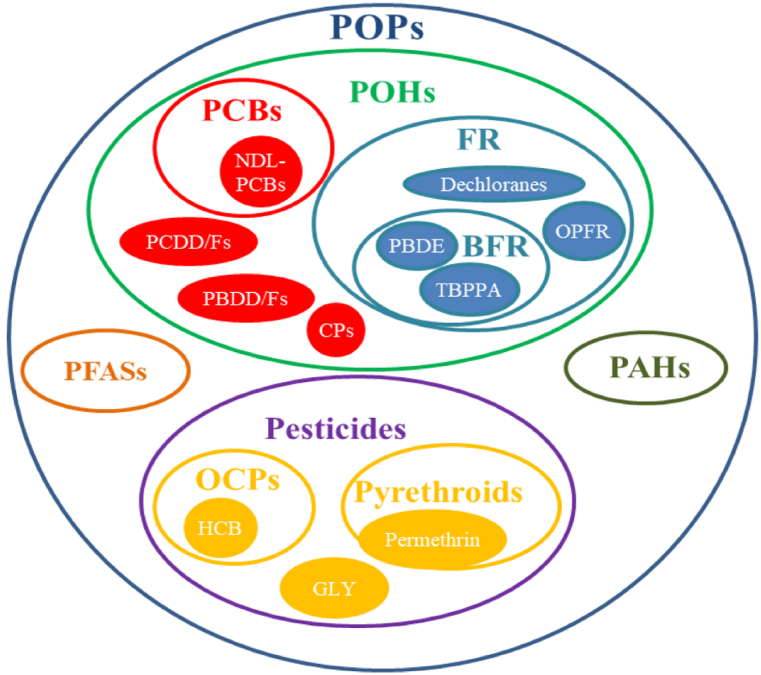
POPs are chemical substances that share the combination of two specific characteristics: persistence and bioaccumulation. Numerous chemical compounds have these characteristics, so the POP group is a miscellaneous group of many different pollutant substances (Fig. 2).
Fig. 2.
Classification of POPs.
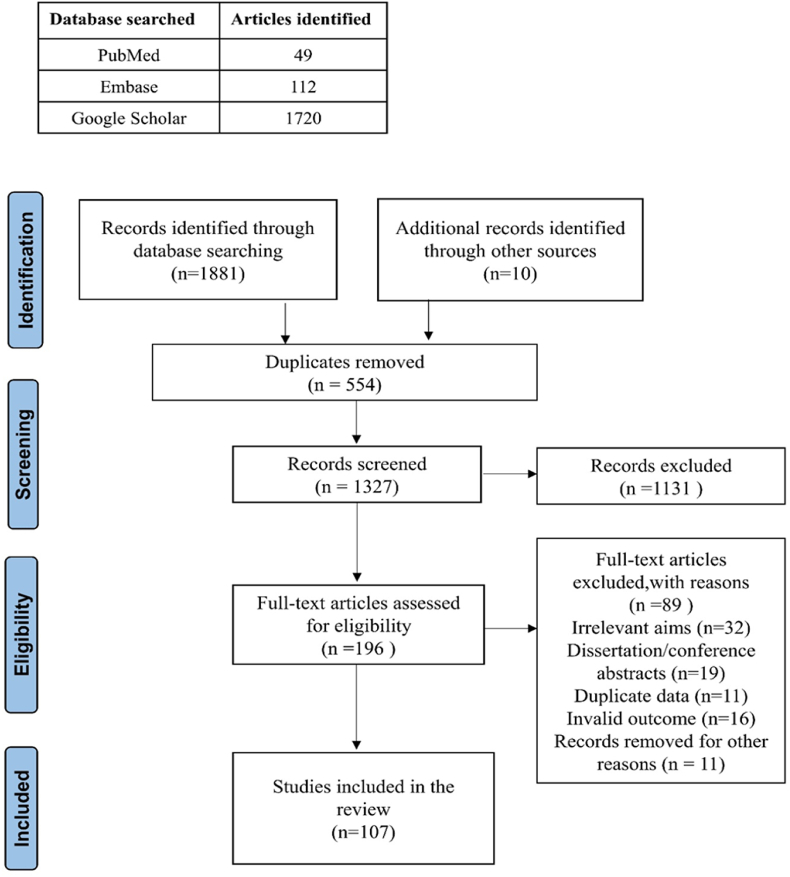
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the search strategy and study selection process of the articles is shown in Fig. 1. As shown in Figs. 1 and 196 articles underwent full-text review, and 107 were included in the final review. The reference lists of included studies were also scanned to ensure complete literature saturation.
Fig. 1.
PRISMA flow diagram.
Note: The figure is derived from https://doi.org/10.3390/ijerph181910236. Abbreviations, phenolic compounds, POHs; polychlorinated biphenyls, PCBs; polybrominated dibenzodioxins/furans, PBDDs/Fs; polychlorinated dibenzodioxins/furans, PCDDs/Fs; Chlorophenols, CPs; Flame retardants, FRs; Brominated flame retardants, BFRs; Polybrominated diphenyl ethers, PBDEs; Tetrabromobisphenol A, TBBPA; Organophosphate flame retardants, OPFRs; Polycyclic aromatic hydrocarbons, PAHs; Perfluorinated alkylated substances, PFASs; Organochlorine pesticides, OCPs; Hexachlorobenzene, HCB; Glyphosate, GLY.
Based on literature review, we describe the relationship between exercise, POP exposure, human POPs levels and exposure risk.
3.1. Exercise and exposure to POPs
During exercise, the human body adjusts metabolic programs by the following ways, such as increasing pulmonary ventilation volume, exchanging mouth and nasal breathing, enhancing lung diffusion capacity, improving cardiac output and muscle microcirculation blood perfusion, etc., to meet the changing energy demands [28,29]. At the same time, individuals exposed to POPs via inhalation or dermal adsorption. And dust acts as the major exposure pathways to POPs for outdoor or indoor exercisers (Table 1). Besides, polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), per- and polyfluoroalkyl substances (PFASs), and polybrominated diphenyl ethers (PBDEs) represent primary classes of POPs exposed [[30], [31], [32], [33], [34]]. Although the production and use of some POPs have been banned, they were detected frequently in environmental media, food and biospecimen due to the environmental release of legacy residues, the by-products associated with industrial production and the generation in nature by biological and abiotic processes.
Table 1.
