Abstract
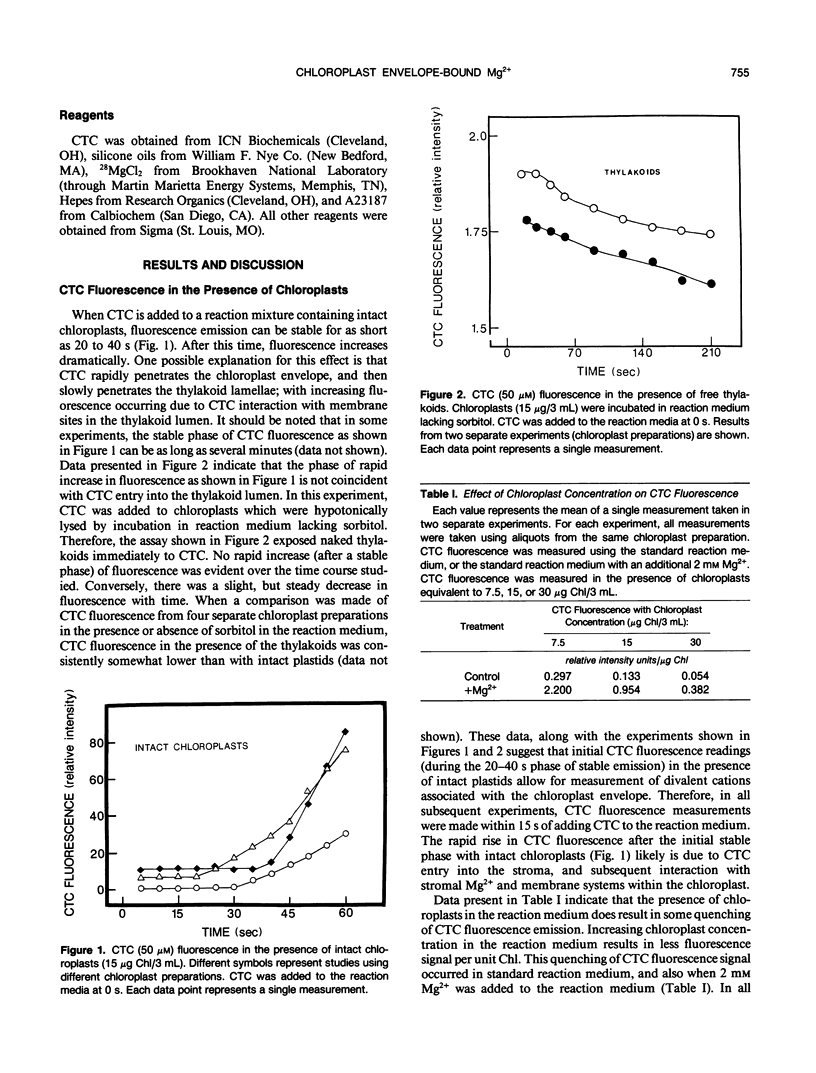
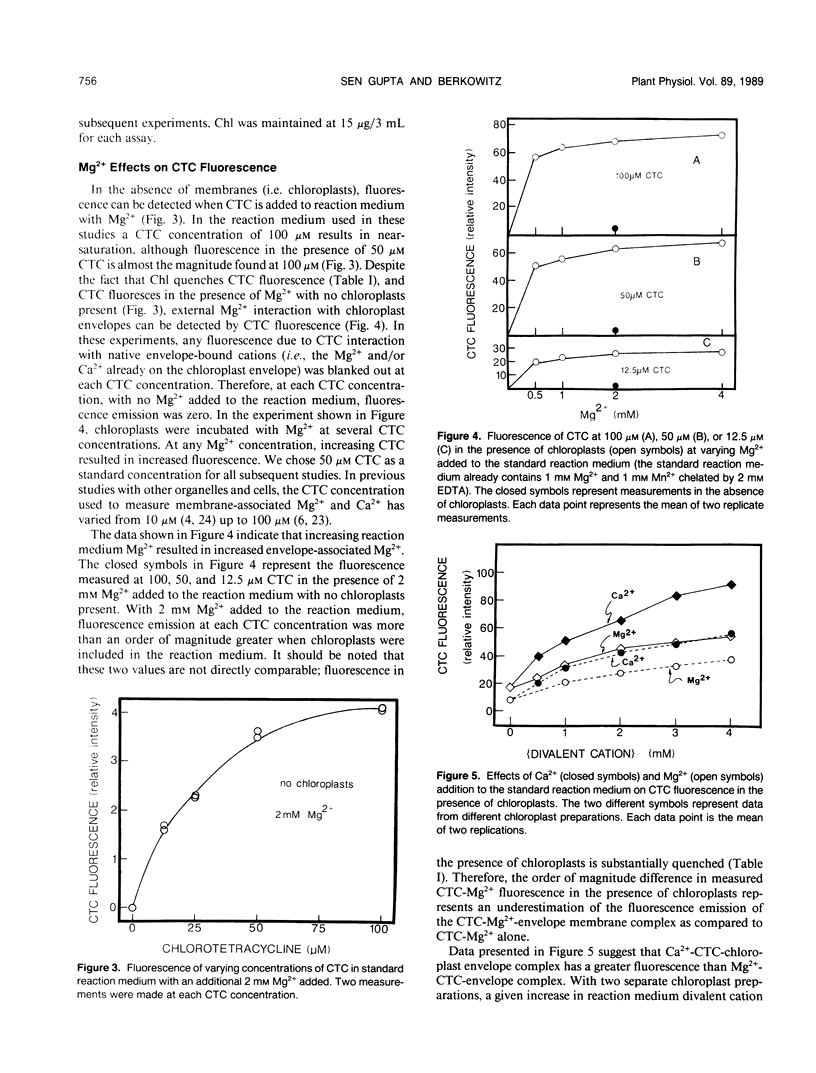
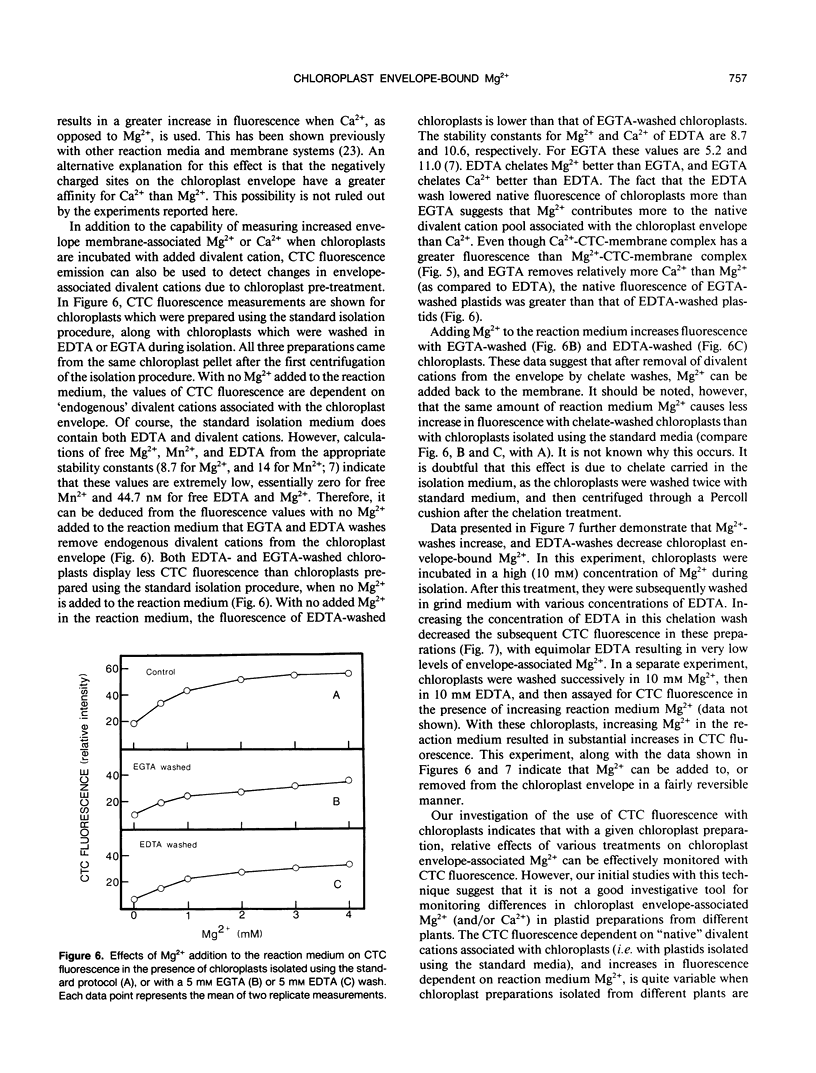
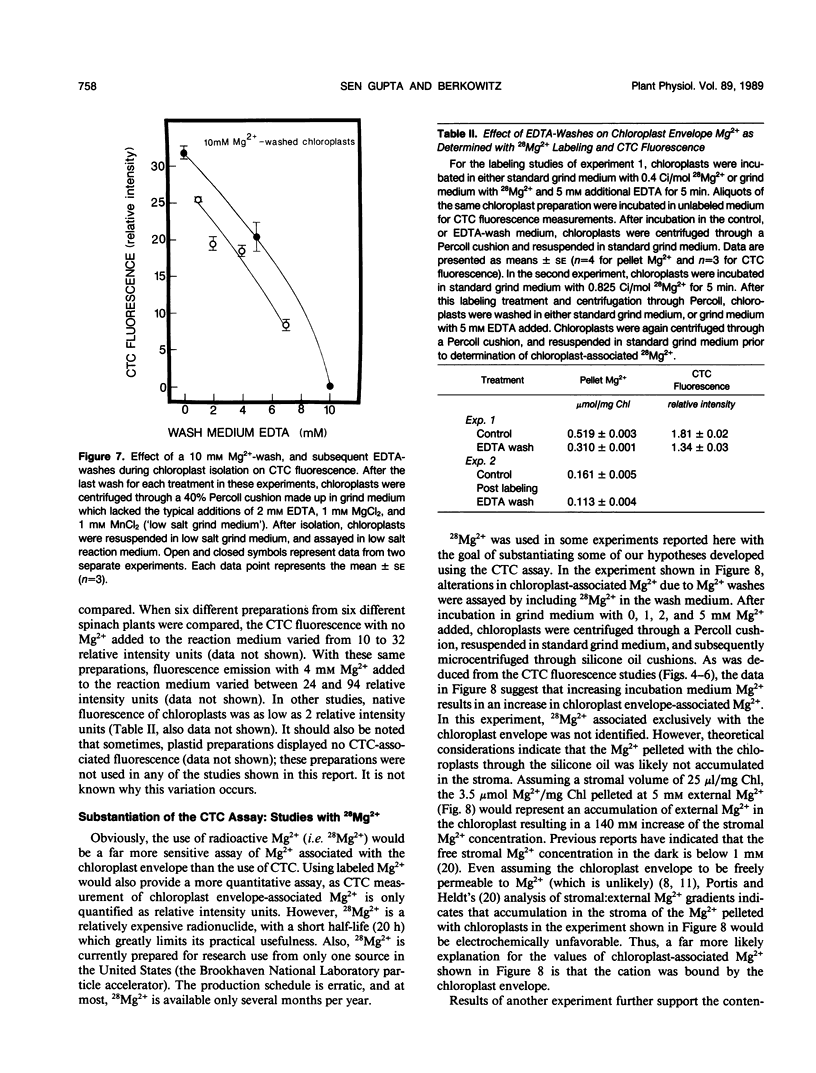
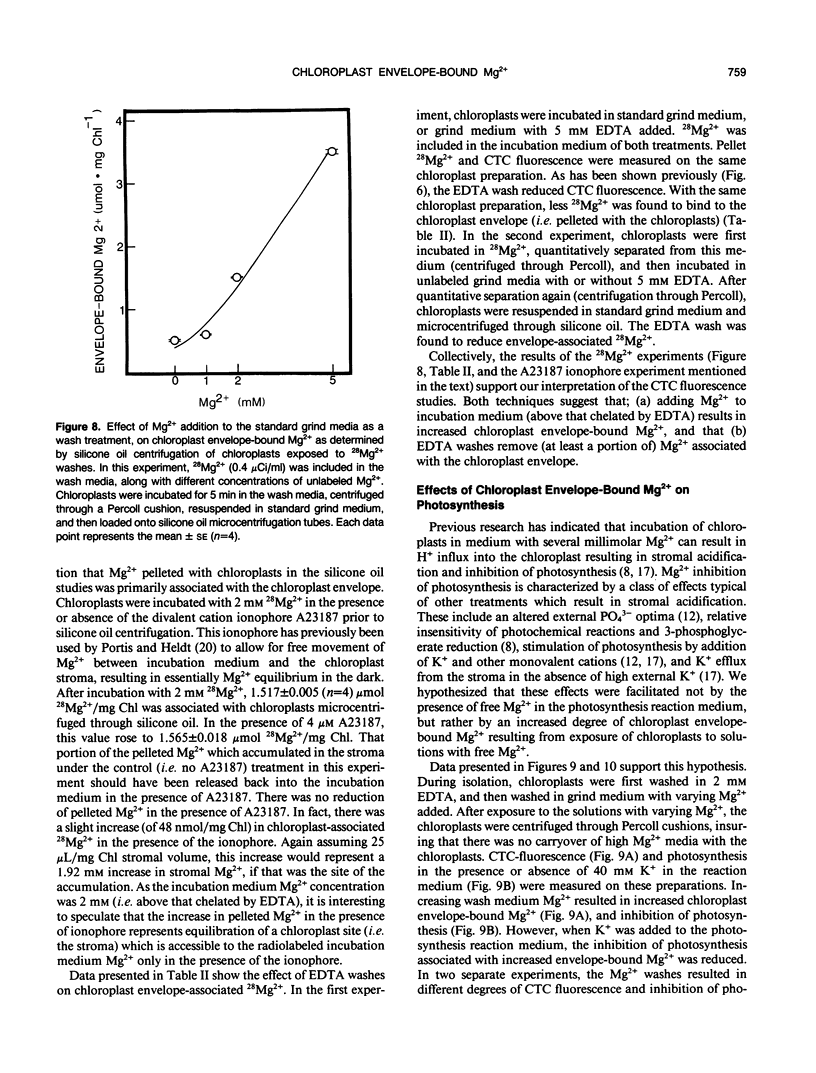
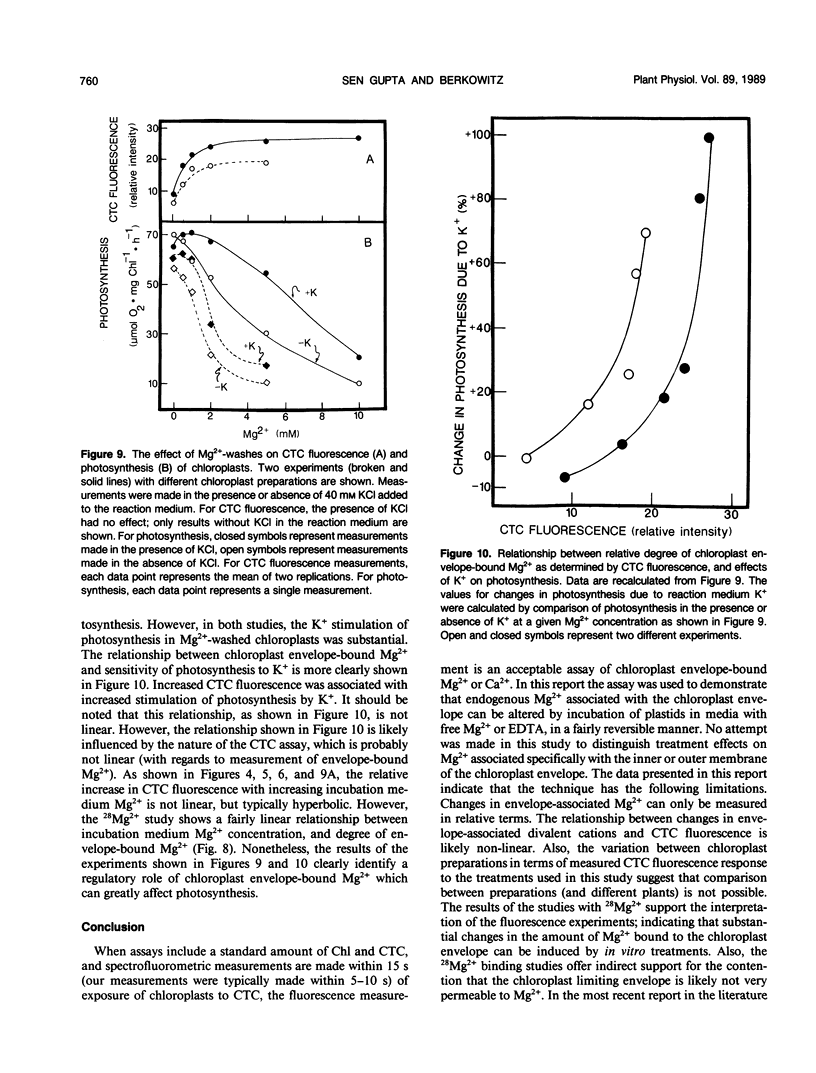
Experiments were conducted to develop chlorotetracycline (CTC) fluorescence as an assay of Mg2+ bound to the envelope of the intact chloroplast. This assay technique has been widely used to measure envelope associated divalent cations in animal cell and subcellular systems, but has not been used with chloroplasts. Chloroplast envelope-associated Mg2+ was altered by pretreatment with Mg2+ and divalent cation chelating agents and by additions of Mg2+ to the CTC assay medium. Results indicated that for a given chloroplast preparation, relative changes in envelope-associated Mg2+ can be effectively monitored with CTC fluorescence. It was concluded that the limitations of this assay system are: (a) chlorophyll strongly quenches CTC