Abstract
Background
Neoadjuvant immunotherapy, the focus of current research and treatment modality for long-term survival, has become one of the main options in supporting primary treatment interventions in early NSCLC.
Methods
This was a retrospective analysis of patients with locally resectable NSCLC who received the neoadjuvant drug pembrolizumab combined with chemotherapy and underwent surgical resection. Pathological responses, PFS and OS in the whole sample and subgroups were analyzed.
Results
Of the 61 patients included in this retrospective analysis, 31 (50.82%) achieved a pCR, and 38 (62.30%) obtained an MPR. Patients with a pCR had significantly higher OS than the non-pCR group (HR = 0.093, P = 0.0227); patients with an MPR also had significantly elevated OS compared with the non-MPR group (HR = 0.05357, P = 0.0169). Patients with lymph node metastasis after surgery had significantly reduced OS (HR = 0.01607, p = 0.0004) and PFS (HR = 0.08757, p = 0.0004) than those without lymph node metastasis. There was no significant difference in OS and PFS between squamous cell carcinomas (SCC) group and adenocarcinomas (AD) group. No significant differences in OS and PFS were found between patients administered 2 and 3 cycles of neoadjuvant therapy before surgery, between those administered ≤5 and > 5 cycles of adjuvant therapy post-surgery, and between patients with TPS <50% and ≥50% (all P > 0.05).
Conclusion
Neoadjuvant immunochemotherapy with pembrolizumab plus chemotherapy in non-small cell lung cancer is safe and tolerable. Both pCR and MPR were closely associated with OS and PFS, reflecting a good response of tumor tissues to drug therapy. Lymph node metastasis after surgery was a poor prognostic factor, reducing OS and PFS.
Keywords: Chemoimmunotherapy, Neoadjuvant therapy, Non-small cell lung cancer, Surgery
1. Introduction
Lung cancer is one of the commonest cancers with high morbidity and mortality worldwide [1,2]. Surgical resection is the primary option for the treatment of early-stage non-small cell lung cancer (NSCLC), providing a high cure rate. However, stage II-III NSCLC cases have a poor prognosis even with complete removal of the tumor [3]. Improving survival in these patients is of utmost priority across the globe. Neoadjuvant therapy, especially neoadjuvant immunotherapy, may benefit cancer patients by supporting primary treatment interventions. Based on recent clinical trials (NADIM, NCT02716038, NCT04304248, and CheckMate 816), neoadjuvant immunotherapy yielded pathological complete response (pCR) rates of 24–63% and major pathologic response (MPR) rates of 36–83% [4]. When neoadjuvant immunotherapy and chemotherapy are combined, the outcome is significantly improved, compared with neoadjuvant chemotherapy or immunotherapy alone. However, long-term survival data for neoadjuvant immunotherapy are lacking, particularly in translating pCR into survival. Gathering relevant data on neoadjuvant immunotherapies, including information on comparing squamous cell carcinoma and adenocarcinoma for prognosis, data on the number of cycles of adjuvant therapy before the primary surgical intervention, details on pathological remission of lymph nodes and primary lesions, and data on survival, is crucial. We aimed to perform a retrospective study in this context to address some of these important unanswered questions.
2. Methods
2.1. Patients
Patient data from January 2019 to June 2022 were retrieved retrospectively from the database of Tianjin Medical University Cancer Institute and Hospital (TJMUCH). This retrospective study involving human participants was reviewed and approved by the ethics committee of TJMUCH (approval number, bc2020060) and the institutional review board of TJMUCH (approval number, bc2020060) following the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all patients before the onset of data analysis. The criteria for patients to receive neoadjuvant immunotherapy in our hospital generally include: age between 20 and 75 years, no common contraindications for lung cancer resection, IIA-IIIC stage resectable non-small cell lung cancer, and volunteered for neoadjuvant immunochemotherapy. The immunotherapy drugs of choice usually include pembrolizumab, tirellizumab, sindillizumab et al.
Inclusion criteria for this retrospective study were: age of 20–75 years, initial diagnosis of resectable NSCLC at the clinical stages of IIA-IIIC, being treatment-naive before neoadjuvant therapy prior to surgical resection, Karnofsky performance status (KPS) of ≥80. Exclusion criteria included intolerance to surgery, contraindications to surgery, autoimmune disease, long-term use of immunosuppressive drugs, tumor progression to unresectable status or distant metastasis after neoadjuvant therapy, or refusal to undergo follow-up at the preoperative assessment. The eighth UICC/AJCC TNM edition for NSCLC staging was used to evaluate the tumors.
Contrast-enhanced computed tomography (CT) of the chest, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET-CT), and fiberoptic bronchoscopy or percutaneous lung puncture were performed for pathological diagnosis.
Overall survival (OS) was defined as the time from the first pathological diagnosis of lung cancer to the last follow-up or death. Disease-free survival (DFS) was defined as the time from the first pathological diagnosis of lung cancer to recurrence or metastasis.
2.2. Pathological assessment
Pathological response was assessed by pathologists and the percentage of residual viable tumor cells in primary tumors resected from each patient was calculated via previously reported methods [4]. All tumor bed samples with the largest diameter below 6 cm were submitted in their entirety. For tumor bed samples with the largest diameter of 6 cm or more, at least one section per cm of the greatest dimension of the tumor bed was evaluated. The samples were sectioned, and the percentage of viable tumor tissue was determined for each tumor section, with the average percentage of viable tumor tissue for each patient being further analyzed. All resected lymph nodes were also assessed. Tumors with <10% of viable tumor cells were considered to have a major pathologic response (MPR) and those with no viable tumor cells were deemed to have a pathological complete response (pCR). If there is lymph node metastasis, regardless of whether the primary tumor is PCR or MPR, it cannot be evaluated as PCR or MPR.
Immunohistochemistry was performed to detect the expression of the PD-L1 protein in tumor cells using a mouse monoclonal anti-human PD-L1 antibody clone 22C3 (Dako North America Inc., Carpentaria, CA, USA). The tumor proportion score (TPS) was provided by a pathologist at TJMUCH, calculating the percentage for at least 100 viable cells with complete or partial membrane protein-specific staining.