Exposure pathways and concentrations of POPs in the air or dust where people do exercise.
| Medium | Pathways | POPs | Concentrations (Mean, Country) |
|---|---|---|---|
| Air | Inhalation | ΣPCBs | 9.624 pg/m3(Pakistan) [35], 3.2 ng/m3(China) [36], 62.1 μg/m3(Indian) [37], 79.925 pg/m3(American) [38], 81 pg/m3(Czech) [39], 0.49 pg/m3(Denmark) [40], 2.33 μg/m3(Denmark) [41], 7.71 pg/m3(American) [42], 51.25 pg/m3(Czech) [43], 46.6 pg/m3(Italy) [44] 69.7 pg/m3(Istanbul) [34], 207 pg/m3(Izmir) [34] |
| ΣPCDDs | 0.001 pg/m3(Pakistan) [35], 10.8 pg/m3(China) [36], 73.7 fg/m3(Denmark) [40], 0.522 pg/m3(American) [45], 2.044 pg/m3(Poland) [45], 14.267 fg/m3(Australia) [46] | ||
| ΣPCDFs | 0.005 pg/m3(Pakistan) [35], 3.2 pg/m3(China) [36], 9.7 fg/m3(Denmark) [40], 0.02 pg/m3(American) [42], 1.189 pg/m3(Poland) [45], 46.117 fg/m3(Australia) [46] | ||
| ΣOCPs | 280 pg/m3(American) [42], 231 pg/m3(Italy) [44] 532.85 pg/m3(China) [47], 264 pg/m3(Istanbul) [34], 323 pg/m3(Izmir) [31], 0.94 ng/m3(Lancaster) [48] | ||
| ΣPFOSs | 3906.2pg/PUF(Africa) [49], 145.7pg/PUF (Asia) [49], 516.7pg/PUF (GRULAC) [49], 213.5pg/PUF(Pacific Islands) [49], 0.793 pg/m3(American) [50], 14 pg/m3(Ireland) [51], 5.45 pg/m3(China) [52] |
||
| ΣPBDEs | 4.97 pg/m3(Czech) [43], 10.8 pg/m3(Istanbul) [34], 10.8 pg/m3(Izmir) [34], 172.2 pg/m3(China) [39], 444.8 pg/m3(American) [53], 167 pg/m3 (Norway) [54] | ||
| Dust | Inhalation, dermal adsorption | ΣPCBs | 24.84 ng/g(Pakistan) [55], 18 ng/g(Nigeria) [56], 6.29 ng/g(Greece) [57], 126 ng/g(Saudi Arabia) [58], 11854 ng/g(Denmark) [41], 76.4 ng/g(Italy) [59], 12.34 ng/g(China) [60], 35 ng/g(Romania) [61] |
| ΣPCDDs | 153 pg/g(Germany) [62], 325 pg/g(Australia) [63], | ||
| ΣPCDFs | 26 pg/g(Germany) [62], 18.5 pg/g(Australia) [63], | ||
| ΣOCPs | 87 ng/g(Nepal) [64], 6.625 ng/g(Serbia) [65], 90.2 ng/g(Pakistan) [66], 111 ng/g(American) [67], 83.42 ng/g(China) [60], 1300 ng/g(Romania) [61] | ||
| ΣPFOS | 140 ng/g(American) [67], 13 ng/g(Sweden) [68], 2.16 ng/g(China) [69], 4.4 ng/g(American)[70] | ||
| ΣPBDEs | 300 ng/g(Nigeria) [56], 564 ng/g(Greece) [57], 70.47 ng/g(China) [60], 1800 ng/g(South Korea) [71], 6894.4 ng/g(American) [53], 152.86 ng/g(Australia) [72], 408.55 ng/g(Kuwait) [73], 16 ng/g(Vietnam) [74] |
In agricultural areas of Argentina, PCBs and HCHs were 100% detected in indoor air particles larger than 2.5 μm, while pM.2.5 showed higher concentrations of PCBs (40.2 pg/m3) and HCHs (113.3 pg/m3) [21]. And the total concentration of PCBs in the air is about 2.9–318 fg/m3 in the southern Argentine city of Cordoba. The estimated daily exposure for adults and children was 8.61 and 10.2 pg/kg﹒bw/d, respectively [35]. PBDEs have been widely used in electronic products and carpets as flame retardants, and they are widely found in indoor environments due to their escape migration in the process of item loss. Concentrations of halogenated compounds have been reported to be higher indoor than in outdoor environments, and PBDEs have been detected in both indoor air and indoor dust samples [[75], [76], [77]]. A study in Canada showed that the average concentration of methyl-N-(2-hydroxyethyl)-perfluorooctanesulfonamide (MeFOSE), Ethyl-N-(2-hydroxyethyl)-perfluorooctanesulfonamide (EtFOSE) and total polybrominated diphenyl ethers (PBDEs) in local indoor air was 2590 pg/m3, 770 pg/m3 and 630 pg/m3 respectively, which were 110 times, 85 times and 15 times of that in outdoor air respectively [78]. Products containing PFASs are released into the environment during production and use. These substances are difficult to be degraded in the natural environment, and have a high detection rate in air and indoor dust samples, as well as higher accumulation level in human body than PCBs and PAHs [[79], [80], [81], [82]]. As reported, the total concentration of PFASs in indoor and outdoor air of a kindergarten ranged from 14.8 to 536.7 pg/m3 in Hong Kong [39].
The increased ventilation rate during exercise and continuous bronchiectasis after exercise make the amount of the air volume and the proportion of air pollution particles deposited in the respiratory tract much higher than the resting state. During exercise, the diffusion ability of the lungs gradually increases, and pollutants enter deeper tissues and areas of the body. Moreover, a large number of alveolar cells increase the contact area of pollutants, leading to more pollutants entering the human blood circulation system through alveolar oxygen replacement. Meanwhile, as the blood flow speed accelerated, harmful substances spread at a faster rate to various organs of the human body [83]. However, although there are reports on exercise and air pollution, such as particulate matter, and even if POP levels are reported in air, there does seem to be little report on the evidence that exercise affect exposure of POPs through air. It might be difficult to detect an increase in blood levels of POPs during exercise due to the release from internal stores.
In addition, sports field types are also important factors affecting POPs exposure. The majority of elderly population keen on the square dance outdoor in China. Square dance is an outdoor fitness dance activity organized by citizens, which integrates physical fitness, entertainment and performance, and is based on aerobic exercise with the characteristics of group and spontaneity. Generally, square dancing takes place in open spaces near streets. Such outdoor exercisers face the potential hazards of POPs from automobile exhaust and street dust. Also, the risk of exposure to POPs for professional athletes such as marathon, triathlon and road cycling in sports training venues cannot be ignored. Indoor sports venues also have problems with air pollution caused by equipment and finishing materials [84,85]. Research on the microenvironment has shown that the mode of transport can also affect air pollution exposure, with cyclists in bike lanes in central cities likely to be exposed to more PM2.5 than pedestrians [85,86].
For outdoor sports, catering fume, motor vehicle and industrial exhaust gas caused health hazards significantly. The total mass emission factors of PCBs and PBDEs were 91.8 ± 9.49 ng/L and 398 ± 110 pg/L in the exhaust from diesel generators, accounting for 8.2% and 4.6% of the total dioxin toxicity, respectively [87]. Combined exercise and diesel exhaust particulate exposure can significantly increase the airway levels of SPD, as well as the production of typical airway neutrophils, DC recruitment and pro-inflammatory cytokines. These are risk factors for the occurrence and development of exercise-induced bronchoconstriction [88]. Indoor dust, incomplete combustion, the use of pesticides, and the escape of flame retardants from household appliances are the main sources of POPs exposure for household duties and leisure exercisers. Compared with rural areas, people who exercise in urban environments are at higher risk of POPs exposure [89,90].
