Abstract
The ability of cartilage to regenerate and repair is limited. N-acetyl- d-glucosamine (GlcNAc) is a nutritional supplement commonly used to activate chondrocytes. To prolong the duration of action of GlcNAc and improve its curative effect after cartilage injury, a GlcNAc thermosensitive hydrogel is prepared based on Pluronic F127 (PF127). The physicochemical properties results indicate that this hydrogel is injectable and retards the release of GlcNAc. Further, the therapeutic benefits of GlcNAc hydrogel are detected through intra-articular injection in rat specimens with cartilage injury. Behavioral experiments results indicate that the rats treated with GlcNAc hydrogel had longer step lengths, smaller foot angles and slower fall times. Compared with the sham group, the expression of Sox9 was 1.5 times and the level of collagen II was 2.4 times in the hydrogel treated group. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining result confirmed that the GlcNAc hydrogel reduce apoptosis by about 50%. Our results of immunohistochemical staining, Western blotting assays and enzyme activity detection all suggested that GlcNAc hydrogel reduce the expression of cleaved-caspase3 and caspase8 (Compared to the sham group, the protein contents were reduced by about 50% in the GlcNAc hydrogel group). We also found that GlcNAc hydrogel activates autophagy through ERK signal pathway. The results of Western blotting indicated that GlcNAc hydrogel increase the levels of LC3B and Becline1 (hydrogel group & sham group, LC3B: 1.56 ± 0.07 & 1.00 ± 0.14; Becline1: 1.98 ± 0.07 & 1.00 ± 0.13). Whereas, the content of P62 reduced after GlcNAc hydrogel treatment, the relative level in sham group and hydrogel group are 1.00 ± 0.02 and 0.73 ± 0.06. Our results revealed that the number of P-ERK positive cells in the hydrogel group (57.36 ± 3.56%) was higher when compared with the sham (24.82 ± 2.72%). And, the ratio of P-ERK and ERK was higher than that in the sham group (1.48 ± 0.07 & 1.00 ± 0.08). The GlcNAc thermosensitive hydrogel is a promising and sustainable drug delivery system for intra-articular injection in the treatment of cartilage injury.
Keywords: Cartilage injury, Autophagy, Apoptosis, Hydrogel, N-acetyl- d-glucosamine
1. Introduction
Articular cartilage injury is a common disease that is often caused by excessive exercise, aging, osteoarthritis, and trauma [1]. Articular cartilage is a highly specialized tissue with rich water but no blood fluid supply, nerve tissue, and lymphatic vessels. It is composed of chondrocytes and rich extracellular matrix. The content of chondrocytes in it is approximately only 1%. Once injured, it is difficult to heal [2,3]. Currently, the methods used for treating cartilage injury include microfracture, autologous chondrocyte transplantation and allogeneic chondrocyte transplantation. Although these methods are widely used in clinical practice, they clearly still have limitations and shortcomings. Microfracture will lead to the formation of fibrocartilage and chondrocyte transplantation is not only difficult to operate, but also prone to rejection reactions and cannot be widely used.
The extracellular matrix of articular cartilage is mainly composed of type-II collagen and glycosaminoglycan (GAG) [4]. Glucosamine is a natural aminoglycan and a component of GAG [5]. Glucosamine has been proven to be a common drug for the treatment of osteoarthritis and has a protective effect on cartilage [6]. N-acetyl-d-glucosamine (GlcNAc) is one of the three forms of glucosamine and is more stable. Studies have indicated that GlcNAc can increase proteoglycan synthesis and have anti-inflammatory properties [7,8].
Recently, the use of biomaterials to repair cartilage damage has become a trend. Hydrogels are promising biomaterials with good biocompatibility, degradability, water content, and elasticity [9]. Pluronic F127 (PF127, also known as Poloxamer 407) is a copolymer of poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide), which is injectable, biodegradable, and thermally reversible [10]. PF127 is a liquid at low temperature and becomes a gel at higher temperatures. It has been widely used in drug delivery systems to treat various diseases [11,12].