2.3. Statistical analysis
Data analysis was performed with SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Continuous data presented as mean ± standard deviation (SD) were analyzed by the two-tailed t-test or rank sum test. Categorical data expressed as frequency or percentage (%) were analyzed by the chi-square test or Fisher's exact test. The survival curves obtained by the Kaplan-Meier method were compared by the log-rank test. The F test was first used to extract features related to the target task and eliminate the noise that may affect irrelevant features. P < 0.05 was considered statistically significant.
3. Results
3.1. Baseline and clinical characteristics
Sixty-one cases were collected from hospital records, including 42 squamous cell carcinomas, 15 adenocarcinomas, one large-cell lung carcinoma, one sarcomatoid carcinoma, one mucoepidermoid carcinoma, and one adenoid cystic carcinoma. Totally 54 and 7 patients were male and female, respectively, with a median age of 61.10 ± 7.99 (41–73) years. According to the TNM staging system, there were 5 IIA, 8 IIB, 36 IIIA, 10 IIIB and 2 IIIC stage cases. All patients underwent baseline tumor staging based on pretreatment biopsy, pathological evaluation of mediastinal lymph nodes (if indicated) by bronchoscopy or mediastinoscopy, positron-emission tomography-computed tomography (PET-CT), and contrast-enhanced CT or magnetic resonance imaging of the brain and chest. Patients with squamous carcinoma received 2–3 courses of pembrolizumab at 200 mg intravenous (IV) q3w with cisplatin at 75 mg/m2 IV q3w plus paclitaxel liposome at 135 mg/m2 q3w. Those with non-squamous carcinoma received pemetrexed at 500 mg/m2 IV q3w instead of paclitaxel liposome (Table 1).
Table 1.
Characteristics of the patients according to pathological response.
| Factors | All patients | MPR | non-MPR | p value | N+# | N-* | p value |
|---|---|---|---|---|---|---|---|
| Sex — no. (%) | 0.259 | 0.881 | |||||
| Male | 54 | 35 | 19 | 14 | 40 | ||
| Female | 7 | 3 | 4 | 2 | 5 | ||
| Age — yr | |||||||
| Mean ± SD | 61.10 ± 7.99 | 61.43 ± 8.53 | 60.89 ± 7.75 | 58.76 ± 8.77 | 61.78 ± 7.68 | ||
| Histologic subtype | 0.029 | 0.205 | |||||
| SCC$ | 42 | 30 | 12 | 9 | 33 | ||
| non-SCC& | 19 | 8 | 11 | 7 | 12 | ||
| TNM Stage —no. (%) | 0.045 | 0.01 | |||||
| IIA | 5 | 4 | 1 | 0 | 5 | ||
| IIB | 8 | 6 | 2 | 2 | 6 | ||
| IIIA | 36 | 25 | 11 | 7 | 29 | ||
| IIIB | 10 | 2 | 8 | 7 | 3 | ||
| IIIC | 2 | 1 | 1 | 0 | 2 | ||
| Smoking status —no. (%) | 0.237 | 0.595 | |||||
| Never | 16 | 8 | 8 | 5 | 11 | ||
| Former or current | 45 | 30 | 15 | 11 | 34 | ||
| PD-L1 (TPS) | 0.055 | 0.36 | |||||
| <50% | 24 | 17 | 7 | 4 | 20 | ||
| ≥50% | 14 | 11 | 3 | 4 | 10 | ||
| unknown | 23 | 10 | 13 | 8 | 15 | ||
| cycles pre-operation | 0.711 | 0.802 | |||||
| 2 | 43 | 28 | 15 | 12 | 31 | ||
| 3 | 15 | 8 | 7 | 3 | 12 | ||
| 4 | 3 | 2 | 1 | 1 | 2 | ||
| cycles post-operation | 0.972 | 0.048 | |||||
| ≤5 | 29 | 18 | 11 | 11 | 18 | ||
| >5 | 32 | 20 | 12 | 5 | 27 |
N+#, lymph node metastasis based on postoperative pathology; N-*, no lymph node metastasis based on postoperative pathology; SCC$, squamous cell carcinomas; non-SCC&, non-squamous cell carcinomas. MPR, major pathological response; pCR, complete pathological response, PD-L1, programmed-death ligand 1; TPS, tumor-proportion score.
3.2. Surgical and postoperative outcomes
Totally 60 eligible patients (100%) underwent complete tumor resection (R0 resection), and 1 patient diagnosed with adenoid cystic carcinoma only had exploratory surgery. No surgical delays associated with the treatment occurred and all surgeries were performed within 40 days after treatment completion. Of the 61 patients included, 31 (50.82%) achieved a pCR, and 38 (62.30%) reached an MPR. Of 42 patients with SCC, 25 (59.52%) achieved a pCR, and 30 (71.43%) achieved an MPR. Among 19 patients with non-SCC, 6 (31.58%) obtained a pCR, and 8 (42.11%) achieved an MPR. Of 43 patients administered 2 treatment cycles before surgery, 23 (53.49%) obtained a pCR, and 28 (65.12%) reached an MPR. Among 15 patients who received 3 treatment cycles before surgical intervention, 7 (46.67%) obtained a pCR, and 8 (53.33%) reached an MPR. Totally 14 of 38 (36.84%) patients had TPS ≥50% based on preoperative tracheoscopy or puncture pathology, 8 of 14 (57.14%) patients obtained a pCR and 11 (78.57%) patients achieved an MPR; 24 of 38 (63.16%) patients had TPS <50%, 14 of 24 (58.3%) patients obtained a pCR and 17 (70.83%) patients reached an MPR (Supplementary Fig. 1).
Totally 2 to 4 cycles of neoadjuvant therapy were administered, with 2, 3 and 4 treatment cycles administered in 43, 15 and 3 patients, respectively. Postoperatively, 29 and 32 patients were administered ≤5 and >5 cycles of immunotherapy, respectively. Postoperative immunotherapy cycles ranged from 0 to 24, with an average of 8.7 cycles.
Totally 16 patients, including 3 N1, 10 N2 and 3 N3 stage cases had lymph node metastasis based on postoperative pathology, while 51 patients had lymph node metastasis based on imaging at the initial diagnosis, including 14 N1, 29 N2 and 8 N3 cases (Table 1). Meanwhile, 1 patient with primary tumor lesions reaching pCR, had residual lymph node metastases.