3.2. Exercise and blood levels of POPs
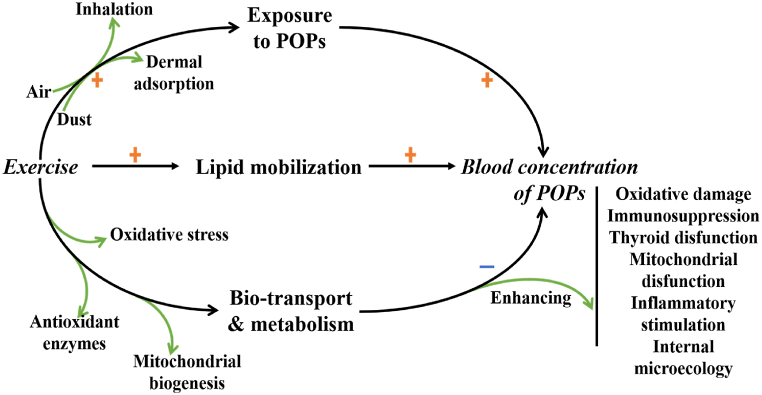
Most of POPs and their metabolites have been reported frequently detected in environmental and biological samples, including soil, air, breast milk, blood, and urine specimens [[91], [92], [93], [94]]. The exposure way of POPs when physical exercise and the possible health risks show in Fig. 3. Low-dose exposure to POPs has been known risk factors that damage blood vessels and endocrine function [95]. Blood levels of POPs largely dictated by the release from adipose tissue and metabolic transformation in liver. Compared to the external environmental exposure, internal exposure levels of POPs provide an accurate depiction of the body burden of POPs, which act key role in revealing connection between pollutants and health risk assessment. However, on the one hand, habitual physical exercise helps to remove POPs from the bloodstream [96]. On the other hand, physical exercise enhances lipid mobilization and promotes release of POPs storage in the lipid, elevating the endogenous exposure levels of POPs temporarily [97].
Fig. 3.
Physical exercise and health risks of exposure to POPs.
Meanwhile, research results of physical exercise and blood concentration varies of POPs were inconsistent. In a cross-sectional study involving 1850 healthy adults, Lee found that serum OCPs concentrations in physically active subjects (312.8 ng/g lipid) were significantly lower than those in inactive subjects (538.0 ng/g lipid) [96]. Among physically active subjects, the serum levels of OCPs decreased as the exercise time increased. But the lipid-corrected serum levels of PCBs were much higher in physically active subjects after adjustment for potential confounders. Of course, although both OCPs and PCBs are lipophilic POPs, their toxicokinetic differences of which may lead to different redistribution and concentration. Meanwhile, OCPs and PCBs are eliminated in different ways through sweat and urine during exercise [98,99]. In another study, compared to the sedentary lean or obese people, the plasma levels of certain POPs were much lower in exercisers with resistance training for 30 years. Similarly, physical exercise seemed to be a new way of eliminating benzopyrene in vivo [100]. Significant fat loss in the first year after bariatric surgery was associated with continued increases in circulating concentrations of lipophilic POPs [101].
Aerobic exercise is also an important factor in the fluctuation of serum POPs level. It takes lots of energy during exercise and activating transcription factor and gene expression of PPAR γ, PGC1α and other enzymes related to lipid transport and catabolism [98,99,[102], [103], [104]]. Not only that, the metabolic enzyme activity increased in liver and promoted POPs biotransformation and excretion. Liver and adipose tissue are the main fat pools in human body. Lipolysis and bile secretion proved to be the main pathways that get rid of POPs [105]. After exercise for 12 min, metabolites associated with insulin resistance, such as glutamate and dimethylguanidino valeric acid decreased 29% and 18%, respectively, as well as metabolites associated with lipolysis, such as 1-methylnicotinamide, increased by 33%, according to exercise tests and metabolic profiles analysis [106]. Obesity, insulin resistance and inflammatory response were improved through various metabolic biological pathways, and the improvement effect was related to the amount of exercise, gender and BMI. These results are also found in the animal experiments. At the initial stage of exercise, the concentration of DDT in blood of mice was significantly lower than that of mice without exercise, while the concentration of DDT in liver was significantly higher than that of mice without exercise, showing a trend of first increasing and then decreasing [107]. Aerobic exercise effectively up-regulated superoxide dismutase activity in liver, enhanced catalase and glutathione peroxidase activity, and promoted DDT biotransformation and metabolism through liver. Finally, the concentrations of DDT in blood and liver of mice were significantly lower than that of mice without exercise. Exercise turned out to be an effective way to reduce POPs body burden.
3.3. Exercise performance and health risk assessment of POPs
The toxicokinetic of POPs is closely bound up with the kinetics of lipid oxidation. The storage of POPs in lipid reduces the burden of other important organs or tissues, and protects against the health threat of POPs. However, the uncontrolled release of POPs from lipids caused by the lipid mobilization or insulin resistance increases the uptake of circulating POPs and harms heart or brain. Studies have shown that losing weight goes against the protective effect of obesity that reducing risk of dementia, due to the lipid mobilization and POPs re-emission [101]. The storage and re-emission of POPs in lipid should be taken into account in the future research on the role of environmental pollutants in the etiology of diseases related to lipid metabolic disorders [101].
Additionally, the moderate endurance exercise improves resistance to the exogenous stressors by means of sustainably ROS production and oxidative stress in blood and skeleton muscle [108]. The endogenous POPs would mimic or block hormones activity, and disrupt the homeostasis of the endocrine system. In addition, the oxidative stress of DNA, protein and lipid aggravates the bad effects of pollutants exposure on cardiovascular diseases. OCPs had a detrimental effect on various tissues and organs via multiple pathways, including oxidative stress, epigenetic modification, mitochondrial dysfunction, endoplasmic reticulum stress and so on [109]. Toxicity of PFASs has been observed, including oxidative DNA damage, immunosuppression, thyroid and mitochondria disfunction [110]. These POPs binding to and activating polycyclic aromatic hydrocarbon receptor (AHR) with high affinity. They upregulated the expression of IL-33, IL-25, and TSLP, promoted the secretion of Th2 cytokines, and lead to tissue oxidative damage [111,112]. Activation of AHR may also affect the function and differentiation of macrophages, dendritic cells and T lymphocytes [113]. Exposure to POPs increased oxidative stress, immune impairment, glucose intolerance, hypercholesterolemia, and endothelial dysfunction characterized by endothelium-dependent atonia. Exercise would protect blood vessels from POPs-induced oxidative stress and inflammatory stimulation, and effectively prevent adverse health effects caused by POPs exposure [114].
The high levels of ROS produced by moderate intensity exercise promoted positive physiological adaptation of active skeletal muscles. Mitochondrial biogenesis, the synthesis of antioxidant enzymes and stress proteins induced DNA repair and contributed to the alleviation of oxidative stress and oxidative damage. A recent study showed that exercise increased the activity of SOD, CAT and GSH-Px in rat liver tissue, and effectively increased the antioxidant capacity of rats exposed to TCDD, thus reducing the hepatotoxicity of pollutants [115]. The up-regulation of exercise-induced DNA repair mechanism was conductive to counteract the oxidative damage of DNA induced by PBDEs and ultrafine particles [[116], [117], [118]].
