This cohort study analyzes the risk of incident autoimmune and autoinflammatory connective tissue disorder following COVID-19.
Key Points
Question
Is COVID-19 associated with an increased risk of autoimmune and autoinflammatory disorders?
Findings
This cohort study including 354 527 individuals with COVID-19 and 6 134 940 controls identified a significant elevation in the risk of multiple incident autoimmune and autoinflammatory disorders subsequent to COVID-19. Notably, certain disease risks exhibited a positive association with the severity of COVID-19.
Meaning
These findings suggest that autoimmune and autoinflammatory connective tissue disorders may manifest as post–COVID-19 sequelae, highlighting the potential long-term health ramifications associated with COVID-19; long-term management should include evaluating the development of such disorders in patients who had COVID-19.
Abstract
Importance
Multiple cases of autoimmune and autoinflammatory diseases after COVID-19 have been reported. However, their incidences and risks have rarely been quantified.
Objective
To investigate the incidences and risks of autoimmune and autoinflammatory connective tissue disorders after COVID-19.
Design, Setting, and Participants
This was a retrospective population-based study conducted between October 8, 2020, and December 31, 2021, that used nationwide data from the Korea Disease Control and Prevention Agency COVID-19 National Health Insurance Service cohort and included individuals who received a diagnosis of COVID-19 via polymerase chain reaction testing and a control group with no evidence of COVID-19 identified from National Health Insurance Service of Korea cohort. Data analysis was conducted from September 2022 to August 2023.
Exposures
Receipt of diagnosis of COVID-19.
Main Outcomes and Measures
The primary outcomes were the incidence and risk of autoimmune and autoinflammatory connective tissue disorders following COVID-19. A total of 32 covariates, including demographics, socioeconomic statuses, lifestyle factors, and comorbidity profiles, were balanced through inverse probability weighting. The incidences and risks of autoimmune and autoinflammatory connective tissue disorders were compared between the groups using multivariable Cox proportional hazard analyses.
Results
A total of 354 527 individuals with COVID-19 (mean [SD] age, 52.24 [15.55] years; 179 041 women [50.50%]) and 6 134 940 controls (mean [SD] age, 52.05 [15.63] years; 3 074 573 women [50.12%]) were included. The risks of alopecia areata (adjusted hazard ratio [aHR], 1.12; 95% CI, 1.05-1.19), alopecia totalis (aHR, 1.74; 95% CI, 1.39-2.17), antineutrophil cytoplasmic antibody–associated vasculitis (aHR, 2.76; 95% CI, 1.64-4.65), Crohn disease (aHR, 1.68; 95% CI, 1.31-2.15), and sarcoidosis (aHR, 1.59; 95% CI, 1.00-2.52) were higher in the COVID-19 group. The risks of alopecia totalis, psoriasis, vitiligo, vasculitis, Crohn disease, ulcerative colitis, rheumatoid arthritis, adult-onset Still disease, Sjögren syndrome, ankylosing spondylitis, and sarcoidosis were associated with the severity of COVID-19.
Conclusions and Relevance
In this retrospective cohort study, COVID-19 was associated with a substantial risk for autoimmune and autoinflammatory connective tissue disorders, indicating that long-term management of patients with COVID-19 should include evaluation for such disorders.
Introduction
COVID-19 is widespread, and its association with various other diseases have been reported.1,2,3,4,5,6 Possible associations of COVID-19 with autoimmune diseases also have been suggested,7 because SARS-CoV-2 appears to perturb self-tolerance and trigger autoimmune reactions via cross-reactivity that may lead to the development of autoimmune diseases.6 A growing body of literature7,8,9 has reported various disease cases—including alopecia areata, vitiligo, systemic lupus erythematosus (SLE), vasculitis, and pediatric inflammatory multisystemic syndrome—that involve immunologic responses following SARS-CoV-2 infection, suggesting the potential existence of underlying immune dysregulations in individuals with COVID-19.
Due to the potential involvement of SARS-CoV-2 infection in cardiopulmonary failure, with its severity being a crucial factor for patients’ overall mortality, extensive evaluation of cardiovascular and respiratory outcomes following COVID-19 infection has been conducted.2,10 Although similarities between COVID-19 and several autoimmune diseases have been suggested,6 a comprehensive evaluation of autoimmune or inflammatory diseases as postacute COVID-19 sequelae has not yet been established. Thus, this nationwide, population-based study aimed to estimate the incidence and risk of various autoimmune and autoinflammatory connective tissue disorders following COVID-19.
Methods
Data Source
This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline and was approved by the Korean National Institute for Bioethics Policy with a waiver of informed consent due to the use of deidentified data. We used nationwide, population-based data from the Korea Disease Control and Prevention Agency (KDCA) COVID-19 National Health Insurance Service (NHIS) registry. The NHIS COVID-19 registry is managed by the Korean government and collates information regarding the date of diagnosis, route of infection, and mortality outcomes of individuals with confirmed COVID-19. Korea has a single health care insurance system (NHIS) that covers more than 99% of the entire Korean population and provides comprehensive information regarding socioeconomic status, inpatient and outpatient care, diagnoses of disease, procedures, and prescriptions of the enrolled patients.11
Data Setting and Study Population
Because Korea was 1 of the last countries to exhibit the nationwide spread of COVID-19, the number of confirmed COVID-19 cases before October 2020 was very small (24 352 cases [0.047%] estimated on the basis of the total population of Korea in 2020).12,13 Owing to the data anonymization policy established by the Korean government, our database excluded the information collected on or before October 7, 2020. Among the 581 500 individuals who received a confirmed COVID-19 diagnosis between October 8, 2020, and December 31, 2021, we extracted the data of only those who underwent a general health examination for further covariate control (354 886 individuals). The general health examination is provided by the Korean government annually or biannually to all employees, householders, and citizens aged 40 years or older.14,15 It includes health assessments such as blood and urine tests, anthropometric measurements, and also gathers information about an individual’s lifestyle and behavior through structured questionnaires.15 Finally, individuals who had tested positive for COVID-19 via polymerase chain reaction testing and were alive at the date of diagnosis were identified (Figure 1). All individuals underwent polymerase chain reaction testing at government-operated triage rooms located across various cities, including both individuals who exhibited symptoms and individuals who were asymptomatic and working or residing in high-risk environments. The date of positive COVID-19 test result served as the study index date for the COVID-19 cohort.
Figure 1. Flowchart of Study Population Selection.