Regarding immunotherapy drug-related toxicity, 2 patients displayed immune-associated pneumonia that developed within one week of surgery, i.e., after 3 cycles of neoadjuvant immunochemotherapy. Two other patients developed rashes of more than three degrees, and one developed vitiligo. Another patient developed a degree III or higher hepatic injury and two developed hypothyroidism.
3.3. Survival data
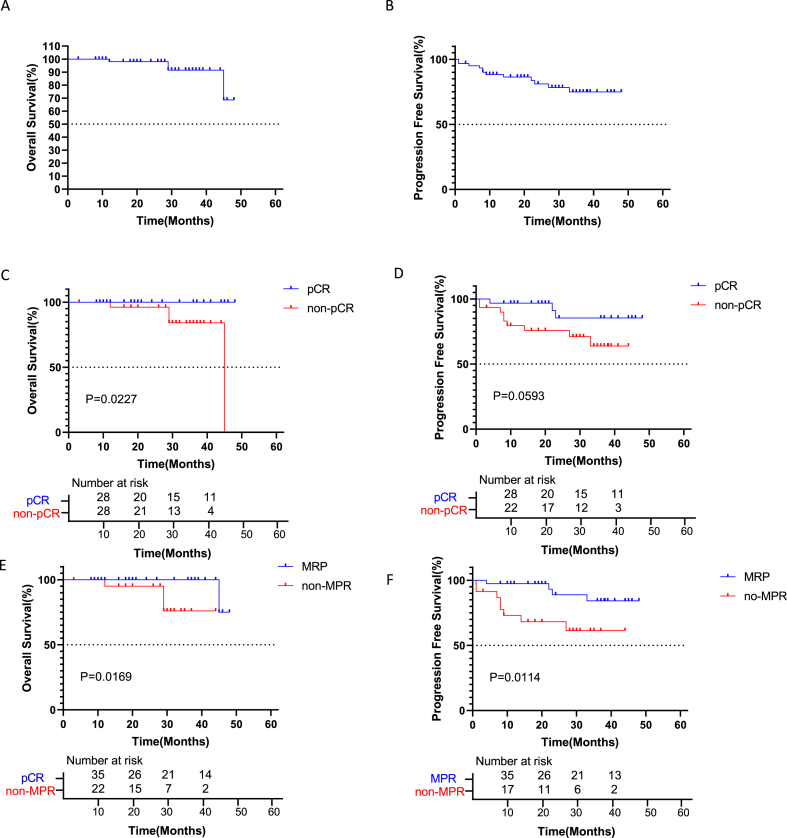
Regarding overall survival, there were 8 deaths from some cause, 2 of which were due to tumor recurrence or progression. Totally 11 patients had tumor recurrence and metastasis. Totally 2 patients died from pulmonary infection secondary to respiratory aspiration, 1 died from pulmonary embolism due to venous thrombosis, and 1 died from immune pneumonia. All patients underwent CT and PET-CT during follow-up, which ended in December 2022, with a median follow-up of 28 months (3–48 months). The 1-, 2- and 3-year OS were 98.36%, 98.36% and 95.08%, respectively. The 1-, 2- and 3- year PFS were 88.52%, 83.61% and 80.33%, respectively (Fig. 1A and B). Comparing patients with a pathological complete response (pCR) versus those with no pCR, 1-year OS rates were 100% and 96.2%, 2-year OS rates were 100% and 96.2%, and 3-year OS rates were 100% and 84.1%, respectively. Patients who achieved a pCR experienced a higher OS than the non-pCR group. A significant difference in OS was found between the two groups (HR = 0.093, P = 0.0227; Fig. 1C). One-year PFS rates with a pCR and no pCR were 96.8% and 79.5%, 2-year PFS rates were 85.4% and 75.7%, and 3-year PFS rates were 85.4% and 63.9%, respectively. Patients who achieved a pCR showed a higher PFS than those with no pCR, but no significant difference in survival was found between the two groups (HR = 0.335, P = 0.0593; Fig. 1D).
Fig. 1.
Kaplan-Meier curves for survival stratified by clinical parameters. A: Overall survival in all patients. B: Progression-free survival in all patients. C: Overall survival stratified by pCR. Patients who achieved pCR had significantly higher OS than the non-pCR group (HR = 0.093, CI 0.01199 to 0.7171; p = 0.0227). D: Progression-free survival stratified by pCR. Patients with pCR had higher PFS than the non-pCR group, with no significant difference (HR = 0.335, CI 0.1073 to 1.044; p = 0.0593). E: Overall survival stratified by MPR. Patients who achieved MPR had significantly increased OS than the non-MPR group (HR = 0.05357, CI 0.004849 to 0.5917; p = 0.0169). F: Progression-free survival stratified by MPR. Patients who achieved MPR had significantly better PFS than the non-MPR group (HR = 0.2076, CI 0.06149 to 0.7012; p = 0.0114). pCR, pathological complete response. MPR, major pathological remission.
In patients with an MPR and no MPR, 1-year OS was 100% vs. 95.0%, 2-year OS was 100% vs. 95.0%, and 3-year OS was 100% vs. 76.0%. Patients who achieved an MPR had significantly higher OS than those with no MPR (HR = 0.05357, P = 0.0169; Fig. 1E). One-year PFS rates in the MPR and non-MPR groups were 97.4% and 73.0%, 2-year PFS rates were 88.9% and 68.2%, and 3-year OS rates were 84.2% and 61.4%, respectively. Patients who achieved an MPR had significantly higher PFS than those with no MPR, with a significant difference in survival between the two groups (P = 0.0114, Fig. 1F).