Moreover, the regulation of intestinal microecology and mitochondrial function might be one of the strategies of exercise to ameliorate the cardiovascular damage caused by POPs exposure. The gut microbiome is a dynamic bacteria community that interacts with the host to regulate energy metabolism and immune function, and is essential to human health. The gut microbiome might also play an important role in toxicological risk of environmental pollutants. For example, TCDD and other AHR ligands not only promoted the expression of inflammatory cytokines and induced oxidative stress, but also caused or aggravated obesity and disturbed the intestinal microecology balance of mice [119,120]. After exposure to the majority of POPs, the expression of genes related to TCA cycle and pyruvate metabolism were significantly changed in cecal bacteria, and the metabolic activity of microorganisms decreased dramatically [121]. Some scholars put forward the opinion that exercise might improve the reduction of intestinal microbial population and diversity caused by POPs exposure by reestablishing microbial flora balance of the intestine and regulating the metabolism [122]. Exercise-induced microbiome modification could mediate host-microbe interactions [123]. Biotransformation involving enzymes produced by intestinal microorganisms would help mitigate toxicity of POPs such as PCBs [124].
Mitochondrial disfunction induced by POPs closely related to the development and progression of metabolic syndrome, of which insulin resistance was the main pathophysiological mechanism. Thus, regulating micro flora and maintaining micro-ecological equilibrium in gut through exercise attributed to improve glucose homeostasis and insulin sensitivity [125]. More than that, exercise provoked mitochondrial biogenesis, and initiated organelle turnover through mitochondrial phagocytosis. This accelerated turnover ensures the efficient functioning of the mitochondrial network, providing sufficient ATP for lipid metabolism and muscle mass maintenance, and reducing susceptibility to apoptosis for an extended period of time [126].
However, uncontrolled lipolytic metabolism and continuing release of POPs are common in cardiovascular and metabolic diseases. The lipid metabolism conditions between different populations with confounding variables (age, gender, serum lipid levels, and BMI) should be considered [127,128]. And the inconsistent findings in numerous epidemiologic studies may result from the uncontrolled or unadjusted confounding variables [101,[129], [130], [131], [132], [133], [134]]. Release and redistribution of POPs in lipid-rich organs such as the brain, kidneys and liver require further investigation. More attention should be paid to limit the transient high exposure to POPs and its acute damage when weight loss or weight back on [20,135]. Developing optimal strategies for weight management requires consideration of dynamics of lipid metabolism and POPs concentration simultaneously.
4. Conclusion
In this study, we comprehensively summarized main exposure pathways and concentrations of POPs during physical exercise, then identified the role and function of exercise in the health concerns of POPs. Although contaminated air or indoor dust increases total exposure to POPs and fat mobilization accelerates POPs diffusion into the bloodstream, the overall risk benefit ratio of regular physical exercise benefits health care. Physical exercise reduces POPs human body burden and combats their health hazard through multiple ways. Significantly, exercise status and lipid metabolism in epidemiologic studies of POPs risk assessments may be overlooked confounding factors. More efforts should be made to prevent and control POPs pollution during physical exercise. To gain the greatest health benefits from exercise, it may be wise to take professional suggestions from both exercise specialist and epidemiologist for population with different age, gender and lipid metabolism characteristics. Exercising outdoors in rural areas pose the least risk of exposure and that might not be afraid of the internal doses that may increase, because exercise seems to promote elimination from the body and has other beneficial effects that can inhibit the negative consequences of POPs.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Berntsen H.F., et al. Effects of a human-based mixture of persistent organic pollutants on the in vivo exposed cerebellum and cerebellar neuronal cultures exposed in vitro. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106240. [DOI] [PubMed] [Google Scholar]
- 3.Miguel Pérez-Carrascosa F., et al. Historical exposure to persistent organic pollutants and cardiovascular disease: a 15-year longitudinal analysis focused on pharmaceutical consumption in primary care. Environ. Int. 2021;156 doi: 10.1016/j.envint.2021.106734. [DOI] [PubMed] [Google Scholar]
- 4.De Angelis M., Schramm K.W. Perinatal effects of persistent organic pollutants on thyroid hormone concentration in placenta and breastmilk. Mol. Aspect. Med. 2021 doi: 10.1016/j.mam.2021.100988. [DOI] [PubMed] [Google Scholar]
- 5.Güil-Oumrait N., et al. Prenatal exposure to persistent organic pollutants and markers of obesity and cardiometabolic risk in Spanish adolescents. Environ. Int. 2021;151 doi: 10.1016/j.envint.2021.106469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi, S.; et al. Association between female reproductive health and mancozeb: systematic review of experimental models. Int. J. Environ. Res. Publ. Health, 17, 2580.doi:10.3390/ijerph17072580. [DOI] [PMC free article] [PubMed]
- 7.Bianchi, S. et al. Exposure to persistent organic pollutants during tooth formation: molecular mechanisms and clinical findings. Environ. Health, 35, 303-310. doi: 10.1515/reveh-2019-0093. [DOI] [PubMed]
- 8.Bányiová K., et al. Long-term time trends in human intake of POPs in the Czech Republic indicate a need for continuous monitoring. Environ. Int. 2017;108:1–10. doi: 10.1016/j.envint.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Aaseth, J.; et al. The role of persistent organic pollutants in obesity: a review of laboratory and epidemiological studies. Toxics 10, 65. doi:10.3390/toxics10020065. [DOI] [PMC free article] [PubMed]
- 10.Vandenberg L.N., et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin E.M., Fry R.C. Environmental influences on the epigenome: exposure- associated DNA methylation in human populations. Annu Rev Public Health. 2018;39:309–333. doi: 10.1146/annurev-publhealth-040617-014629. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y., et al. Air pollution and DNA methylation in adults: a systematic review and meta-analysis of observational studies. Environ Pollut. 2021;284 doi: 10.1016/j.envpol.2021.117152. [DOI] [PubMed] [Google Scholar]
- 13.Kowluru R.A., Mohammad G. Epigenetic modifications in diabetes. Metabolism. 2022;126 doi: 10.1016/j.metabol.2021.154920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donate P.B., et al. Cigarette smoke induces miR-132 in Th17 cells that enhance osteoclastogenesis in inflammatory arthritis. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2017120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratakis N., et al. Prenatal exposure to persistent organic pollutants and childhood obesity: a systematic review and meta-analysis of human studies. Obes. Rev. 2022;23(Suppl 1) doi: 10.1111/obr.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X., et al. Identification and prioritization of the potent components for combined exposure of multiple persistent organic pollutants associated with gestational diabetes mellitus. J. Hazard Mater. 2021;409 doi: 10.1016/j.jhazmat.2020.124905. [DOI] [PubMed] [Google Scholar]