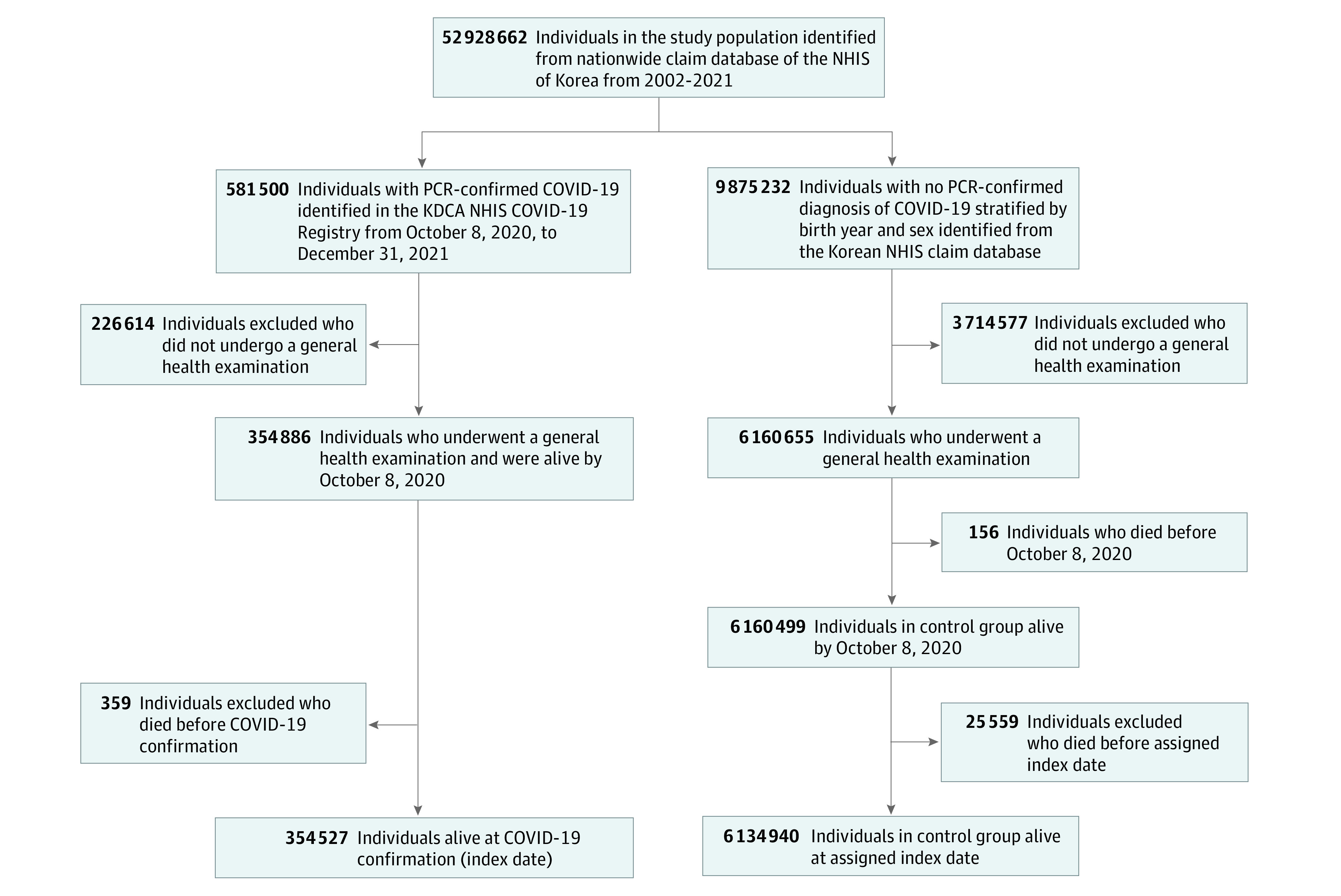
A total of 354 527 individuals with COVID-19 and 6 134 940 individuals without COVID-19 (as controls) were selected from the Korean Disease Control and Prevention Agency (KDCA) COVID-19 National Health Insurance Service (NHIS) cohort. PCR indicates polymerase chain reaction.
For comparison, we identified 9 875 232 individuals stratified by birth year and sex who had no evidence of SARS-CoV-2 infection (ie, those in the NHIS database who were not registered in the NHIS COVID-19 registry) as the primary control cohort (approximately 20% of the total Korean population) from the entire Korean population in 2020.13 Then, we extracted data from only those who had general health examination data (6 160 655 individuals), and those who were alive by October 8, 2020 (6 160 499 individuals). To ensure that the control group had a similar observational period as the COVID-19 group, we randomly assigned the study index date for the control participants according to the distribution of the study index date in the COVID-19 group; hence, the proportion of people enrolled on a certain date was the same in both the control and COVID-19 groups. The study population was followed up from the study index date to each disease diagnosis, emigration, death, or the end of the study period (December 31, 2021).
Outcomes
The incidences and risks of autoimmune and autoinflammatory connective tissue disorders were assessed during the follow-up of those without a history of such outcomes before the study index date. The occurrence of outcome diseases was defined as at least 3 medical visits with the corresponding International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnostic code. To validate our cohort and analyses, outcomes of cardiovascular diseases that were reported to be associated with COVID-1910 and outcomes less likely to be associated with COVID-19 were set as the positive and negative control outcomes, respectively, and were examined. The predefined outcomes and corresponding ICD-10 codes are summarized in eTable 1 in Supplement 1.
Covariates
The demographics, socioeconomic statuses, lifestyle factors, and comorbidity profiles of the study population were obtained from the NHIS database. We set covariates that may potentially be associated with the disease outcome on the basis of previous literature and the biological plausibility of associations.10,14,16,17,18 The predefined covariates are listed in the eMethods in Supplement 1.
Statistical Analyses
The propensity scores for individuals were estimated as the probability of belonging to the COVID-19 cohort on the basis of the covariates and were used to calculate the inverse probability weights, which were calculated as follows: probability of belonging to the COVID-19 cohort / (1 − the probability of being in the COVID-19 cohort). Covariate balances before and after the application of probability weights were assessed using standardized mean differences. We then estimated the risks of predefined outcomes for COVID-19 vs control cohorts. Statistical estimates were derived using the multivariable Cox proportional hazard analysis after adjusting for all covariates used for inverse probability weighting. For each analysis, individuals who had already received a diagnosis with the target outcome at the index date or before were excluded; hence, the analysis included only individuals at risk. To further investigate specific populations within both groups, we then conducted subgroup analyses according to age, sex, severity of COVID-19 (intensive care unit [ICU] care vs non-ICU care), and COVID-19 vaccination status. Viral vector vaccines (ChAdOx1, Oxford-AstraZeneca; Ad26.COV2.S, Janssen-Johnson & Johnson), mRNA vaccines (BNT162b2, Pfizer-BioNTech; mRNA-1273, Moderna), and protein subunit vaccines (NVX-CoV2373, Novavax) were supplied on a national basis. Vaccination completion was assessed according to the schedules recommended for each vaccine. All statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute) and R statistical software version 3.4.1 (R Project for Statistical Computing) from September 2022 to August 2023.
In sensitivity analyses, given the exclusion of a substantial number of individuals without health examination data, we compared demographic and clinical characteristics between the examined and unexamined populations to assess potential selection bias. Next, we repeated our analysis including only those alive at least 60 days after their COVID-19 diagnosis or index date, with a more stringent observation period considering the lag time in the outcome disease developments. This 60-day threshold was determined by our preliminary analysis, which indicated a markedly elevated mortality rate in the COVID-19 cohort during that period (eFigure 1 in Supplement 1), thereby excluding early mortality cases as a competing risk for outcome disease development.
Results
Study Population
In total, 354 527 individuals in the COVID-19 group (mean [SD] age, 52.24 [15.55] years; 179 041 women [50.50%]) and 6 134 940 individuals (mean [SD] age, 52.05 [15.63] years; 3 074 573 women [50.12%]) in the control group were analyzed (Table and Figure 1). Assessment of covariate balance after the application of inverse probability weighting suggested that the covariates were well balanced (eFigure 2 in Supplement 1). The mean (SD) follow-up times for the COVID-19 and control cohorts were 119.70 (117.90) and 121.40 (118.70) days, respectively.