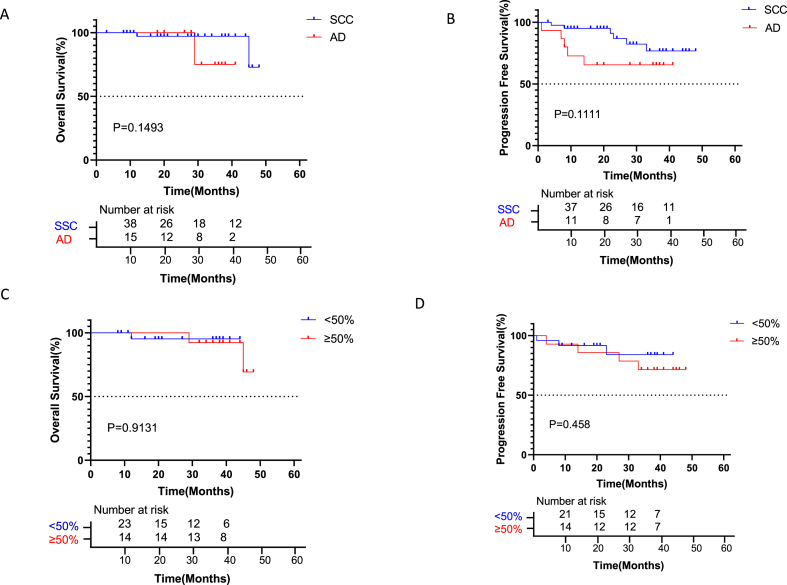
In patients with squamous cell carcinomas (SCC) and adenocarcinomas (AD), 1-year OS was 97.6% vs. 100%, 2-year OS was 97.61% vs. 100%, and 3-year OS was 97.6% vs. 86.7%. The OS of patients with SCC was similar to that of the AD group (P > 0.05, Fig. 2A). One-year PFS rates in the SCC and AD groups were 95.24% and 73.33%, 2-year PFS rates were 90.48% and 66.67%, and 3-year OS rates were 88.10% and 66.67%, respectively. PFS of patients with SCC was similar to that of the AD group (HR = 0.3278, p = 0.1111; Fig. 2B).
Fig. 2.
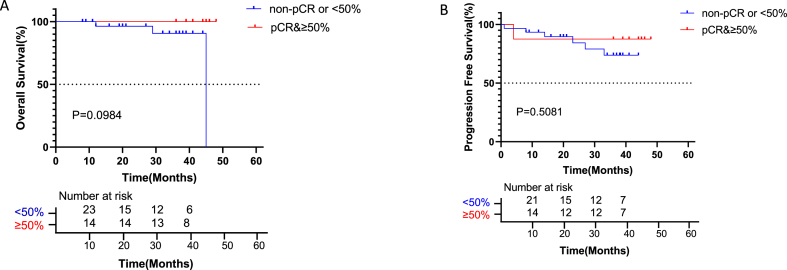
Kaplan-Meier curves for survival stratified by pathology and TPS. A: Overall survival stratified by SCC or AD. OS in patients with SCC was similar to that of patients with AD (HR = 0.2814, CI 0.02637 to 3.002; p = 0.1493). B: Progression-free survival stratified by SCC or AD. PFS was elevated in patients with SCC compared with the AD group, with no significant difference (HR = 0.3278, CI 0.08312 to 1.293; p = 0.1111). C: Overall survival stratified by PD-L1<50 or ≥50%. OS was comparable between patients with TPS <50% and TPS ≥50 (P = 0.9131). D: Progression-free survival stratified by PD-L1<50 or ≥50%. PFS was comparable between patients with TPS<50% and TPS ≥50 (P = 0.458).
PD-L1 protein expression was evaluated, in relation with patient outcomes. As shown in Fig. 2C–D, OS and PFS in patients with TPS <50% were similar to those of patients with TPS ≥50 (P > 0.05).
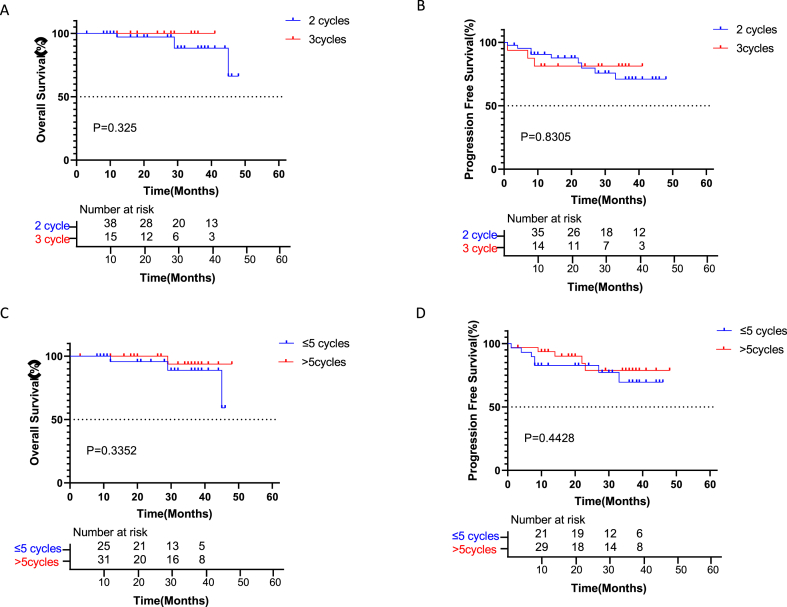
The difference in the effect of neoadjuvant therapy before surgery between cases administered 2 and 3 treatment cycles was examined. As shown in Fig. 3A–B, OS and PFS in patients administered 3 treatment cycles were similar to those of cases administered 2 treatment cycles (both P > 0.05). Further, the difference in the effect of adjuvant therapy post-surgery between cases administered ≤5 and > 5 treatment cycles was analyzed. As shown in Fig. 3C–D, OS and PFS in patients administered ≤5 treatment cycles were similar to those of patients who received >5 cycles of therapy (both P > 0.05).
Fig. 3.
Kaplan-Meier curves for survival stratified by treatment cycles before and after surgery. A: Overall survival stratified by 2 or 3 cycles before surgery. OS was non-significantly higher in patients administered 3 treatment cycles than in those with 2 cycles (HR = 3.865, CI 0.2607 to 57.31; p = 0.3258). B: Progression-free survival stratified by 2 or 3 cycles before surgery. PFS was comparable between patients with 2 and 3 treatment cycles (HR = 1.149, CI 0.3231 to 4.084; p = 0.8305). C: Overall survival stratified by ≤ 5 or >5 cycles post-surgery. OS was comparable between patients with ≤5 and > 5 treatment cycles (P = 0.3352). D: Progression-free survival stratified by ≤ 5 or >5 cycles post-surgery. OS was comparable between patients with ≤5 and > 5 cycles (P = 0.4428).