- 17.Bailey M.J., Naik N.N., Wild L.E., Patterson W.B., Alderete T.L. Exposure to air pollutants and the gut microbiota: a potential link between exposure, obesity, and type 2 diabetes. Gut Microb. 2020;11:1188–1202. doi: 10.1080/19490976.2020.1749754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones K.C. Persistent organic pollutants (POPs) and related chemicals in the global environment: some personal reflections. Environ. Sci. Technol. 2021;55:9400–9412. doi: 10.1021/acs.est.0c08093. [DOI] [PubMed] [Google Scholar]
- 19.Horri K., et al. Fish life-history traits are affected after chronic dietary exposure to an environmentally realistic marine mixture of PCBs and PBDEs. Sci. Total Environ. 2018;610–611:531–545. doi: 10.1016/j.scitotenv.2017.08.083. [DOI] [PubMed] [Google Scholar]
- 20.Cheikh Rouhou M., Karelis A.D., St-Pierre D.H., Lamontagne L. Adverse effects of weight loss: are persistent organic pollutants a potential culprit? Diabetes Metab. 2016;42:215–223. doi: 10.1016/j.diabet.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Tames F., Miglioranza K.S.B., Rodriguez Nuñez M., Carreras H. Indoor persistent organic pollutants in agricultural areas from Argentina. Indoor Air. 2020;30:725–734. doi: 10.1111/ina.12649. [DOI] [PubMed] [Google Scholar]
- 22.Sohail M., et al. Persistent organic pollutant emission via dust deposition throughout Pakistan: spatial patterns, regional cycling and their implication for human health risks. Sci. Total Environ. 2018;618:829–837. doi: 10.1016/j.scitotenv.2017.08.224. [DOI] [PubMed] [Google Scholar]
- 23.Cassilhas R.C., Tufik S., de Mello M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell. Mol. Life Sci. 2016;73:975–983. doi: 10.1007/s00018-015-2102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith P.J., Merwin R.M. The role of exercise in management of mental health disorders: an integrative review. Annu. Rev. Med. 2021;72:45–62. doi: 10.1146/annurev-med-060619-022943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syed N., et al. Effects of traffic-related air pollution on exercise endurance, dyspnea, and cardiorespiratory responses in health and copd: a randomized, placebo-controlled, crossover trial. Chest. 2021 doi: 10.1016/j.chest.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Giles L.V., Koehle M.S. The health effects of exercising in air pollution. Sports Med. 2014;44:223–249. doi: 10.1007/s40279-013-0108-z. [DOI] [PubMed] [Google Scholar]
- 27.Tainio M., et al. Air pollution, physical activity and health: a mapping review of the evidence. Environ. Int. 2021;147 doi: 10.1016/j.envint.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellsten Y., Nyberg M. Cardiovascular adaptations to exercise training. Compr. Physiol. 2015;6:1–32. doi: 10.1002/cphy.c140080. [DOI] [PubMed] [Google Scholar]
- 29.Roman M.A., Rossiter H.B., Casaburi R. Exercise, ageing and the lung. Eur. Respir. J. 2016;48:1471–1486. doi: 10.1183/13993003.00347-2016. [DOI] [PubMed] [Google Scholar]
- 30.Whitehead T.P., et al. Concentrations of persistent organic pollutants in California children's whole blood and residential dust. Environ. Sci. Technol. 2015;49:9331–9340. doi: 10.1021/acs.est.5b02078. [DOI] [PubMed] [Google Scholar]
- 31.Miralles-Marco A., Harrad S. Perfluorooctane sulfonate: a review of human exposure, biomonitoring and the environmental forensics utility of its chirality and isomer distribution. Environ. Int. 2015;77:148–159. doi: 10.1016/j.envint.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Byrne S., et al. Exposure to polybrominated diphenyl ethers and perfluoroalkyl substances in a remote population of Alaska Natives. Environ Pollut. 2017;231:387–395. doi: 10.1016/j.envpol.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vrijheid M., et al. Early-life environmental exposures and childhood obesity: an exposome-wide approach. Environ. Health Perspect. 2020;128 doi: 10.1289/ehp5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gungormus E., et al. Selected persistent organic pollutants in ambient air in Turkey: regional sources and controlling factors. Environ. Sci. Technol. 2021;55:9434–9443. doi: 10.1021/acs.est.0c06272. [DOI] [PubMed] [Google Scholar]
- 35.López A., Coscollà C., Hernández C.S., Pardo O., Yusà V. Dioxins and dioxin-like PCBs in the ambient air of the Valencian Region (Spain): levels, human exposure, and risk assessment. Chemosphere. 2021;267 doi: 10.1016/j.chemosphere.2020.128902. [DOI] [PubMed] [Google Scholar]
- 36.Chakraborty P., et al. Passive air sampling of PCDD/fs, PCBs, PAEs, DEHA, and PAHs from informal electronic waste recycling and allied sectors in Indian megacities. Environ. Sci. Technol. 2021;55:9469–9478. doi: 10.1021/acs.est.1c01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H., et al. Comprehensive subchronic inhalation toxicity assessment of an indoor school air mixture of PCBs. Environ. Sci. Technol. 2020;54:15976–15985. doi: 10.1021/acs.est.0c04470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu C., Niu L., Zou D., Zhu S., Liu W. Congener-specific composition of polychlorinated biphenyls (PCBs) in soil-air partitioning and the associated health risks. Sci. Total Environ. 2019;684:486–495. doi: 10.1016/j.scitotenv.2019.05.334. [DOI] [PubMed] [Google Scholar]
- 39.Li M., et al. Evaluation of atmospheric sources of PCDD/Fs, PCBs and PBDEs around an MSWI plant using active and passive air samplers. Chemosphere. 2021;274 doi: 10.1016/j.chemosphere.2021.129685. [DOI] [PubMed] [Google Scholar]
- 40.Degrendele C., et al. Multiyear levels of PCDD/Fs, dl-PCBs and PAHs in background air in central Europe and implications for deposition. Chemosphere. 2020;240 doi: 10.1016/j.chemosphere.2019.124852. [DOI] [PubMed] [Google Scholar]
- 41.Andersen H.V., Gunnarsen L., Knudsen L.E., Frederiksen M. PCB in air, dust and surface wipes in 73 Danish homes. Int. J. Hyg Environ. Health. 2020;229 doi: 10.1016/j.ijheh.2019.113429. [DOI] [PubMed] [Google Scholar]
- 42.Hu Z., Li J., Li B., Zhang Z. Annual changes in concentrations and health risks of PCDD/Fs, DL-PCBs and organochlorine pesticides in ambient air based on the Global Monitoring Plan in São Paulo. Environ Pollut. 2019;255 doi: 10.1016/j.envpol.2019.113310. [DOI] [PubMed] [Google Scholar]
- 43.Melymuk L., et al. Seasonality and indoor/outdoor relationships of flame retardants and PCBs in residential air. Environ Pollut. 2016;218:392–401. doi: 10.1016/j.envpol.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 44.Qu C., et al. The occurrence of OCPs, PCBs, and PAHs in the soil, air, and bulk deposition of the Naples metropolitan area, southern Italy: implications for sources and environmental processes. Environ. Int. 2019;124:89–97. doi: 10.1016/j.envint.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 45.Węgiel M., Chrząszcz R., Maślanka A., Grochowalski A. Seasonal variations of PCDD/Fs congeners in air, soil and eggs from a Polish small-scale farm. Chemosphere. 2018;199:89–97. doi: 10.1016/j.chemosphere.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Rahman M.M., Kim K.H., Brown R.J., Bae I.S., Park C.G. PCDD and PCDF concentrations in a traffic tunnel environment. Sci. Total Environ. 2014;493:773–780. doi: 10.1016/j.scitotenv.2014.06.073. [DOI] [PubMed] [Google Scholar]