Table. Demographic and Clinical Characteristics of Individuals With COVID-19 and Controls Before and After Inverse Probability Treatment Weighting.
| Characteristic | Preweighting, patients, No. (%) | Postweighting, weighted % | ||||
|---|---|---|---|---|---|---|
| COVID-19 (n = 354 527) | Control (n = 6 134 940) | ASD | COVID-19 | Control | ASD | |
| Age, mean (SD), y | 52.24 (15.55) | 52.05 (15.63) | 0.0097 | 52.00 (65.90) | 52.00 (16.01) | 0.0010 |
| Sex | ||||||
| Female | 179 041 (50.50) | 3 074 573 (50.12) | 0.0055 | 50.26 | 50.25 | 0.0003 |
| Male | 175 486 (49.50) | 3 060 367 (49.88) | 49.74 | 49.75 | ||
| Insurance type | ||||||
| Standard | 341 665 (96.37) | 5 925 309 (96.58) | 0.0084 | 97.11 | 97.08 | 0.0015 |
| Medicaid | 12 862 (3.63) | 209 631 (3.42) | 2.89 | 2.92 | ||
| Income level quartilea | ||||||
| 1 (Highest) | 102 008 (28.77) | 1 836 937 (29.94) | 0.0342 | 30.03 | 30.25 | 0.0006 |
| 2 (Higher) | 100 392 (28.32) | 1 764 919 (28.77) | 29.09 | 29.06 | ||
| 3 (Lower) | 93 035 (26.24) | 1 541 440 (25.13) | 25.94 | 25.39 | ||
| 4 (Lowest) | 57 718 (16.28) | 957 348 (15.60) | 14.94 | 15.29 | ||
| Area of residence | ||||||
| Urban | 193 943 (54.70) | 2 735 002 (44.58) | 0.2028 | 54.39 | 54.76 | 0.0074 |
| Rural | 160 584 (45.30) | 3 399 938 (55.42) | 45.61 | 45.24 | ||
| No. of health care encounters per year, mean (SD) | 15.90 (16.73) | 15.03 (16.75) | 0.0514 | 15.09 (64.96) | 14.94 (17.09) | 0.0091 |
| COVID-19 vaccination status | ||||||
| Vaccinated at least 1 time | 194 194 (54.78) | 3 954 348 (64.46) | 0.0221 | 63.39 | 63.90 | 0.0083 |
| Unvaccinated | 160 333 (45.22) | 2 180 592 (35.54) | 36.61 | 36.10 | ||
| General health examination data, mean (SD) | ||||||
| Height, cm | 164.40 (9.36) | 164.20 (9.35) | 0.0158 | 164.30 (39.63) | 164.30 (9.60) | 0.0002 |
| Weight, kg | 66.28 (13.37) | 65.62 (13.30) | 0.0506 | 65.65 (55.72) | 65.66 (13.69) | 0.0007 |
| Body mass indexb | 24.40 (3.70) | 24.20 (3.92) | 0.0540 | 24.21 (15.41) | 24.21 (4.12) | 0.0001 |
| Waist circumference, cm | 81.95 (11.20) | 81.38 (11.77) | 0.0504 | 81.41 (44.73) | 81.38 (11.95) | 0.0024 |
| Smoking status | ||||||
| Current smoker | 68 162 (19.23) | 1 161 460 (18.93) | 0.0080 | 19.04 | 18.96 | 0.0020 |
| Former smoker | 52 678 (14.86) | 1 086 653 (17.71) | 17.55 | 17.54 | ||
| Never smoker | 233 687 (65.92) | 3 886 827 (63.36) | 63.41 | 63.50 | ||
| Blood pressure, mean (SD), mm Hg | ||||||
| Systolic | 123.00 (15.14) | 123.00 (15.04) | 0.0009 | 123.00 (64.61) | 123.00 (15.48) | 0.0013 |
| Diastolic | 75.88 (10.36) | 75.90 (10.20) | 0.0015 | 75.88 (44.10) | 75.90 (10.49) | 0.0020 |
| Laboratory values, mean (SD) | ||||||
| Hemoglobin, g/dL | 14.17 (1.58) | 14.20 (1.59) | 0.0177 | 14.19 (6.72) | 14.19 (1.64) | 0.0025 |
| Fasting serum glucose, mg/dL | 101.60 (25.88) | 101.30 (25.14) | 0.0122 | 101.30 (109.50) | 101.30 (25.90) | 0.0006 |
| Aspartate aminotransferase, U/L | 26.68 (25.47) | 26.49 (23.70) | 0.0075 | 26.72 (131.20) | 26.53 (28.09) | 0.0078 |
| Alanine transaminase, U/L | 26.30 (28.92) | 26.17 (27.39) | 0.0049 | 26.46 (183.40) | 26.17 (28.12) | 0.0104 |
| γ-Glutamyltransferase, U/L | 36.81 (50.82) | 36.53 (50.97) | 0.0060 | 36.55 (212.10) | 36.54 (52.56) | 0.0002 |
| Creatinine, mg/dL | 0.86 (0.63) | 0.86 (0.53) | 0.0097 | 0.86 (2.70) | 0.86 (0.54) | 0.0013 |
| Underlying disease | ||||||
| Hypertension | 106 702 (30.1) | 1 759 640 (28.68) | 0.0290 | 28.71 | 28.62 | 0.0018 |
| Diabetes | 61 160 (17.25) | 966 435 (15.75) | 0.0388 | 15.78 | 15.73 | 0.0013 |
| Dyslipidemia | 143 511 (40.48) | 2 363 824 (38.53) | 0.0374 | 38.67 | 38.57 | 0.0020 |
| Chronic obstructive pulmonary disease | 5676 (1.60) | 94 600 (1.54) | 0.0037 | 1.50 | 1.50 | 0.0002 |
| Chronic kidney disease | 4881 (1.38) | 68 218 (1.11) | 0.0225 | 1.13 | 1.11 | 0.0019 |
| Liver disease | 10 113 (2.85) | 169 071 (2.76) | 0.0056 | 2.73 | 2.75 | 0.0010 |
| Atopic dermatitis | 4114 (1.16) | 68 358 (1.11) | 0.0039 | 1.12 | 1.12 | 0.0004 |
| Allergic rhinitis | 17 869 (5.04) | 297 662 (4.85) | 0.0085 | 4.87 | 4.87 | 0.0001 |
| Asthma | 8427 (2.38) | 136 555 (2.23) | 0.0096 | 2.23 | 2.22 | 0.0009 |
| Hepatitis B | 7217 (2.04) | 123 402 (2.01) | 0.0014 | 2.02 | 2.02 | 0.0003 |
| Hepatitis C | 1318 (0.37) | 18 817 (0.31) | 0.0107 | 0.31 | 0.31 | 0.0002 |
| HIV infection | 18 (0.01) | 276 (<0.01) | 0.0005 | 0 | 0 | 0.0005 |
Abbreviation: ASD, absolute standardized difference.
SI conversion factors: To convert γ-glutamyltransferase to microkatals per liter, multiply by 0.0167; alanine transaminase to microkatals per liter, multiply by 0.0167; aspartate aminotransferase to microkatals per liter, multiply by 0.0167; creatinine to micromoles per liter, multiply by 76.25; fasting glucose serum, multiply by 0.0555; hemoglobin to grams per liter, multiply by 10.
The income level was divided into quartiles on the basis of health insurance premiums.
Body mass index was calculated as weight in kilograms divided by height in meters squared.
Autoinflammatory and Autoimmune Connective Disorders Following COVID-19
The incidences and risks of predefined diseases in the COVID-19 and control cohorts during the follow-up were estimated (Figure 2). Individuals with COVID-19 had significantly higher risks of alopecia areata (adjusted hazard ratio [aHR], 1.12; 95% CI, 1.05-1.19), alopecia totalis (aHR, 1.74; 95% CI, 1.39-2.17), antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (aHR, 2.76; 95% CI, 1.64-4.65), Crohn disease (aHR, 1.68; 95% CI, 1.31-2.15), and sarcoidosis (aHR, 1.59; 95% CI, 1.00-2.52). However, the risk of SLE was lower (aHR, 0.47; 95% CI, 0.36-0.61) in the COVID-19 cohort. The positive and negative outcome suggested minimal overdetection bias in the COVID-19 cohort. The cumulative incidence of each outcome disease is shown in eFigure 3 in Supplement 1.
Figure 2. Risks of Incident Autoimmune and Autoinflammatory Disease Outcomes in the COVID-19 Cohort Compared With the Control Cohort.