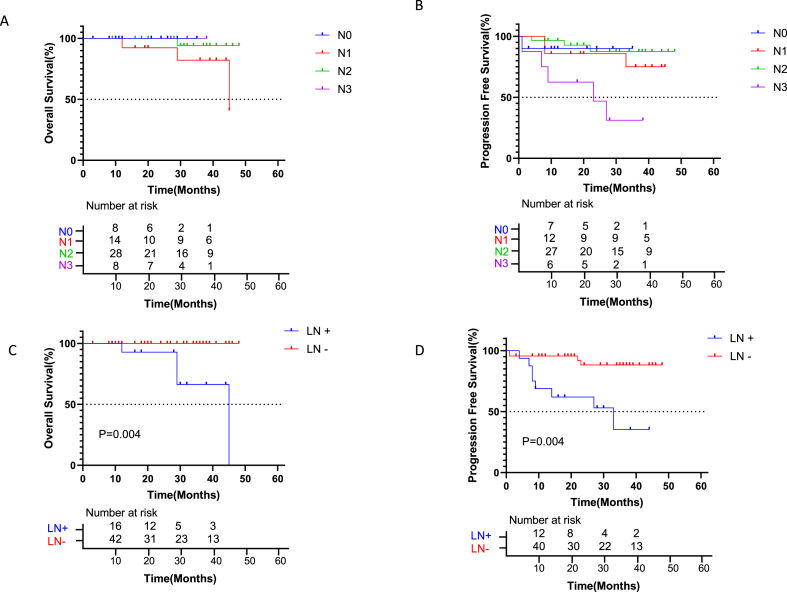
Considering clinical N stage before surgery, OS was significantly worse in N1 stage cases than N0, N2, and N3 stage cases. PFS was worse in patients with stages N1 and N3 compared with N0 and N2 stage cases (Fig. 4A–B). The effect of N staging on patient prognosis after surgery was further evaluated, and patients with lymph node metastasis based on postoperative pathology (N+) had significantly reduced OS than those without lymph node metastasis (N-) (P = 0.004, Fig. 4C). No death was reported in the N- group after surgery, while higher mortality was found in the N+ group. Patients with lymph node metastasis (N+) based on postoperative pathology had significantly reduced PFS than those without lymph node metastasis (N-) (P = 0.004, Fig. 4D). The N+ group had significantly higher rate of disease progression than the N- group. To assess whether patients with PD-L1 expression and pCR benefit more from neoadjuvant immune combination chemotherapy, we also studied OS and PFS in these cases. OS and PFS in patients with pCR and TPS ≥50% were similar to those of patients with non-pCR and TPS <50 (P > 0.05, Fig. 5A and B).
Fig. 4.
Kaplan-Meier curves for survival stratified by lymph node stage and metastasis. A: Overall survival stratified by lymph node stage (N stage). OS was significantly worse in patients with stage N1 than in the N0, N2, and N3 groups. B: Progression-free survival stratified by N stage. PFS was significantly decreased in patients with stage N1 and N3 than in the N0 and N2 groups. C: Overall survival stratified by lymph node metastasis. Patients with lymph node metastasis after surgery (N+) had significantly worse OS than those without lymph node metastasis (N-) (HR = 0.01607, CI 0.001620 to 0.1594; p = 0.0004). D: Progression-free survival stratified by lymph node metastasis. Patients with lymph node metastasis (N+) had significantly worse PFS than those without lymph node metastasis (N-) (HR = 0.08757, CI 0.02284 to 0.3357; p = 0.0004). The N+ group had a significantly higher rate of disease progression than the N- group.
Fig. 5.
Kaplan-Meier curves for survival stratified by pCR in combination with PD-L1. OS (A) and PFS (B) in patients with pCR and TPS ≥50% were similar to those of patients with no pCR and TPS <50 (P > 0.05).
Regarding the clinical TNM stage before surgery, OS was significantly decreased in patients with stage IIB compared with stage IIA and IIIA cases (P = 0.016, Supplementary Fig. 2A). PFS was significantly elevated in patients with stage IIIA disease than in stage IIA, IIB IIIB, and IIIC cases (P = 0.005, Supplementary Fig. 2B).
Differences among patients with distinct T stages before surgery were also evaluated. As shown in Supplementary Figs. 2C–D, no significant differences were observed in OS and PFS among patients with different T stages (P > 0.05).
4. Discussion
Based on the improved efficacy of immune checkpoint inhibitors in the treatment of advanced non-small cell lung cancer, these drugs are gradually used in preoperative neoadjuvant therapy. In clinical studies, neoadjuvant immunochemotherapy resulted in improved pathological response than neoadjuvant chemotherapy or immunotherapy alone, which might be a predictor of long-term survival. According to recent reports, MPR rates for neoadjuvant mono-immunotherapy and immunochemotherapy are 18–45% and 57–80%, respectively, with pCR rates of 5–15% and 33–63%, respectively [[5], [6], [7]]. Long-term survival data and subgroup analyses of neoadjuvant pembrolizumab in combination with chemotherapy are unavailable.
In the current retrospective study, 50.82% of patients achieved a pCR and 62.30% exhibited an MPR. In the pCR and non-pCR groups, 3-year OS rates were 100% and 84.1%, respectively. Patients who achieved a pCR had significantly elevated OS than those with no pCR, corroborating a prospective trial of neoadjuvant immunotherapy and chemotherapy [5]. The 3-year PFS rates were 85.4% and 63.9%, respectively, in the pCR and non-pCR groups. Patients who achieved a pCR had elevated PFS than the non-pCR group, without significant difference. A longer observation period and/or a larger sample size may cause such a difference to become significant. Additionally, OS and PFS showed significant differences between the MPR and non-MPR groups. Both pCR and MPR reflect the response of tumor tissues to therapy. With postoperative maintenance therapy, long-term survival can be achieved. Interestingly, none of the patients with a pCR died due to tumor progression by the end of follow-up, but three patients relapsed with disease progression. More advanced molecular diagnostic evaluations including biomarkers and gene expression in the tumor microenvironment may be needed to ensure the accuracy of a pCR and the detection of tumor residues [[7], [8], [9]].