- 47.Tian L., et al. DDT, chlordane, and Hexachlorobenzene in the air of the pearl river delta revisited: a tale of source, history, and monsoon. Environ. Sci. Technol. 2021;55:9740–9749. doi: 10.1021/acs.est.1c01045. [DOI] [PubMed] [Google Scholar]
- 48.Aliyeva G., Kurkova R., Hovorkova I., Klánová J., Halsall C. Organochlorine pesticides and polychlorinated biphenyls in air and soil across Azerbaijan. Environ. Sci. Pollut. Res. Int. 2012;19:1953–1962. doi: 10.1007/s11356-012-0944-7. [DOI] [PubMed] [Google Scholar]
- 49.Camoiras González P., Sadia M., Baabish A., Sobhanei S., Fiedler H. Air monitoring with passive samplers for perfluoroalkane substances in developing countries (2017-2019) Chemosphere. 2021;282 doi: 10.1016/j.chemosphere.2021.131069. [DOI] [PubMed] [Google Scholar]
- 50.Zhou J., et al. PFOS dominates PFAS composition in ambient fine particulate matter (PM(2.5)) collected across North Carolina nearly 20 years after the end of its US production. Environ Sci Process Impacts. 2021;23:580–587. doi: 10.1039/d0em00497a. [DOI] [PubMed] [Google Scholar]
- 51.Harrad S., Wemken N., Drage D.S., Abdallah M.A., Coggins A.M. Perfluoroalkyl substances in drinking water, indoor air and dust from Ireland: implications for human exposure. Environ. Sci. Technol. 2019;53:13449–13457. doi: 10.1021/acs.est.9b04604. [DOI] [PubMed] [Google Scholar]
- 52.Lin H., et al. Per- and polyfluoroalkyl substances in the air particles of asia: levels, seasonality, and size-dependent distribution. Environ. Sci. Technol. 2020;54:14182–14191. doi: 10.1021/acs.est.0c03387. [DOI] [PubMed] [Google Scholar]
- 53.Watkins D.J., et al. Associations between PBDEs in office air, dust, and surface wipes. Environ. Int. 2013;59:124–132. doi: 10.1016/j.envint.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakhi A.K., et al. Concentrations of selected chemicals in indoor air from Norwegian homes and schools. Sci. Total Environ. 2019;674:1–8. doi: 10.1016/j.scitotenv.2019.04.086. [DOI] [PubMed] [Google Scholar]
- 55.Aslam I., et al. Polychlorinated biphenyls in indoor dust from urban dwellings of Lahore, Pakistan: congener profile, toxicity equivalency, and human health implications. Indoor Air. 2021;31:1417–1426. doi: 10.1111/ina.12788. [DOI] [PubMed] [Google Scholar]
- 56.Akinrinade O.E., et al. Concentrations of halogenated flame retardants and polychlorinated biphenyls in house dust from Lagos, Nigeria. Environ Sci Process Impacts. 2021;23:1696–1705. doi: 10.1039/d1em00316j. [DOI] [PubMed] [Google Scholar]
- 57.Besis A., Botsaropoulou E., Balla D., Voutsa D., Samara C. Toxic organic pollutants in Greek house dust: implications for human exposure and health risk. Chemosphere. 2021;284 doi: 10.1016/j.chemosphere.2021.131318. [DOI] [PubMed] [Google Scholar]
- 58.Ali N., et al. Semi-volatile organic compounds in car dust: a pilot study in jeddah, Saudi arabia. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18094803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simonetti G., et al. Occurrence of halogenated pollutants in domestic and occupational indoor dust. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17113813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng G., et al. Typical halogenated persistent organic pollutants in indoor dust and the associations with childhood asthma in Shanghai, China. Environ Pollut. 2016;211:389–398. doi: 10.1016/j.envpol.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Dirtu A.C., Ali N., Van den Eede N., Neels H., Covaci A. Country specific comparison for profile of chlorinated, brominated and phosphate organic contaminants in indoor dust. Case study for Eastern Romania. Environ. Int. 2012;49:1–8. doi: 10.1016/j.envint.2012.08.002. 2010. [DOI] [PubMed] [Google Scholar]
- 62.Klees M., Hiester E., Bruckmann P., Molt K., Schmidt T.C. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and dibenzofurans in street dust of North Rhine-Westphalia, Germany. Sci. Total Environ. 2015;511:72–81. doi: 10.1016/j.scitotenv.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 63.Hinwood A.L., et al. Polychlorinated biphenyl (PCB) and dioxin concentrations in residential dust of pregnant women. Environ Sci Process Impacts. 2014;16:2758–2763. doi: 10.1039/c4em00383g. [DOI] [PubMed] [Google Scholar]
- 64.Chandra Yadav I., Devi N.L., Li J., Zhang G. Polychlorinated biphenyls and organochlorines pesticides in indoor dust: an exploration of sources and health exposure risk in a rural area (Kopawa) of Nepal. Ecotoxicol. Environ. Saf. 2020;195 doi: 10.1016/j.ecoenv.2020.110376. [DOI] [PubMed] [Google Scholar]
- 65.Škrbić B.D., Marinković V. Occurrence, seasonal variety of organochlorine compounds in street dust of Novi Sad, Serbia, and its implication for risk assessment. Sci. Total Environ. 2019;662:895–902. doi: 10.1016/j.scitotenv.2019.01.133. [DOI] [PubMed] [Google Scholar]
- 66.Ali N., Van den Eede N., Dirtu A.C., Neels H., Covaci A. Assessment of human exposure to indoor organic contaminants via dust ingestion in Pakistan. Indoor Air. 2012;22:200–211. doi: 10.1111/j.1600-0668.2011.00757.x. [DOI] [PubMed] [Google Scholar]
- 67.Schildroth S., et al. Per-and polyfluoroalkyl substances (PFAS) and persistent chemical mixtures in dust from U.S. colleges. Environ. Res. 2022;206 doi: 10.1016/j.envres.2021.112530. [DOI] [PubMed] [Google Scholar]
- 68.Weiss J.M., Jones B., Koekkoek J., Bignert A., Lamoree M.H. Per- and polyfluoroalkyl substances (PFASs) in Swedish household dust and exposure of pet cats. Environ. Sci. Pollut. Res. Int. 2021;28:39001–39013. doi: 10.1007/s11356-021-13343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y., et al. Chlorinated polyfluoroalkyl ether sulfonic acids in fish, dust, drinking water and human serum: from external exposure to internal doses. Environ. Int. 2021;157 doi: 10.1016/j.envint.2021.106820. [DOI] [PubMed] [Google Scholar]
- 70.Hall S.M., et al. Per- and polyfluoroalkyl substances in dust collected from residential homes and fire stations in north America. Environ. Sci. Technol. 2020;54:14558–14567. doi: 10.1021/acs.est.0c04869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee H.K., et al. Human exposure to legacy and emerging flame retardants in indoor dust: a multiple-exposure assessment of PBDEs. Sci. Total Environ. 2020;719 doi: 10.1016/j.scitotenv.2020.137386. [DOI] [PubMed] [Google Scholar]
- 72.Stasinska A., et al. Concentrations of polybrominated diphenyl ethers (PBDEs) in residential dust samples from Western Australia. Chemosphere. 2013;91:187–193. doi: 10.1016/j.chemosphere.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 73.Al-Harbi M., Al-Enzi E., Al-Mutairi H., Whalen J.K. Human health risks from brominated flame retardants and polycyclic aromatic hydrocarbons in indoor dust. Chemosphere. 2021;282 doi: 10.1016/j.chemosphere.2021.131005. [DOI] [PubMed] [Google Scholar]