fluorescence signal, so a constant chlorophyll concentration must be maintained, (b) measurements must be made quickly, and (c) use of the technique to compare different chloroplast preparations may not be valid. Studies with 28Mg2+ confirmed our interpretation of the fluorescence results, and also suggested that the chloroplast envelope is fairly impermeable to Mg2+. It was concluded that changes in Mg2+ associated with the chloroplast due to incubation of plastids in solutions containing up to 5 millimolar Mg2+ may be exclusively due to increased envelope-associated Mg2+. The CTC assay was used in experiments to demonstrate that increases in chloroplast envelope-associated Mg2+ inhibit photosynthetic capacity. This inhibition can be partially overcome by the presence of K+ in the photosynthetic reaction media.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz G. A., Gibbs M. Reduced osmotic potential effects on photosynthesis : identification of stromal acidification as a mediating factor. Plant Physiol. 1983 Apr;71(4):905–911. doi: 10.1104/pp.71.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet A., Volfin P. ADP requirement for prevention by a cytosolic factor of Mg2+ and Ca2+ release from rat liver mitochondria. Arch Biochem Biophys. 1974 Oct;164(2):756–764. doi: 10.1016/0003-9861(74)90090-3. [DOI] [PubMed] [Google Scholar]
- Carvalho C. A. Chlorotetracycline as an indicator of the interaction of calcium with brain membrane fractions. J Neurochem. 1978 May;30(5):1149–1155. doi: 10.1111/j.1471-4159.1978.tb12410.x. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Hutchison J. D. Selectivity of cation chelation to tetracyclines: evidence for special conformation of calcium chelate. Biochem Biophys Res Commun. 1971 May 7;43(3):625–630. doi: 10.1016/0006-291x(71)90660-7. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Intracellular divalent cation release in pancreatic acinar cells during stimulus-secretion coupling. I. Use of chlorotetracycline as fluorescent probe. J Cell Biol. 1978 Feb;76(2):371–385. doi: 10.1083/jcb.76.