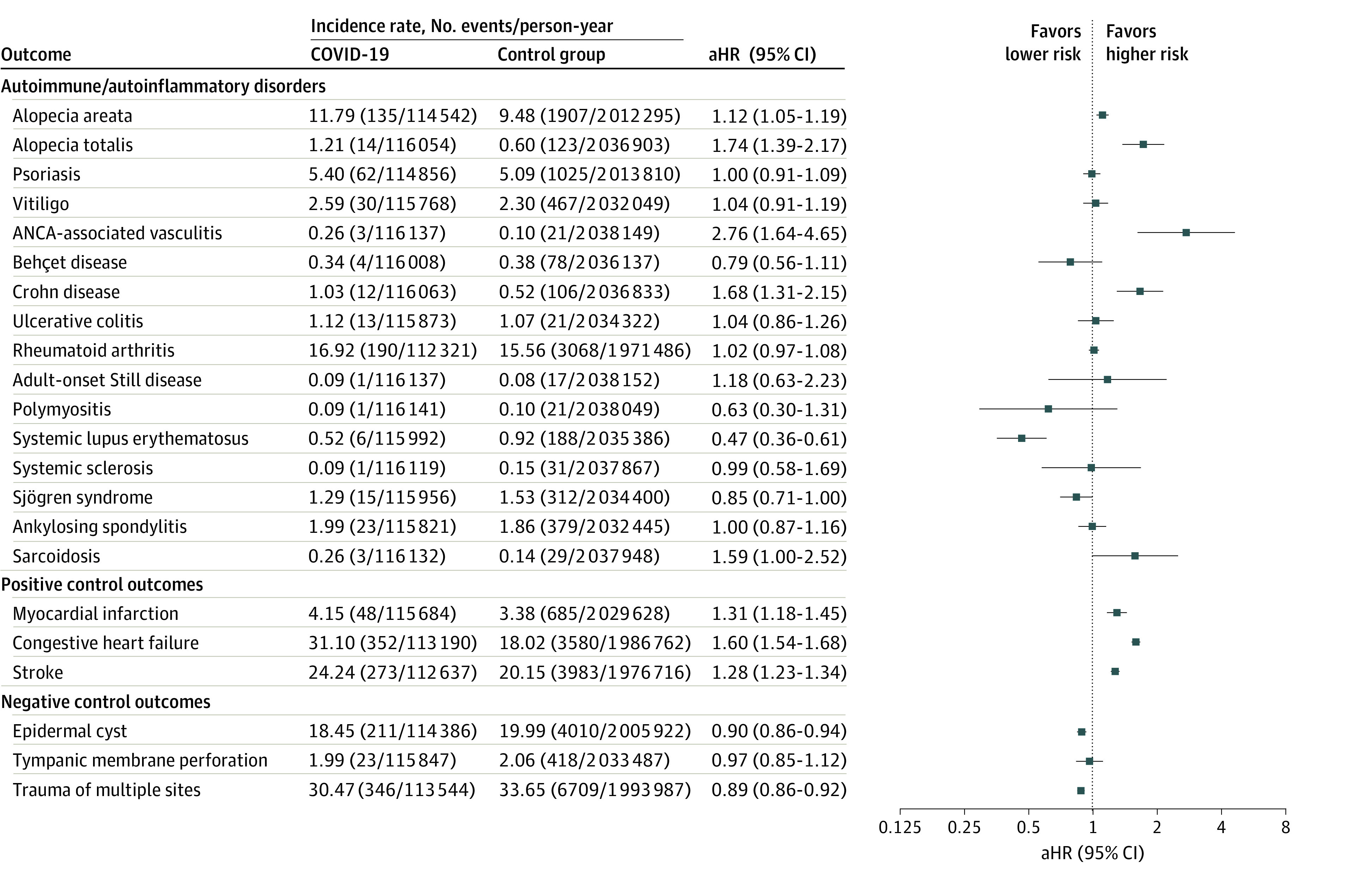
The forest plot depicts adjusted hazard ratios (aHRs) and 95% CIs of individuals with COVID-19 compared with control participants. The hazard estimates were adjusted for all 32 covariates used for the inverse probability of treatment weighting. ANCA indicates antineutrophilic cytoplasmic antibody.
Subgroup Analyses
We further examined the risks of incident disease outcomes in the subgroups according to age, sex, and severity of COVID-19 (Figure 3 and Figure 4). The subgroup comprising women showed increased risks of alopecia areata, alopecia totalis, vitiligo, ANCA-associated vasculitis, Crohn disease, and sarcoidosis in the COVID-19 cohort; the subgroup comprising men in the COVID-19 cohort revealed increased risks of alopecia totalis, psoriasis, Crohn disease, adult-onset Still disease, systemic sclerosis, and ankylosing spondylitis. With age stratification, the risks of alopecia areata, totalis, and ANCA-associated vasculitis were higher in individuals aged 40 years or older, whereas the risks of Crohn disease, rheumatoid arthritis, adult-onset Still disease, and sarcoidosis were higher in individuals aged younger than 40 years (Figure 3).
Figure 3. Subgroup Analyses of the Risks of Incident Autoimmune and Autoinflammatory Disease Outcomes Stratified by Age and Sex .
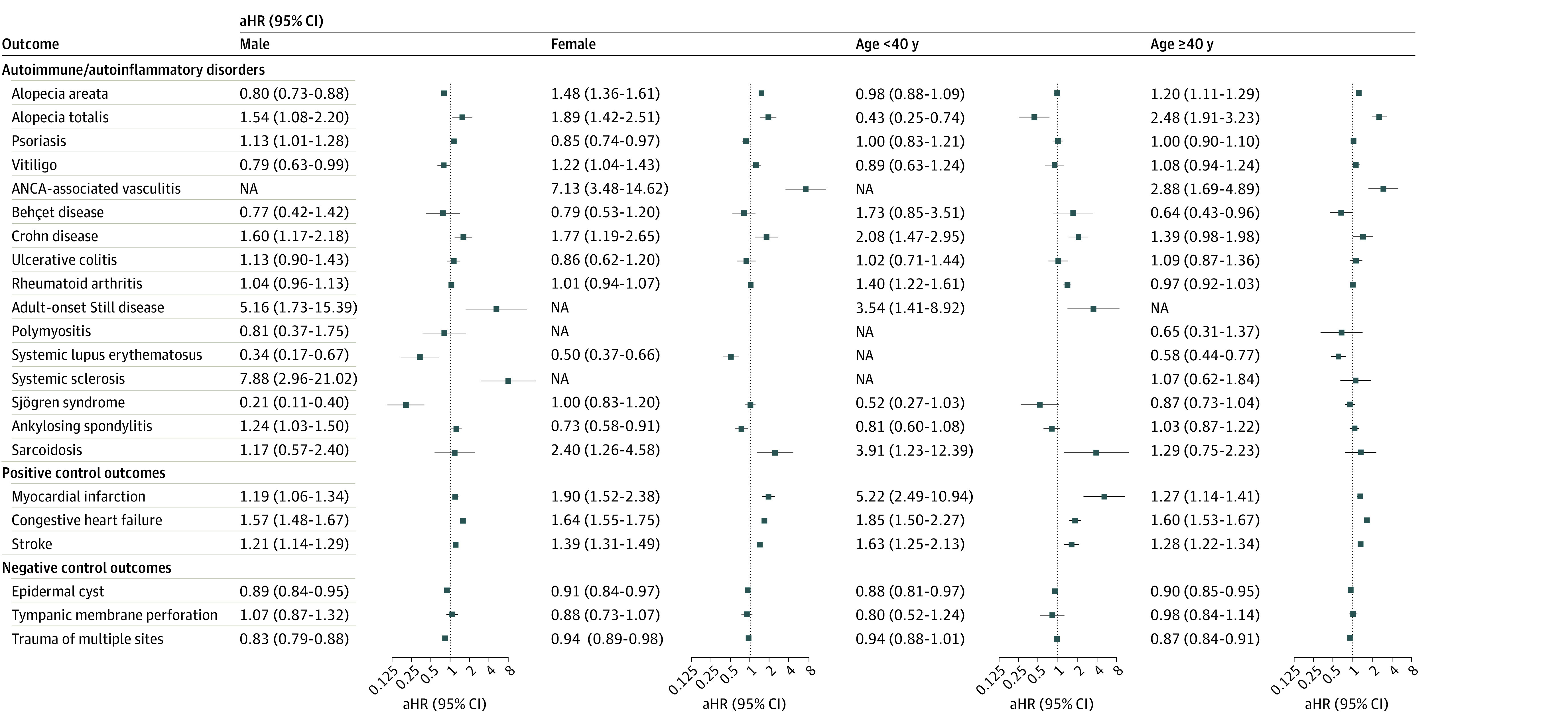
The forest plot depicts adjusted hazard ratios (aHRs) and 95% CIs of individuals with COVID-19 compared with control participants. The hazard estimates were adjusted for all 32 covariates used for the inverse probability of treatment weighting. ANCA indicates antineutrophilic cytoplasmic antibody; and NA, not available.