- 74.Anh H.Q., et al. PBDEs and novel brominated flame retardants in road dust from northern Vietnam: levels, congener profiles, emission sources and implications for human exposure. Chemosphere. 2018;197:389–398. doi: 10.1016/j.chemosphere.2018.01.066. [DOI] [PubMed] [Google Scholar]
- 75.Harrad S., Hazrati S., Ibarra C. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: implications for human exposure. Environ. Sci. Technol. 2006;40:4633–4638. doi: 10.1021/es0609147. [DOI] [PubMed] [Google Scholar]
- 76.Hwang H.M., Park E.K., Young T.M., Hammock B.D. Occurrence of endocrine-disrupting chemicals in indoor dust. Sci. Total Environ. 2008;404:26–35. doi: 10.1016/j.scitotenv.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takigami H., Suzuki G., Hirai Y., Sakai S. Brominated flame retardants and other polyhalogenated compounds in indoor air and dust from two houses in Japan. Chemosphere. 2009;76:270–277. doi: 10.1016/j.chemosphere.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 78.Shoeib M., Harner T., Ikonomou M., Kannan K. Indoor and outdoor air concentrations and phase partitioning of perfluoroalkyl sulfonamides and polybrominated diphenyl ethers. Environ. Sci. Technol. 2004;38:1313–1320. doi: 10.1021/es0305555. [DOI] [PubMed] [Google Scholar]
- 79.Makey C.M., et al. Airborne precursors predict maternal serum perfluoroalkyl acid concentrations. Environ. Sci. Technol. 2017;51:7667–7675. doi: 10.1021/acs.est.7b00615. [DOI] [PubMed] [Google Scholar]
- 80.De Silva A.O., Allard C.N., Spencer C., Webster G.M., Shoeib M. Phosphorus-containing fluorinated organics: polyfluoroalkyl phosphoric acid diesters (diPAPs), perfluorophosphonates (PFPAs), and perfluorophosphinates (PFPIAs) in residential indoor dust. Environ. Sci. Technol. 2012;46:12575–12582. doi: 10.1021/es303172p. [DOI] [PubMed] [Google Scholar]
- 81.Harada K., et al. The influence of time, sex and geographic factors on levels of perfluorooctane sulfonate and perfluorooctanoate in human serum over the last 25 years. J. Occup. Health. 2004;46:141–147. doi: 10.1539/joh.46.141. [DOI] [PubMed] [Google Scholar]
- 82.Du J., et al. Pesticides in human milk of Western Australian women and their influence on infant growth outcomes: a cross-sectional study. Chemosphere. 2017;167:247–254. doi: 10.1016/j.chemosphere.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Münzel T., Hahad O., Daiber A. Running in polluted air is a two-edged sword - physical exercise in low air pollution areas is cardioprotective but detrimental for the heart in high air pollution areas. Eur. Heart J. 2021;42:2498–2500. doi: 10.1093/eurheartj/ehab227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reche C., et al. Athletes' exposure to air pollution during World Athletics Relays: a pilot study. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2020.137161. [DOI] [PubMed] [Google Scholar]
- 85.Salonen H., Salthammer T., Morawska L. Human exposure to air contaminants in sports environments. Indoor Air. 2020;30:1109–1129. doi: 10.1111/ina.12718. [DOI] [PubMed] [Google Scholar]
- 86.Grana M., Toschi N., Vicentini L., Pietroiusti A., Magrini A. Exposure to ultrafine particles in different transport modes in the city of Rome. Environ Pollut. 2017;228:201–210. doi: 10.1016/j.envpol.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 87.Chen S.J., et al. Emission factors and congener-specific characterization of PCDD/Fs, PCBs, PBDD/Fs and PBDEs from an off-road diesel engine using waste cooking oil-based biodiesel blends. J. Hazard Mater. 2017;339:274–280. doi: 10.1016/j.jhazmat.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 88.Decaesteker T., et al. Differential effects of intense exercise and pollution on the airways in a murine model. Part. Fibre Toxicol. 2021;18:12. doi: 10.1186/s12989-021-00401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mørck T.A., et al. PCB concentrations and dioxin-like activity in blood samples from Danish school children and their mothers living in urban and rural areas. Basic Clin. Pharmacol. Toxicol. 2014;115:134–144. doi: 10.1111/bcpt.12214. [DOI] [PubMed] [Google Scholar]
- 90.Harner T., Shoeib M., Diamond M., Stern G., Rosenberg B. Using passive air samplers to assess urban-rural trends for persistent organic pollutants. 1. Polychlorinated biphenyls and organochlorine pesticides. Environ. Sci. Technol. 2004;38:4474–4483. doi: 10.1021/es040302r. [DOI] [PubMed] [Google Scholar]
- 91.Suk W.A., et al. Human exposure monitoring and evaluation in the Arctic: the importance of understanding exposures to the development of public health policy. Environ. Health Perspect. 2004;112:113–120. doi: 10.1289/ehp.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu Y., et al. Co-exposure to polycyclic aromatic hydrocarbons and phthalates and their associations with oxidative stress damage in school children from South China. J. Hazard Mater. 2021;401 doi: 10.1016/j.jhazmat.2020.123390. [DOI] [PubMed] [Google Scholar]
- 93.Lukina A.O., et al. Temporal variation of total mercury levels in the hair of pregnant women from the Maternal-Infant Research on Environmental Chemicals (MIREC) study. Chemosphere. 2021;264 doi: 10.1016/j.chemosphere.2020.128402. [DOI] [PubMed] [Google Scholar]
- 94.Hyland C., et al. Organophosphate pesticide dose estimation from spot and 24-hr urine samples collected from children in an agricultural community. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murphy M.O., et al. Exercise protects against PCB-induced inflammation and associated cardiovascular risk factors. Environ. Sci. Pollut. Res. Int. 2016;23:2201–2211. doi: 10.1007/s11356-014-4062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee Y.M., Shin J.Y., Kim S.A., Jacobs D.R., Jr., Lee D.H. Can habitual exercise help reduce serum concentrations of lipophilic chemical mixtures? Association between physical activity and persistent organic pollutants. Diabetes Metab. J. 2020;44:764–774. doi: 10.4093/dmj.2019.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.La Merrill M., et al. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ. Health Perspect. 2013;121:162–169. doi: 10.1289/ehp.1205485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Genuis S.J., Beesoon S., Birkholz D. Biomonitoring and elimination of perfluorinated compounds and polychlorinated biphenyls through perspiration: blood, urine, and sweat study. ISRN Toxicol. 2013 doi: 10.1155/2013/483832. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Genuis S.J., Lane K., Birkholz D. Human elimination of organochlorine pesticides: blood, urine, and sweat study. BioMed Res. Int. 2016 doi: 10.1155/2016/1624643. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu Y., et al. A new approach for reducing pollutants level: a longitudinal cohort study of physical exercises in young people. BMC Publ. Health. 2022;22:223. doi: 10.1186/s12889-022-12621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fénichel P., et al. Sustained bloodstream release of persistent organic pollutants induced by extensive weight loss after bariatric surgery: implications for women of childbearing age. Environ. Int. 2021;151 doi: 10.1016/j.envint.2021.106400. [DOI] [PubMed] [Google Scholar]