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Gimmler H. Properties of the Isolated Intact Chloroplast at Cytoplasmic K Concentrations : I. Light-Induced Cation Uptake into Intact Chloroplasts is Driven by an Electrical Potential Difference. Plant Physiol. 1983 Sep;73(1):169–174. doi: 10.1104/pp.73.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Fish L. E., Jagendorf A. T. Permeability of chloroplast envelopes to mg: effects on protein synthesis. Plant Physiol. 1984 Apr;74(4):956–961. doi: 10.1104/pp.74.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Maury W. Effects of Magnesium on Intact Chloroplasts: I. EVIDENCE FOR ACTIVATION OF (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE CHLOROPLAST ENVELOPE. Plant Physiol. 1980 Feb;65(2):350–354. doi: 10.1104/pp.65.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D. W., Shi G. Y., Brierley G. P. Induction of passive monovalent cation-exchange activity in heart mitochondria by depletion of endogenous divalent cations. Arch Biochem Biophys. 1981 Jul;209(2):356–361. doi: 10.1016/0003-9861(81)90292-7. [DOI] [PubMed] [Google Scholar]
- Laban E., Charbon G. A. Magnesium and cardiac arrhythmias: nutrient or drug? J Am Coll Nutr. 1986;5(6):521–532. doi: 10.1080/07315724.1986.10720154. [DOI] [PubMed] [Google Scholar]
- Luthra R., Olson M. S. The effects of chlorotetracycline on calcium movements in isolated rat liver mitochondria. Arch Biochem Biophys. 1978 Dec;191(2):494–502. doi: 10.1016/0003-9861(78)90388-0. [DOI] [PubMed] [Google Scholar]
- Maury W. J., Huber S. C., Moreland D. E. Effects of Magnesium on Intact Chloroplasts : II. CATION SPECIFICITY AND INVOLVEMENT OF THE ENVELOPE ATPase IN (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE ENVELOPE. Plant Physiol. 1981 Dec;68(6):1257–1263. doi: 10.1104/pp.68.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki K., Kasai M. Magnesium permeability of sarcoplasmic reticulum vesicles monitored in terms of chlortetracycline fluorescence. J Biochem. 1980 Mar;87(3):709–716. doi: 10.1093/oxfordjournals.jbchem.a132799. [DOI] [PubMed] [Google Scholar]
- Piazza G. J., Gibbs M. Influence of adenosine phosphates and magnesium on photosynthesis in chloroplasts from peas, sedum, and spinach. Plant Physiol. 1983 Mar;71(3):680–687. doi: 10.1104/pp.71.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Schneider A. S., Herz R., Sonenberg M. Chlortetracycline as a probe of membrane-associated calcium and magnesium: interaction with red cell membranes, phospholipids, and proteins monitored by fluorescence and circular dichroism. Biochemistry. 1983 Mar 29;22(7):1680–1686. doi: 10.1021/bi00276a025. [DOI] [PubMed] [Google Scholar]
- Täljedal I. B. Interaction of Na+ and Mg2+ with Ca2+ in pancreatic islets as visualized by chlorotetracycline fluorescence. Biochim Biophys Acta. 1974 Nov 4;372(1):154–161. [PubMed] [Google Scholar]