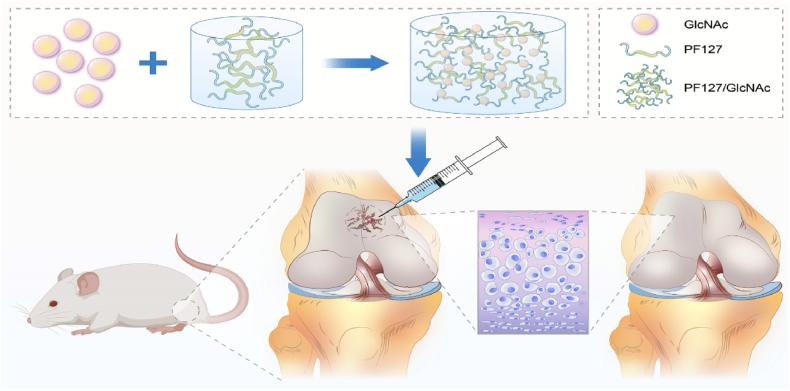
The protective effect of GlcNAc on cartilage has been confirmed [13]. However, its absorption is minimal, whether oral, intravenous or through intra-articular injection [[14], [15], [16]]. Therefore, it cannot achieve the ideal repair effect. The intra-articular injection of GlcNAc and Poloxamer hydrogels has been reported for the treatment of osteoarthritis [17]. Based on this report, in this study, a thermosensitive gel delivery system is developed for the intra-articular injection of GlcNAc, in which GlcNAc is encapsulated in a PF127 thermosensitive hydrogel to explore its therapeutic effect on cartilage injury. In addition, it is examined whether PF127/GlcNAc could reduce apoptosis and matrix degradation of chondrocytes by activating autophagy (Fig. 1).
Fig. 1.
Schematic of the GlcNAc hydrogel under local treatment.
2. Materials and methods
2.1. Preparation of GlcNAc hydrogel
PF127 (BASF, Shanghai, China) was prepared using the cold method. To 800 μL of aseptic phosphate buffer saline (PBS), 200 mg of PF127 was added and completely dissolved. 50 mg of GlcNAc (Sigma-Aldrich, St. Louis, USA) was added to 1 mL PF127 solution and stirred until completely dissolved.
2.2. Characterization
2.2.1. The structure of hydrogel
The morphology and structure of the hydrogel were observed using scanning electron microscopy. The sample was freeze-dried, coated with a thin layer of gold and then examined using a scanning electron microscope (Hitachi SU8020, Tokyo, Japan).
2.2.2. Gelation temperature and time
The tube containing different PF127/GlcNAc hydrogel concentrations was removed from 4 °C and placed in a warm water bath, starting at a temperature of 10 °C and increasing by 1 °C each time. The gelation temperature was defined as the temperature at which the hydrogels stopped flowing upon inverting the tube.
The hydrogel tubes containing PF127/GlcNAc were removed from 4 °C and quickly placed in a 37 °C water bath. The instant of time at which the hydrogels no longer flowed after inversion was recorded as the gel time.
2.2.3. Swelling
The gels were incubated in 1 ml of PBS for 0.5, 1, 4, 8, 12, and 24 h at 37 °C. PBS was removed entirely after the incubation period, and the gels were weighed to obtain mswollen. Next, the gels were lyophilized and weighed again to obtain mdry. The swelling (1) ratio Q was calculated as follows:
| (1) |
2.3. Drug release
PF127/GlcNAc and GlcNAc (3 ml) were placed in a test tube and placed in a water bath at 37 °C until PF127/GlcNAc formed gelation with 5 ml of pre-equilibrated PBS (pH = 7.4) was gently placed on the surface of the gel. The experiment was conducted on a constant-temperature shaking table (100 r/min, 37 °C). At a predetermined time interval, the equilibrium medium was removed, the same amount of release medium was added to the test tube, and the drug content in the release medium was determined through UV–vis spectrophotometry (UV-2550, Shimadzu, Japan).
2.4. Animal surgery procedure
A total of 32 male Sprague Dawley (SD) rats (250–300 g) were used in this study. They were randomly divided into four groups (Table 1). Before surgery, use 2% pentobarbital sodium (0.2 ml/100g) for anesthesia, The successfully anesthetized rats were placed on a small animal operating table, fixed in a supine position, and skin preparation was performed at the distal end of the rat femur. After routine medical iodine disinfection, cartilage defects (1.5 mm in diameter and 1 mm in depth) were made in the trochlear groove of the distal femur in each rat with dental bit [18]. All animal studies comply with ARRIVE guidelines. Thereafter, the intra-articular injection (50 μg/μL) was administered twice a week, and this treatment was continued for 4 or 8 weeks. After the experiment, the rats were euthanized by injecting excessive anesthetics. All animal experiments were approved by the Institutional Animal Care and Use Committee of Jinzhou Medical University (SYXK[Liao]2019-0007).
Table 1.
Treatment groups.
| Group | Factor |
|---|---|
| Control | Normal articular cartilage |
| Sham | Make articular cartilage injury |
| GlcNAc | Intra-articular injection of 50 μl GlcNAc solution, twice a week |
| PF127/GlcNAc | Intra-articular injection of 50 μl PF127/GlcNAc hydrogel, twice a week |
2.5. Behavioral experiments
2.5.1. Rotarod test
After three days of training, each group was subjected to a rotarod test. The rats were placed on a rotating rod instrument, which was set to acceleration mode. The rotational speed was increased from 5 to 43 r/min in 5 min, and the fall time of the rats was recorded.