According to a recent report by Forde et al. describing the Checkmate 816 clinical trial, patients with non-squamous histological cancer types had better outcomes than those with squamous histological types [10]. The pCR with nivolumab plus chemotherapy and chemotherapy in patients with squamous cell carcinoma was 25% vs. 4%, with a median event-free survival (EFS) of 31 months vs. 23 months (HR 0.77). Patients with non-squamous cell carcinoma benefited more from the combined immunotherapy plus chemotherapy than individuals with squamous cell carcinoma and had a pCR rate of 23% vs. 0%, with a median EFS of NR (not reached) vs. 20 months (HR 0.50). However, in the current analysis, 59.52% of patients with SCC (Squamous Cell Carcinoma) achieved a pCR and 71.43% achieved an MPR, while only 26.67% of patients with AD achieved a pCR and 33.33% achieved an MPR. However, there was no significant difference in OS and PFS between SCC group and AD group. This indicates that neoadjuvant treatment with nivolumab and pembrolizumab in combination with chemotherapy has complex and possibly different mechanisms of action in squamous cell carcinoma and adenocarcinoma.
The association of PD-L1 level with pathological reaction in neoadjuvant immunochemotherapy remains controversial. In the CheckMate-816 study, patients with greater than 50% of PD-L1 expression had increased OS and EFS than those with <50% of PD-L1 expression. In the NADIM clinical trial, 58% of patients whose PD-L1 tumor proportion scores were below 25% achieved MPR or pCR; and patients with PD-L1 expression and pCR benefited more significantly from neoadjuvant immune combination chemotherapy [10]. According to our data, the prognoses of patients with high and low PD-L1 levels were similar after neoadjuvant immunotherapy. No significant differences were found in OS and PFS. However, we found that patients with PD-L1 expression and pCR did not benefit more from neoadjuvant immune combination chemotherapy. Considering that the effect of PD-L1 positivity on immunotherapy is controversial in adjuvant therapy, our finding may be related to the population enrolled. Furthermore, more in-depth studies are needed to analyze the relationship between PD-L1 expression and immunotherapy.
In the neoSCORE study, the three-treatment cycle group achieved a higher MPR rate (41.4%, 12/29) compared with the two-treatment cycle group (26.9%, 7/26) (p = 0.260) and 24.1% (7/29) and 19.2% (5/26) of cases achieved a pCR, respectively (p = 0.660). Three cycles of neoadjuvant treatment achieved a numerically higher MPR rate compared with the two-treatment cycle group [11]. According to our data, no difference in efficacy was found between 2 and 3 cycles of neoadjuvant therapy. However, OS in the 3-cycle group was slightly higher than that of the 2-cycle group. Whether immunotherapy should be continued after surgery and for how long remains unknown. According to our data, no significant differences were found between cases administered >5 and < 5 treatment cycles. This indicates that 5 cycles of immunotherapy after surgery may be sufficient in these patients; as they all have major R0 resection, there are no residual tumor cells in the body and tumor antigens are heavily reduced. Therefore, it may not make sense continuing the treatment.
Nodal downstaging is widely regarded as a positive prognostic factor in NSCLC after neoadjuvant therapy. Corsini et al. reported that only MPRypN0 instead of MPRypN1-2 could accurately predict DFS in NSCLC after complete resection [12]. Based on the evidence in neoadjuvant chemotherapy, node downstaging or nodal clearance may be a critical factor in assessing long-term survival after neoadjuvant immunotherapy. In this study, lymph node metastases after surgery were critical to determine patient prognosis. Patients with no lymph node metastasis after surgery had significantly higher OS and PFS than those with metastasis. In 45 patients with no lymph node metastasis after surgery, no patients died by the end of follow-up, and they showed a significantly lower recurrence rate than individuals with lymph node metastasis. Another interesting finding is that patients diagnosed preoperatively with N1 stage disease had a poorer prognosis than those diagnosed preoperatively at the N0 and N2 stages. Station N1 lymph nodes are located between the lung lobes, close to the preserved lobes and closer to the primary tumor than station N2 lymph nodes. Further studies are needed to determine the cause of poor outcome in patients with metastases at station N1 lymph nodes.
Metastatic lymph nodes also do not respond to chemotherapy combined with immunotherapy in the same way as the primary lesion does. In a trial of the neoadjuvant drug sintilimab, only 3 of 6 patients with primary tumor pCR had complete tumor clearance in lymph nodes [13]. In another trial of neoadjuvant nivolumab monotherapy, 1 patient with primary tumor pCR had persistent lymph node metastasis [10,14]. According to the current data, 10 patients with primary tumors did not reach a pCR, but lymph nodes had no tumor residues. Certain patients might benefit from neoadjuvant immunotherapy due to the downstaging of the disease and such patients might have nodal downstaging or nodal clearance although not achieving an MPR with their primary tumors based on an improved response of lymph nodes to neoadjuvant immunotherapy [15,16]. Our data support these assumptions that patients with residual primary tumors but without lymph node metastases have a very good prognosis while the prognosis of patients with lymph node metastases but without residual primary tumors is relatively poor.
There are also studies underway to examine whether immunotherapy combined with other drugs can improve the efficacy of immunotherapy [17]. For example, a recent clinical trial of immunotherapy combined with targeted therapy for patients with KRAS-mutated non-small cell lung cancer was designed to investigate whether combined targeted immunotherapy could improve patient survival (NCT03777124). A systematic review and meta-analysis conducted by Alessandro Rizzo found that PPIs and H2RAs were inversely associated with progression-free survival (PFS) and overall survival (OS) in patients treated with immunotherapy for metastatic NSCLC [18].In a systematic review and network meta-analysis (NMA) currently published by Wang et al. [19], the authors collected 22 randomized controlled clinical trials involving a total of 4289 patients with NSCLC. Based on the results of the NMA, Wang and colleagues concluded that chemoimmunotherapy showed a statistically significant improvement in overall response rate (ORR) and progression-free survival (PFS) when compared with immune checkpoint inhibitors, but no significant differences were observed in overall survival (OS). However, Alessandro Rizzo argued that Bayesian NMA might be associated with an amplification of type 1 (false-positive) and type 2 (false-negative) errors, therefore, large-scale, well-designed clinical trials are needed to aim at comparing chemoimmunotherapy and ICIs monotherapy as first-line treatment for advanced NSCLC patients with high PD-L1 expression [20]. Matteo Santoni also conducted a meta-analysis to determine the possibility of achieving complete remission (CR) in cancer patients treated with immunotherapy or immuno-oncology combinations [21]. For 14,249 NSCLC patients treated with immunotherapy (ICIs alone or in combination with other anticancer drugs) (n = 7794) versus control treatment (n = 6455), the collected OR of CR rate was 2.0. It was concluded that the use of ICIs might significantly increase the chance of achieving CR in comparison with control treatments.