Figure 4. Subgroup Analysis of the Risks of Incident Autoimmune and Autoinflammatory Disease Outcomes in the COVID-19 Cohort Stratified by COVID-19 Severity and COVID-19 Vaccination Status.
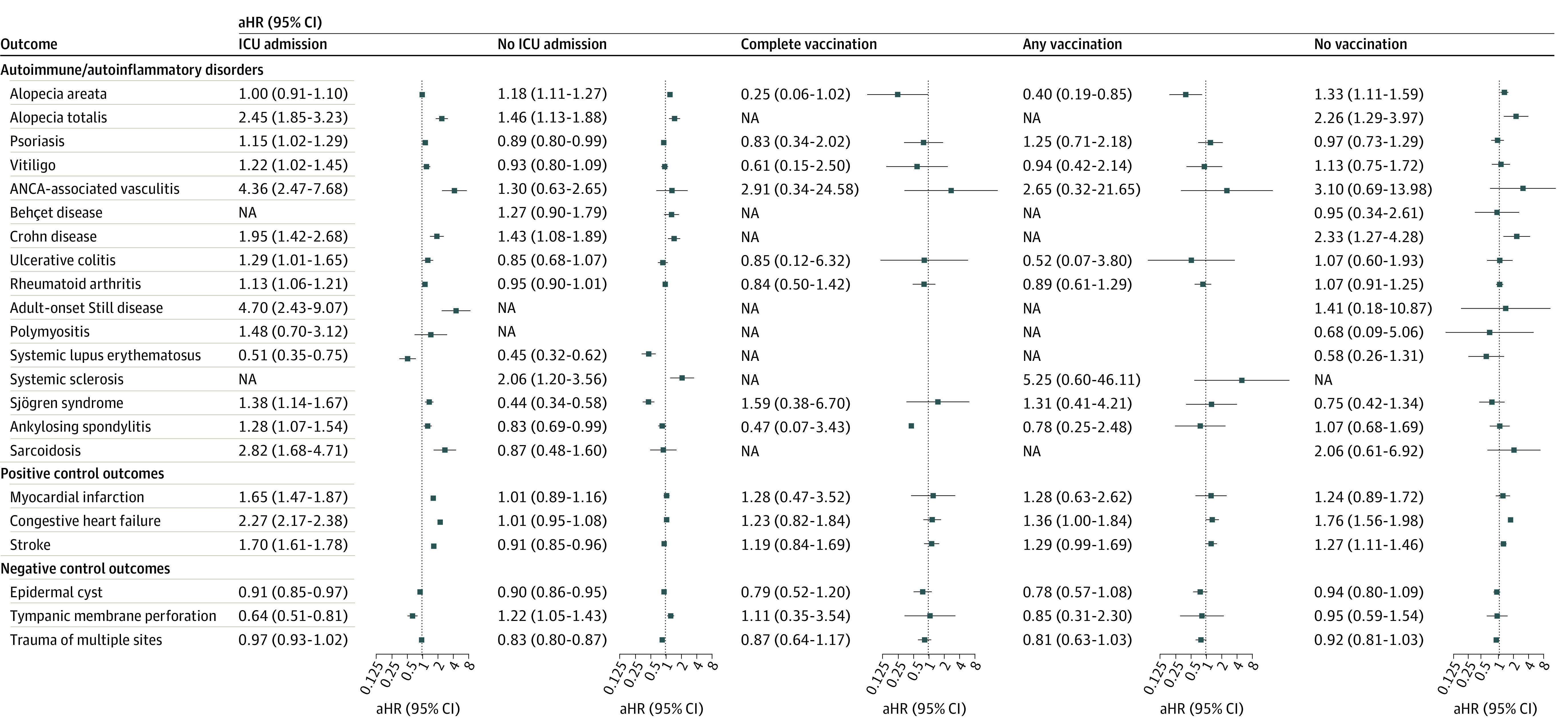
The forest plot depicts adjusted hazard ratios (aHRs) and 95% CIs of individuals with COVID-19 compared with control participants. Subgroup analyses stratified by severity of COVID-19 (intensive care unit [ICU] vs non-ICU) and vaccination status are shown. Vaccination completion was assessed according to the schedules recommended for each vaccine. The hazard estimates were adjusted for 32 covariates used for the inverse probability of treatment weighting. ANCA indicates antineutrophilic cytoplasmic antibody; and NA, not available.
Incident disease outcomes were then evaluated on the basis of the severity of COVID-19 (Figure 4). Despite lower SLE risk in both subgroups and reduced risk of Sjögren syndrome and ankylosing spondylitis in the non-ICU group, the overall risks of incident disease outcomes increased markedly with the severity of the acute stage of COVID-19. When stratified by COVID-19 vaccination status (Figure 4), a greater risk of autoimmune diseases such as alopecia areata, alopecia totalis, and Crohn disease was observed in the unvaccinated group, as well as for positive control outcomes. However, in the vaccinated subgroups, increased risks were diminished for both autoimmune and positive control outcomes.
Sensitivity Analysis
Demographic data and clinical characteristics were compared between those who underwent health examinations and those who did not (eTable 2 in Supplement 1). The examined group predominantly consisted of adults, because health examinations are generally targeted at householders or employed individuals. The COVID-19 positivity rates were similar for both groups. Notably, the COVID-19 vaccination rate was higher in the examined group. This disparity may be attributed to the public health policy in Korea at that time, which involved a cautious approach to vaccinating adolescents and children due to concerns over efficacy and potential risks. This suggested that our findings were predominantly derived from the adult population.
Sensitivity analyses of disease risks in survivors for at least 60 days after COVID-19 diagnosis were performed (eTable 3 and eFigure 4 in Supplement 1). Furthermore, due to the highest standardized mean difference in area of residence, we conducted an additional analysis adjusting only for this variable (eFigure 5 in Supplement 1). Both sets of findings were consistent with the main results.