- 102.Smith R.L., Soeters M.R., Wüst R.C.I., Houtkooper R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018;39:489–517. doi: 10.1210/er.2017-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yiamouyiannis C.A., Sanders R.A., Watkins J.B., 3rd, Martin B.J. Chronic physical activity: hepatic hypertrophy and increased total biotransformation enzyme activity. Biochem. Pharmacol. 1992;44:121–127. doi: 10.1016/0006-2952(92)90045-k. [DOI] [PubMed] [Google Scholar]
- 104.Watkins J.B., 3rd, Crawford S.T., Sanders R.A. Chronic voluntary exercise may alter hepatobiliary clearance of endogenous and exogenous chemicals in rats. Drug Metab. Dispos. 1994;22:537–543. [PubMed] [Google Scholar]
- 105.Macdonald T.L. Chemical mechanisms of halocarbon metabolism. Crit. Rev. Toxicol. 1983;11:85–120. doi: 10.3109/10408448309089849. [DOI] [PubMed] [Google Scholar]
- 106.Nayor M., et al. Metabolic architecture of acute exercise response in middle-aged adults in the community. Circulation. 2020;142:1905–1924. doi: 10.1161/circulationaha.120.050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li K., Zhu X., Wang Y., Zheng S., Dong G. Effect of aerobic exercise intervention on DDT degradation and oxidative stress in rats. Saudi J. Biol. Sci. 2017;24:664–671. doi: 10.1016/j.sjbs.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li D., Suh S. Health risks of chemicals in consumer products: a review. Environ. Int. 2019;123:580–587. doi: 10.1016/j.envint.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 110.Buckley J.P., et al. Prenatal phthalate exposures and body mass index among 4- to 7-Year-old children: a pooled analysis. Epidemiology. 2016;27:449–458. doi: 10.1097/ede.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weng C.M., et al. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy. 2018;73:2192–2204. doi: 10.1111/all.13462. [DOI] [PubMed] [Google Scholar]
- 112.Vogel C.F.A., Van Winkle L.S., Esser C., Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors - implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rothhammer V., Quintana F.J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019;19:184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 114.Murphy M.O., et al. Exercise protects against PCB-induced inflammation and associated cardiovascular risk factors. Environ. Sci. Pollut. Res. Int. 2016;23:2201–2211. doi: 10.1007/s11356-014-4062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yan H., et al. Effect of exercise on liver redox status in continuously 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-Exposed rats. Asian J. Ecotoxicol. 2017;12:81–87. doi: 10.7524/aje.1673-5897.20170321001. [DOI] [Google Scholar]
- 116.Tryfidou D.V., McClean C., Nikolaidis M.G., Davison G.W. DNA damage following acute aerobic exercise: a systematic review and meta-analysis. Sports Med. 2020;50:103–127. doi: 10.1007/s40279-019-01181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bhargava A., et al. Ultrafine particulate matter impairs mitochondrial redox homeostasis and activates phosphatidylinositol 3-kinase mediated DNA damage responses in lymphocytes. Environ Pollut. 2018;234:406–419. doi: 10.1016/j.envpol.2017.11.093. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y., et al. Polybrominated diphenyl ethers quinone-induced intracellular protein oxidative damage triggers ubiquitin-proteasome and autophagy-lysosomal system activation in LO2 cells. Chemosphere. 2021;275 doi: 10.1016/j.chemosphere.2021.130034. [DOI] [PubMed] [Google Scholar]
- 119.Zhang L., et al. Persistent organic pollutants modify gut microbiota-host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environ. Health Perspect. 2015;123:679–688. doi: 10.1289/ehp.1409055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moyer B.J., et al. Obesity and fatty liver are prevented by inhibition of the aryl hydrocarbon receptor in both female and male mice. Nutr. Res. 2017;44:38–50. doi: 10.1016/j.nutres.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tian Y., et al. Metabolic impact of persistent organic pollutants on gut microbiota. Gut Microb. 2020;12:1–16. doi: 10.1080/19490976.2020.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Potera C. Running interference? Exercise and PCB-induced changes in the gut microbiome. Environ. Health Perspect. 2013;121:A199. doi: 10.1289/ehp.121-a199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choi J.J., et al. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ. Health Perspect. 2013;121:725–730. doi: 10.1289/ehp.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saad R., Rizkallah M.R., Aziz R.K. Gut Pharmacomicrobiomics: the tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathog. 2012;4:16. doi: 10.1186/1757-4749-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Y., et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 2020;31:77–91.e75. doi: 10.1016/j.cmet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 126.Hood D.A., Memme J.M., Oliveira A.N., Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu. Rev. Physiol. 2019;81:19–41. doi: 10.1146/annurev-physiol-020518-114310. [DOI] [PubMed] [Google Scholar]
- 127.Lee D.H., Jacobs D.R., Jr., Lind L., Lind P.M. Lipophilic environmental chemical mixtures released during weight-loss: the need to consider dynamics. Bioessays. 2020;42 doi: 10.1002/bies.201900237. [DOI] [PubMed] [Google Scholar]
- 128.Domazet S.L., Jensen T.K., Grøntved A., Domazet S.L., Jensen T.K., Grøntved A. Higher circulating plasma polychlorinated biphenyls (PCBs) in fit and lean children: the European youth heart study. Environ. Int. 2020;136 doi: 10.1016/j.envint.2020.105481. [DOI] [PubMed] [Google Scholar]
- 129.Raffetti E., et al. Polychlorinated biphenyls (PCBs) exposure and cardiovascular, endocrine and metabolic diseases: a population-based cohort study in a North Italian highly polluted area. Environ. Int. 2018;120:215–222. doi: 10.1016/j.envint.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 130.Van Larebeke N., et al. Internal exposure to organochlorine pollutants and cadmium and self-reported health status: a prospective study. Int. J. Hyg Environ. Health. 2015;218:232–245. doi: 10.1016/j.ijheh.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Z., et al. Associations of serum PFOA and PFOS levels with incident hypertension risk and change of blood pressure levels. Environ. Res. 2022;212 doi: 10.1016/j.envres.2022.113293. [DOI] [PubMed] [Google Scholar]
- 132.Darrow L.A., Stein C.R., Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005-2010. Environ. Health Perspect. 2013;121:1207–1213. doi: 10.1289/ehp.1206372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee D.H., Jacobs D.R., Jr. Firm human evidence on harms of endocrine-disrupting chemicals was unlikely to be obtainable for methodological reasons. J. Clin. Epidemiol. 2019;107:107–115. doi: 10.1016/j.jclinepi.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 134.Lee D.H., Porta M., Jacobs D.R., Jr., Vandenberg L.N. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr. Rev. 2014;35:557–601. doi: 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brown R.H., Ng D.K., Steele K., Schweitzer M., Groopman J.D. Mobilization of environmental toxicants following bariatric surgery. Obesity. 2019;27:1865–1873. doi: 10.1002/oby.22618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.