Due to the complex mechanism, individual factors cannot accurately predict the prognosis without a comprehensive incorporation of multiple factors. Future models predicting the clinical outcome of immune checkpoint inhibitors require the integration of additional factors affecting tumor development, including PD-L1 expression, patient germline genetics, and immune microenvironment composition [[22], [23], [24], [25]]. In case the efficacy of immunotherapy can be accurately predicted in the future, the efficiency of immunotherapy can be improved, and patients may be treated more precisely with immunotherapy and surgery.
Our real-world retrospective study of 61 patients administered neoadjuvant pembrolizumab and chemotherapy is the largest of its kind thus far. In addition, because the current study included a widely varied patient population and was limited to an Asian population with small sample size, the results may have some limitations. More accurate data are needed from multi-center randomized controlled clinical studies with larger sample sizes around the world. Another problem is that we apply PET/CT for the diagnosis of positive N2 lymph nodes before treatment. However, PET/CT had the false-positive diagnostic value in predicting the N2 metastases. If E-BUST is applied, the results will be more accurate.
5. Conclusions
Neoadjuvant immunochemotherapy with pembrolizumab plus chemotherapy in non-small cell lung cancer is safe and tolerable. Both pCR and MPR were closely related to OS and PFS, reflecting the good response of tumor tissues to drug therapy. Lymph node metastasis after surgery was a poor prognostic factor, decreasing OS and PFS, whether the primary tumor achieved an MPR or not.
Data sharing statement
The datasets used and/or analyzed in the current retrospective study are available from the corresponding author upon a reasonable request.
Funding
This retrospective study was funded by Tianjin Key Medical Discipline Construction Project (TJYXZDXK-010A).
Author contribution statement
Yulong Chen: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Bo Yan: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ran Zhang: Gang Zhao: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jian you: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Data availability statement
Data will be made available on request.
Contributors
Conception and design: Dr. Jian You.
Administrative support: Dr. Jian You.
Provision of study material or patients’ information: Dr. Yulong Chen.
Collection and assembly of data: Yulong Chen, Bo Yan, and Ran Zhang.
Data analysis and interpretation: Yulong Chen, Gang Zhao.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Accountable for all aspects of the work: All authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all patients, their families, and caregivers for participating in this study, Mercer (China) Investment Co., Ltd. Medical Department Miao Fu for the help and support of this studys, and Lianmin Zhang, Hui Chen, and Meng Lu for performing surgery after the neoadjuvant treatment. Manuscript writing assistance was provided by Yuanting Guo.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19818.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Uramoto H., Tanaka F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014;3:242–249. doi: 10.3978/j.issn.2218-6751.2013.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Yan B., Xu F., Hui Z., Zhao G., Liu J., Zhang H., Zeng Z., Zhang R., Provencio M., Ren X., You J. Neoadjuvant chemoimmunotherapy in resectable stage IIIA/IIIB non-small cell lung cancer. Transl. Lung Cancer Res. 2021 May;10(5):2193–2204. doi: 10.21037/tlcr-21-329. PMID: 34164269; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faehling M., Witte H., Sebastian M., Ulmer M., Sätzler R., Steinestel K., Brückl W.M., Evers G., Büschenfelde C.M.Z., Bleckmann A. Real-world multicentre analysis of neoadjuvant immunotherapy and chemotherapy in localized or oligometastatic non-small cell lung cancer (KOMPASSneoOP) Ther Adv Med Oncol. 2022 doi: 10.1177/17588359221085333. Mar 25;14:17588359221085333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laza-Briviesca R., Cruz-Bermúdez A., Nadal E., Insa A., García-Campelo M.D.R., Huidobro G., Dómine M., Majem M., Rodríguez-Abreu D., Martínez-Martí A., De Castro Carpeño J., Cobo M., López Vivanco G., Del Barco E., Bernabé Caro R., Viñolas N., Barneto Aranda I., Viteri S., Massuti B., Casarrubios M., Sierra-Rodero B., Tarín C., García-Grande A., Haymaker C., Wistuba, Romero A., Franco F., Provencio M. Blood biomarkers associated to complete pathological response on NSCLC patients treated with neoadjuvant chemoimmunotherapy included in NADIM clinical trial. Clin. Transl. Med. 2021 Jul;11(7):e491. doi: 10.1002/ctm2.491. PMID: 34323406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laza-Briviesca R., Cruz-Bermúdez A., Nadal E., Insa A., García-Campelo M.D.R., Huidobro G., Dómine M., Majem M., Rodríguez-Abreu D., Martínez-Martí A., De Castro Carpeño J., Cobo M., López Vivanco G., Del Barco E., Bernabé Caro R., Viñolas N., Barneto Aranda I., Viteri S., Massuti B., Casarrubios M., Sierra-Rodero B., Tarín C., García-Grande A., Haymaker C., Wistuba, Romero A., Franco F., Provencio M. Blood biomarkers associated to complete pathological response on NSCLC patients treated with neoadjuvant chemoimmunotherapy included in NADIM clinical trial. Clin. Transl. Med. 2021 Jul;11(7):e491. doi: 10.1002/ctm2.491. PMID: 34323406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casarrubios M., Cruz-Bermúdez A., Nadal E., Insa A., García Campelo M.D.R., Lázaro M., Dómine M., Majem M., Rodríguez-Abreu D., Martínez-Martí A., de Castro-Carpeño J., Cobo M., López-Vivanco G., Del Barco E., Bernabé Caro R., Viñolas N., Barneto Aranda I., Viteri S., Massuti B., Barquín M., Laza-Briviesca R., Sierra-Rodero