Discussion
In this cohort study, we comprehensively examined and compared the risks of autoimmune and autoinflammatory connective tissue disorders in COVID-19 and control cohorts. Several previous reports have evaluated the incidence of autoimmune diseases following COVID-19.19,20 One large cohort study19 using a global COVID-19 data set found that the COVID-19 group had a higher risk of various autoimmune diseases compared with a control group. Subgroup analyses stratified by race indicated that White patients generally exhibited a higher risk than Asian patients, except for SLE.19 Another study20 assessing the incidence of autoimmune diseases after COVID-19 in the German population also observed an elevated risk within the COVID-19 cohort (first onset of autoimmune disease incidence rate ratio, 1.43; 95% CI, 1.37-1.48). Given that our study primarily included Asian participants, we hypothesize that their estimated risks were lower, or may appear to be lower, as a result of delayed disease development or progression, compared with those observed in other ethnic groups. Furthermore, the prevalence of SLE in Korea is lower than that in the US (18.8-21.7 cases per 100 000 people vs 78.5-124.0 cases per 100 000 people)21 and the allele frequency of autoimmune disease–associated single-nucleotide variation is more varied among White individuals than in Asian individuals.22
In a biological context, one study23 has suggested that SARS-CoV-2 infection may be associated with autoimmunity. Widely distributed tissue antigens may be a target of cross-reactive antibodies generated against SARS-CoV-2 epitopes.23 Individuals with COVID-19 were reported to have low levels of complements24 and positive autoantibodies.25,26,27,28,29 Another hypothesis suggested that the release of cytokines (ie, a cytokine storm) may trigger autoimmune responses as bystander activation.30,31 Various cytokines, including interleukin (IL)–6 and IL–1, were reported to be associated with immune-mediated damage in individuals with SARS-CoV-2 infection,32,33 and the efficacy of antagonists of such cytokines in severely ill patients have been extensively studied.34,35,36,37 Dysfunctional angiotensin-converting enzyme 2 and its variant were also suggested to contribute to a skewed inflammatory microenvironment.38,39
Patients with COVID-19 mount an early and robust defense through the activation of type 1 and 2 interferon (IFN) responses, which are pivotal against viral infections.40,41 Nevertheless, studies40,41 indicate that these IFN responses may also induce hyperinflammation, exacerbate the severity of COVID-19, and be associated with mortality. A high IFN status has also been associated with the pathogenesis of autoimmune disorders, such as vitiligo and alopecia areata, which exhibit type 1 and type 2 IFN signatures, respectively,42,43 suggesting a potential association of excessive antiviral responses with the subsequent breaching of immune-privileged areas leading to immune responses against self-antigens.
The risk of psoriasis was slightly elevated in the COVID-19 subcohort comprising men and those who had severe COVID-19. Previous reports described aggravation of preexisting psoriatic lesions44,45 and increased levels of T helper (TH) 17–related cytokines in patients with COVID-19.46,47 Thereby, the TH17–skewed milieu induced by SARS-CoV-2 infection may contribute to the pathogenesis of psoriasis.
The incidence of ANCA-associated vasculitis was increased in individuals with COVID-19, which was consistent with previous reports.48,49,50 Plausible mechanisms involve viral-induced hypercoagulability resulting from endothelial infection or injury, complement system activation, and dysregulation of the coagulation cascade, ultimately leading to vasculopathy.51,52 Moreover, the protracted exposure of neutrophil extracellular traps has been postulated to elicit the development of antineutrophil antibodies.50
A few studies have reported Crohn disease53,54 and ulcerative colitis following COVID-19.55,56 Notably, our overall COVID-19 cohort showed increased risk of Crohn disease, whereas the risk of ulcerative colitis was increased in the severe COVID-19 subgroup only. Crohn disease is mainly mediated by TH1 and TH17, whereas ulcerative colitis is an atypical TH2 disease.57 Hyperactivated TH1 response against SARS-CoV-2 may be associated with Crohn disease, and triggering of TH2 response which is associated with poor prognosis of COVID-19 and may contribute to the development of ulcerative colitis.58
The precise association of COVID-19 with rheumatoid arthritis remains unclear, and inconsistent findings have been reported in the literature.59,60,61,62 Although several investigations61,62 failed to detect significant differences in the prevalence of anticitrullinated antibody positivity following COVID-19, some investigations62 suggested that cytokine dysregulation is more likely to be associated with rheumatoid arthritis than antibody-mediated reactions. Our data showed that younger individuals (<40 years) and those who had a severe COVID-19 infection had an increased risk of rheumatoid arthritis.
Development of sarcoidosis following COVID-19 infection has also been reported.63,64 Although the causes of sarcoidosis are not fully understood, evidence suggests that an aberrant TH1 response together with cytokines such as IL–2, IL–12, IL–17, IL–22, IFN–γ, and tumor necrosis factor–α may contribute to sarcoidal granuloma formation.65,66 However, we believe that our findings regarding this should be interpreted with caution because we had only a small number of incident cases in the COVID-19 group.
Our data revealed that the risk of incident SLE was decreased in the COVID-19 cohort, which seems to contradict the results of other autoimmune diseases in our study. The observation period may not have been long enough to capture the full spectrum of SLE development. This is suggested by the cumulative incidence of SLE (eFigure 3 in Supplement 1), which shows a delayed disease onset occurring nearly 100 days after a COVID-19 diagnosis. Moreover, SLE may be associated with the severity of COVID-19; a higher mortality in critical COVID-19 cases may skew toward reduced SLE risk.
The severity of the acute stage of COVID-19 was associated with autoimmune disease outcomes. Overall, the risks of the most common outcome diseases were higher among the ICU-admitted subgroup compared with those with milder (non-ICU) cases. The severity of COVID-19 and death were associated with increased levels of diverse cytokines, such as tumor necrosis factor–α and IL-6,67 suggesting a possible association with sustained autoimmunity.
Whether COVID-19 vaccination triggers autoimmune diseases has been controversial. Our data showed no elevated disease risks in the vaccinated subgroups. Notably, the risk in positive control outcomes also disappeared, suggesting a potential protective effect of vaccination against COVID-19–related disease development.68 This is consistent with a previous report69 that evaluated the risk of autoimmune diseases after mRNA vaccination in Korean population, wherein no significant increase in the risk of autoimmune diseases was noted.
Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19. Although the risks of developing each disease following COVID-19 varied, there was a clear tendency toward increased risk overall, especially in those who experienced a severe case of COVID-19. This suggests the existence of a common pathway, which may involve excessive cytokine storm leading to prolonged autoimmune responses that trigger specific underlying pathophysiology of each disease. Taken together, surveillance for the new development of autoimmune and autoinflammatory diseases for the myriad of COVID-19 survivors globally is suggested.
Limitations and Strengths
This study has several limitations. The demographic composition consisted almost entirely of a single ethnicity, and the age distribution was largely skewed toward adults. Consequently, the results are not generalizable to adolescents and children. Despite rigorous adjustments with covariates, possible misclassifications and residual confounders may exist. There is a possibility that there were some people who had SARS-CoV-2 infection but did not undergo the COVID-19 polymerase chain reaction test and were allocated to the control group. Some diseases included only a small number of incident cases, which could have led to imprecise interpretation. Our data lacked detailed information regarding each participant (eg, information on genetic background) and could not differentiate between individuals who were more susceptible to autoimmune diseases. Early mortality may have acted as a competing factor that could obscure the development of diseases. In addition, the follow-up period was relatively short to fully assess disease development. However, the key strengths of this study lie in its comprehensive statistical analyses, which incorporated diverse covariates with positive and negative controls.
Conclusions
Our study comprehensively investigated the risks of autoimmune and autoinflammatory connective tissue disorders in patients with COVID-19 compared with controls, highlighting these disorders as potential post–COVID-19 sequelae. Long-term management of patients with COVID-19 should include evaluation of subsequent development of autoimmune and autoinflammatory connective tissue disorders.
eTable 1. International Statistical Classification of Diseases, Tenth Revision (ICD-10) Codes of the Included Diseases
eMethods. The Predefined Covariates for Inverse Probability Weighting
eFigure 1. Cumulative Incidence Plot for All-Cause Mortality Among Patients With COVID-19 and Controls
eFigure 2. Covariate Balance After Application of Inverse Probability Weighting
eFigure 3. Cumulative Incidences of Autoinflammatory and Autoimmune Connective Tissue Disorders Outcomes
eTable 2. Sensitivity Analysis: Demographic and Clinical Characteristics of Individuals Who Underwent and Who Did Not Undergo General Health Examination
eTable 3. Sensitivity Analysis: Demographic and Clinical Characteristics of Individuals Who Survived at Least 60 Days Post COVID-19 Diagnosis and Controls Before And After Weighting
eFigure 4. Sensitivity Analyses: Risks of Incident Autoimmune and Autoinflammatory Disease Outcomes in Individuals Survived by at Least 60 Days Post–COVID-19 Diagnosis and the Controls
eFigure 5. Sensitivity Analysis: Risks of Incident Autoimmune and Autoinflammatory Disease Outcomes in COVID-19 Cohort and the Control Cohort, Adjusted for Area of Residency (Urban vs Rural) Status Only
Data Sharing Statement
References
- 1.Lee SC, Son KJ, Han CH, Jung JY, Park SC. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci Rep. 2020;10(1):21805. doi: 10.1038/s41598-020-77791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller-Wieland D, Marx N, Dreher M, Fritzen K, Schnell O. COVID-19 and cardiovascular comorbidities. Exp Clin Endocrinol Diabetes. 2022;130(3):178-189. doi: 10.1055/a-1269-1405 [DOI] [PubMed] [Google Scholar]
- 3.Hua S, Yang Y, Zou D, et al. COVID-19 and metabolic comorbidities: an update on emerging evidences for optimal therapies. Biomed Pharmacother. 2021;140:111685. doi: 10.1016/j.biopha.2021.111685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad A, Ishtiaq SM, Khan JA, Aslam R, Ali S, Arshad MI. COVID-19 and comorbidities of hepatic diseases in a global perspective. World J Gastroenterol. 2021;27(13):1296-1310. doi: 10.3748/wjg.v27.i13.1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi: 10.1016/j.ijid.2020.11.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33(2):155-162. doi: 10.1097/BOR.0000000000000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8):413-414. doi: 10.1038/s41584-020-0448-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt AF, Rubin A, Milgraum D, Wassef C. Vitiligo following COVID-19: a case report and review of pathophysiology. JAAD Case Rep. 2022;22:47-49. doi: 10.1016/j.jdcr.2022.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phong CH, Babadjouni A, Nguyen C, Kraus CN, Mesinkovska NA. Not just thinning: a case of alopecia universalis after mild COVID-19. JAAD Case Rep. 2022;25:1-3. doi: 10.1016/j.jdcr.2022.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583-590. doi: 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheol Seong S, Kim YY, Khang YH, et al. Data resource profile: the national health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46(3):799-800. doi: 10.1093/ije/dyw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korea Disease Control and Prevention Agency; Statistics Korea . COVID-19 dashboard. Updated August 28, 2023. Accessed August 30, 2023. https://kosis.kr/covid/covid_index.do
- 13.Korean Statistical Information Service; Statistics Korea . Population households and housing units. Updated July 27, 2023. Accessed August 30, 2023. https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1IN1502&vw_cd=MT_ETITLE&list_id=A11_2015_1&scrId=&language=en&seqNo=&lang_mode=en&obj_var_id=&itm_id=&conn_path=MT_ETITLE&path=%252Feng%252FstatisticsList%252FstatisticsListIndex.do
- 14.Lee S, Lee YB, Kim BJ, Bae S, Lee WS. All-cause and cause-specific mortality risks associated with alopecia areata: a Korean nationwide population-based study. JAMA Dermatol. 2019;155(8):922-928. doi: 10.1001/jamadermatol.2019.0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. doi: 10.1093/ije/dyv319 [DOI] [PubMed] [Google Scholar]
- 16.Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28(11):2406-2415. doi: 10.1038/s41591-022-02001-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safary A, Esalatmanesh K, Eftekharsadat AT, Jafari Nakjavani MR, Khabbazi A. Autoimmune inflammatory rheumatic diseases post-COVID-19 vaccination. Int Immunopharmacol. 2022;110:109061. doi: 10.1016/j.intimp.2022.109061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez Y, Rojas M, Beltrán S, et al. Autoimmune and autoinflammatory conditions after COVID-19 vaccination: new case reports and updated literature review. J Autoimmun. 2022;132:102898. doi: 10.1016/j.jaut.2022.102898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang R, Yen-Ting Chen T, Wang SI, Hung YM, Chen HY, Wei CJ. Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study. EClinicalMedicine. 2023;56:101783. doi: 10.1016/j.eclinm.2022.101783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tesch F, Ehm F, Vivirito A, et al. Incident autoimmune diseases in association with SARS-CoV-2 infection: a matched cohort study. Clin Rheumatol. Published online June 19, 2023. doi: 10.1007/s10067-023-06670-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju JH, Yoon SH, Kang KY, et al. Prevalence of systemic lupus erythematosus in South Korea: an administrative database study. J Epidemiol. 2014;24(4):295-303. doi: 10.2188/jea.JE20120204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori M, Yamada R, Kobayashi K, Kawaida R, Yamamoto K. Ethnic differences in allele frequency of autoimmune-disease-associated SNPs. J Hum Genet. 2005;50(5):264-266. doi: 10.1007/s10038-005-0246-8 [DOI] [PubMed] [Google Scholar]
- 23.Mohkhedkar M, Venigalla SSK, Janakiraman V. Untangling COVID-19 and autoimmunity: identification of plausible targets suggests multi organ involvement. Mol Immunol. 2021;137:105-113. doi: 10.1016/j.molimm.2021.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mobini M, Ghasemian R, Vahedi Larijani L, Mataji M, Maleki I. Immunologic markers, vasculitis-associated autoantibodies, and complement levels in patients with COVID-19. J Res Med Sci. 2021;26:103. doi: 10.4103/jrms.JRMS_923_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazzaruso C, Carlo Stella N, Mariani G, et al. High prevalence of antinuclear antibodies and lupus anticoagulant in patients hospitalized for SARS-CoV2 pneumonia. Clin Rheumatol. 2020;39(7):2095-2097. doi: 10.1007/s10067-020-05180-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13(6):1077-1086. doi: 10.1111/cts.12805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastard P, Rosen LB, Zhang Q, et al. ; HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort . Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C, Fan J, Luo Y, et al. Prevalence and characteristics of rheumatoid-associated autoantibodies in patients with COVID-19. J Inflamm Res. 2021;14:3123-3128. doi: 10.2147/JIR.S312090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlachoyiannopoulos PG, Magira E, Alexopoulos H, et al. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis. 2020;79(12):1661-1663. doi: 10.1136/annrheumdis-2020-218009 [DOI] [PubMed] [Google Scholar]
- 30.Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19(1):80-94. doi: 10.1128/CMR.19.1.80-94.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zacharias H, Dubey S, Koduri G, D’Cruz D. Rheumatological complications of Covid 19. Autoimmun Rev. 2021;20(9):102883. doi: 10.1016/j.autrev.2021.102883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405-407. doi: 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JS, Lee JY, Yang JW, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316-329. doi: 10.7150/thno.49713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators . Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491-1502. doi: 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shankar-Hari M, Vale CL, Godolphin PJ, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group . Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326(6):499-518. doi: 10.1001/jama.2021.11330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mardi A, Meidaninikjeh S, Nikfarjam S, Majidi Zolbanin N, Jafari R. Interleukin-1 in COVID-19 infection: immunopathogenesis and possible therapeutic perspective. Viral Immunol. 2021;34(10):679-688. doi: 10.1089/vim.2021.0071 [DOI] [PubMed] [Google Scholar]