B., Parra E.R., Sanchez-Espiridion B., Rocha P., Kadara H., Wistuba, Romero A., Calvo V., Provencio M. Pretreatment tissue TCR repertoire evenness is associated with complete pathologic response in patients with NSCLC receiving neoadjuvant chemoimmunotherapy. Clin. Cancer Res. 2021 Nov 1;27(21):5878–5890. doi: 10.1158/1078-0432.CCR-21-1200. Epub 2021 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sierra-Rodero B., Cruz-Bermúdez A., Nadal E., Garitaonaindía Y., Insa A., Mosquera J., Casal-Rubio J., Dómine M., Majem M., Rodriguez-Abreu D., Martinez-Marti A., De Castro Carpeño J., Cobo M., López Vivanco G., Del Barco E., Bernabé Caro R., Viñolas N., Barneto Aranda I., Viteri S., Massuti B., Laza-Briviesca R., Casarrubios M., García-Grande A., Romero A., Franco F., Provencio M. Clinical and molecular parameters associated to pneumonitis development in non-small-cell lung cancer patients receiving chemoimmunotherapy from NADIM trial. J Immunother Cancer. 2021 Aug;9(8) doi: 10.1136/jitc-2021-002804. PMID: 34446577; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forde P.M., Spicer J., Lu S., Provencio M., Mitsudomi T., Awad M.M., Felip E., Broderick S.R., Brahmer J.R., Swanson S.J., Kerr K., Wang C., Ciuleanu T.E., Saylors G.B., Tanaka F., Ito H., Chen K.N., Liberman M., Vokes E.E., Taube J.M., Dorange C., Cai J., Fiore J., Jarkowski A., Balli D., Sausen M., Pandya D., Calvet C.Y., Girard N. CheckMate 816 investigators. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022 May 26;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. 10.1056/NEJMoa2202170. Epub 2022 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu Fuming, Fan Junqiang, Shao Miner, Yao Jie, Zhao Lufeng, et al. Two cycles versus three cycles of neoadjuvant sintilimab plus platinum-doublet chemotherapy in patients with resectable non-small-cell lung cancer (neoSCORE): a randomized, single center, two-arm phase II trial. J. Clin. Oncol. 2022;40(16_suppl):8500. doi: 10.1200/JCO.2022.40.16_suppl.8500. 8500. [DOI] [Google Scholar]
- 12.Corsini E.M., Weissferdt A., Pataer A., et al. Pathological nodal disease defines survival outcomes in patients with lung cancer with tumour major pathological response following neoadjuvant chemotherapy. Eur. J. Cardio. Thorac. Surg. 2021;59:100–108. doi: 10.1093/ejcts/ezaa290. [DOI] [PubMed] [Google Scholar]
- 13.Gao S., Li N., Gao S., et al. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J. Thorac. Oncol. 2020;15:816–826. doi: 10.1016/j.jtho.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Forde P.M., Chaft J.E., Smith K.N., et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 2018;378:1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corsini E.M., Weissferdt A., Pataer A., et al. Pathological nodal disease defines survival outcomes in patients with lung cancer with tumour major pathological response following neoadjuvant chemotherapy. Eur. J. Cardio. Thorac. Surg. 2021;59:100–108. doi: 10.1093/ejcts/ezaa290. [DOI] [PubMed] [Google Scholar]
- 16.Ge S., Huang C. Immune checkpoint inhibitors in neoadjuvant therapy of non-small cell lung cancer: a systematic review and meta-analysis. J. Thorac. Dis. 2022 Feb;14(2):333–342. doi: 10.21037/jtd-21-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamberti G., Andrini E., Sisi M., Rizzo A., Parisi C., Di Federico A., Gelsomino F., Ardizzoni A. Beyond EGFR, ALK and ROS1: current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit. Rev. Oncol. Hematol. 2020 Dec;156 doi: 10.1016/j.critrevonc.2020.103119. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo A., Cusmai A., Giovannelli F., Acquafredda S., Rinaldi L., Misino A., Montagna E.S., Ungaro V., Lorusso M., Palmiotti G. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers. 2022 Mar 9;14(6):1404. doi: 10.3390/cancers14061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Han H., Zhang F., Lv T., Zhan P., Ye M., et al. Immune checkpoint inhibitors alone vs immune checkpoint inhibitors-combined chemotherapy for NSCLC patients with high PD-L1 expression: a network meta-analysis. Br. J. Cancer. 2022 doi: 10.1038/s41416-022-01832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizzo A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: a matter of debate. Br. J. Cancer. 2022 Nov;127(8):1381–1382. doi: 10.1038/s41416-022-01929-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santoni M., Rizzo A., Kucharz J., Mollica V., Rosellini M., Marchetti A., Tassinari E., Monteiro F.S.M., Soares A., Molina-Cerrillo J., Grande E., Battelli N., Massari F. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol. Immunother. 2023 Jun;72(6):1365–1379. doi: 10.1007/s00262-022-03349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havel J.J., Chowell D., Chan T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z., Wang X., Zeng S., Ren X., Yan Y., Gong Z. Applying artificial intelligence for cancer immunotherapy. Acta Pharm. Sin. B. 2021 Nov;11(11):3393–3405. doi: 10.1016/j.apsb.2021.02.007. Epub 2021 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randrian V., Desette A., Emambux S., Derangere V., Roussille P., Frouin E., Godet J., Karayan-Tapon L., Ghiringhelli F., Tougeron D. New artificial intelligence score and immune infiltrates as prognostic factors in colorectal cancer with brain metastases. Front. Immunol. 2021 Oct 18;12 doi: 10.3389/fimmu.2021.750407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johannet P., Coudray N., Donnelly D.M., Jour G., Illa-Bochaca I., Xia Y., Johnson D.B., Wheless L., Patrinely J.R., Nomikou S., Rimm D.L., Pavlick A.C., Weber J.S., Zhong J., Tsirigos A., Osman I. Using machine learning algorithms to predict immunotherapy response in patients with advanced melanoma. Clin. Cancer Res. 2021 Jan 1;27(1):131–140. doi: 10.1158/1078-0432.CCR-20-2415. Epub 2020 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.