- 37.Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27(10):1752-1760. doi: 10.1038/s41591-021-01499-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najafi S, Rajaei E, Moallemian R, Nokhostin F. The potential similarities of COVID-19 and autoimmune disease pathogenesis and therapeutic options: new insights approach. Clin Rheumatol. 2020;39(11):3223-3235. doi: 10.1007/s10067-020-05376-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JS, Shin EC. The type I interferon response in COVID-19: implications for treatment. Nat Rev Immunol. 2020;20(10):585-586. doi: 10.1038/s41577-020-00429-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gadotti AC, de Castro Deus M, Telles JP, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289:198171. doi: 10.1016/j.virusres.2020.198171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi A, Magri F, Michelini S, et al. New onset of alopecia areata in a patient with SARS-CoV-2 infection: possible pathogenetic correlations? J Cosmet Dermatol. 2021;20(7):2004-2005. doi: 10.1111/jocd.14080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertolotti A, Boniface K, Vergier B, et al. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res. 2014;27(3):398-407. doi: 10.1111/pcmr.12219 [DOI] [PubMed] [Google Scholar]
- 44.Shahidi Dadras M, Diab R, Ahadi M, Abdollahimajd F. Generalized pustular psoriasis following COVID-19. Dermatol Ther. 2021;34(1):e14595. doi: 10.1111/dth.14595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozaras R, Berk A, Ucar DH, Duman H, Kaya F, Mutlu H. Covid-19 and exacerbation of psoriasis. Dermatol Ther. 2020;33(4):e13632. doi: 10.1111/dth.13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadeghi A, Tahmasebi S, Mahmood A, et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J Cell Physiol. 2021;236(4):2829-2839. doi: 10.1002/jcp.30047 [DOI] [PubMed] [Google Scholar]
- 47.Martonik D, Parfieniuk-Kowerda A, Rogalska M, Flisiak R. The role of Th17 response in COVID-19. Cells. 2021;10(6):1550. doi: 10.3390/cells10061550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kataria S, Rogers S, Sadia H, et al. Antineutrophil cytoplasmic antibody (ANCA)-associated renal vasculitis after COVID-19 infection: a case report. Cureus. 2022;14(6):e26111. doi: 10.7759/cureus.26111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fireizen Y, Shahriary C, Imperial ME, Randhawa I, Nianiaris N, Ovunc B. Pediatric P-ANCA vasculitis following COVID-19. Pediatr Pulmonol. 2021;56(10):3422-3424. doi: 10.1002/ppul.25612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izci Duran T, Turkmen E, Dilek M, Sayarlioglu H, Arik N. ANCA-associated vasculitis after COVID-19. Rheumatol Int. 2021;41(8):1523-1529. doi: 10.1007/s00296-021-04914-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):e437-e445. doi: 10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGonagle D, Bridgewood C, Ramanan AV, Meaney JFM, Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3(3):e224-e233. doi: 10.1016/S2665-9913(20)30420-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Senthamizhselvan K, Ramalingam R, Mohan P, Kavadichanda C, Badhe B, Hamide A. De novo Crohn’s disease triggered after COVID-19: is COVID-19 more than an infectious disease? ACG Case Rep J. 2021;8(8):e00652. doi: 10.14309/crj.0000000000000652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tursi A, Nenna R. COVID-19 as a trigger for de novo Crohn’s disease. Inflamm Bowel Dis. 2022;28(6):e76-e77. doi: 10.1093/ibd/izab298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aydın MF, Taşdemir H. Ulcerative colitis in a COVID-19 patient: a case report. Turk J Gastroenterol. 2021;32(6):543-547. doi: 10.5152/tjg.2021.20851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cayley WE Jr. New-onset ulcerative colitis in patients with COVID-19. Am Fam Physician. 2022;106(4):362. [PubMed] [Google Scholar]
- 57.Poggi A, Benelli R, Venè R, et al. Human gut-associated natural killer cells in health and disease. Front Immunol. 2019;10:961. doi: 10.3389/fimmu.2019.00961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aleebrahim-Dehkordi E, Molavi B, Mokhtari M, et al. T helper type (Th1/Th2) responses to SARS-CoV-2 and influenza A (H1N1) virus: from cytokines produced to immune responses. Transpl Immunol. 2022;70:101495. doi: 10.1016/j.trim.2021.101495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perrot L, Hemon M, Busnel JM, et al. First flare of ACPA-positive rheumatoid arthritis after SARS-CoV-2 infection. Lancet Rheumatol. 2021;3(1):e6-e8. doi: 10.1016/S2665-9913(20)30396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouzid S, Ben Abdelghani K, Miledi S, Fazaa A, Laatar A. Can SARS-CoV-2 infection trigger rheumatoid arthritis? a case report. Clin Case Rep. 2022;10(4):e05748. doi: 10.1002/ccr3.5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Derksen VFAM, Kissel T, Lamers-Karnebeek FBG, et al. Onset of rheumatoid arthritis after COVID-19: coincidence or connected? Ann Rheum Dis. 2021;80(8):1096-1098. doi: 10.1136/annrheumdis-2021-219859 [DOI] [PubMed] [Google Scholar]
- 62.Taha SI, Samaan SF, Ibrahim RA, El-Sehsah EM, Youssef MK. Post-COVID-19 arthritis: is it hyperinflammation or autoimmunity? Eur Cytokine Netw. 2021;32(4):83-88. doi: 10.1684/ecn.2021.0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592. doi: 10.3390/cells10123592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rabufetti A, Borradori L, Heidemeyer K, et al. New onset of sarcoidosis after COVID-19 infection. J Eur Acad Dermatol Venereol. 2022;36(10):e756-e759. doi: 10.1111/jdv.18313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bennett D, Bargagli E, Refini RM, Rottoli P. New concepts in the pathogenesis of sarcoidosis. Expert Rev Respir Med. 2019;13(10):981-991. doi: 10.1080/17476348.2019.1655401 [DOI] [PubMed] [Google Scholar]
- 66.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155-1167. doi: 10.1016/S0140-6736(13)60680-7 [DOI] [PubMed] [Google Scholar]
- 67.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636-1643. doi: 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang J, Chan L, Kauffman J, et al. ; N3C Consortium . Impact of vaccination on major adverse cardiovascular events in patients with COVID-19 infection. J Am Coll Cardiol. 2023;81(9):928-930. doi: 10.1016/j.jacc.2022.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ju HJ, Lee JY, Han JH, Lee JH, Bae JM, Lee S. Risk of autoimmune skin and connective tissue disorders after mRNA-based COVID-19 vaccination. J Am Acad Dermatol. Published online May 13, 2023. doi: 10.1016/j.jaad.2023.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. International Statistical Classification of Diseases, Tenth Revision (ICD-10) Codes of the Included Diseases
eMethods. The Predefined Covariates for Inverse Probability Weighting
eFigure 1. Cumulative Incidence Plot for All-Cause Mortality Among Patients With COVID-19 and Controls
eFigure 2. Covariate Balance After Application of Inverse Probability Weighting
eFigure 3. Cumulative Incidences of Autoinflammatory and Autoimmune Connective Tissue Disorders Outcomes
eTable 2. Sensitivity Analysis: Demographic and Clinical Characteristics of Individuals Who Underwent and Who Did Not Undergo General Health Examination
eTable 3. Sensitivity Analysis: Demographic and Clinical Characteristics of Individuals Who Survived at Least 60 Days Post COVID-19 Diagnosis and Controls Before And After Weighting
eFigure 4. Sensitivity Analyses: Risks of Incident Autoimmune and Autoinflammatory Disease Outcomes in Individuals Survived by at Least 60 Days Post–COVID-19 Diagnosis and the Controls
eFigure 5. Sensitivity Analysis: Risks of Incident Autoimmune and Autoinflammatory Disease Outcomes in COVID-19 Cohort and the Control Cohort, Adjusted for Area of Residency (Urban vs Rural) Status Only
Data Sharing Statement