2.5.2. Gait analysis
The front and rear claws of the rats were stained with nontoxic dyes of two different colors. After two days of training, they passed through a 1-m-long tunnel and left paw prints on a blotting paper. Four groups of effective noninterference hind paw step lengths and foot angles were measured.
2.6. Morphology staining
The cartilage tissue of rat was embedded in paraffin after decalcification. Then paraffin slices with a thickness of 5 μm were made. The morphology of cartilage tissue and the distribution of collagen were observed by Hematoxylin and eosin (HE) staining, Masson staining and Toluidine blue staining. The experimental steps were carried out according to the reagent kit. The kit of HE staining and Toluidine blue staining were provided by Solarbio (Beijing, China). The kit of Masson staining was provided by ZSGB-BIO (Beijing, China).
2.7. Immunohistochemical staining of type-II collagen and Sox9
The paraffin sections were dewaxed into water. High pressure method was used for repairing antigens. Endogenous peroxidase was blocked with H2O2. After washed the slices, serum was added. Then, the slices were incubated with the first antibody overnight at 4 °C, and rewarmed. Thereafter, the second antibody and horseradish enzyme were added; the mixture was stained with 3,3′-diaminobenzidine and dehydrated and sealed.
2.8. Immunofluorescence staining of LC3B and P-ERK
The paraffin sections were dewaxed into water. High pressure method was used for repairing antigens. After the serum was blocked, the primary antibody was incubated overnight at 4 °C. After rewarming, the fluorescent secondary antibody label and a sealing tablet containing 4′,6-diamidino-2-phenylindole (DAPI) were added to seal the tablet. The sections were examined using a fluorescence microscope and analyzed using ImageJ software.
2.9. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
The slices were dewaxed into water, then 20 μg/mL immunostaining washing solution was added and incubated for 15 min at 37 °C. After blockaded by endogenous peroxidase for 20 min, 50 μL biotin labeling solution was added, and incubated in dark at 37 °C for 60 min. After reaction termination, 50 μL Streptavidin HRP working solution was added incubated for 30 min at room temperature. DAB was used for color rendering. The slices were observed under microscope.
2.10. Western blot assay
The proteins in the cartilage were extracted using RIPA lysis buffer. Protein concentrations were quantified using the BCA Protein Assay Kit. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to separated protein samples from different groups. After gel electrophoresis, the protein samples were transferred to the polyvinylidene fluoride (PVDF) membrane, and blocked in 5% bovine serum albumin for 2 h. Thereafter, the first antibody was incubated at 4 °C overnight. The primary antibodies used were as follows: anti-β actin (1:1000, ZSGB-BIO, Beijing, China), antibody-collagen Ⅱ (1:1000, Invitrogen, Carlsbad, USA), Sox9 rabbit monoclonal antibody (1:1000, Beyotime, Shanghai, China), cleaved-caspase3 antibody (1:1000, Biorbyt, Cambridge, UK), caspase8 antibody (1:500, BOSTER, Wuhan, China), p44/42 Erk1/2 antibody (1:1000, Cell Signaling, Beverly, USA), phospho-p44/42 Erk1/2 antibody (1:1000, Cell Signaling), LC3B antibody (1:1000, Cell Signaling), Beclin1 mouse monoclonal antibody (1:1000, Proteintech, Rosemont, USA), and P62 antibody (1:1000, Affinity, Shanghai, China). Next day, after washed with tris-buffered saline with 0.1% Tween® 20 detergent (TBST), the samples incubated along with the corresponding secondary antibody at room temperature for 2 h. Thereafter, the enhanced chemiluminescence (ECL) developer was added to the mixture, and the bands were visually analyzed using the Bio-Rad developer.
2.11. Caspase3 activity assay
The activity of the caspase3 enzyme in the cartilage tissue was studied as follows. The tissue was lysed with lytic solution and centrifuged in 12000 rpm at 4 °C for 10–15 min. The activity of the enzyme in the supernatant was detected using a caspase3 activity detection kit (Beyotime, Shanghai, China) and through colorimetry. The level of activity was calculated using an absorbance value of 405 nm and the standard curve and expressed as the percentage of enzyme activity.
2.12. Statistical analysis
The software SPSS25 was used for statistical analysis. All the data were represented as mean ± standard deviation. One-way analysis of variance was used to assess the significance of between-group differences.
3. Results
3.1. Performance of PF127/GlcNAc hydrogels
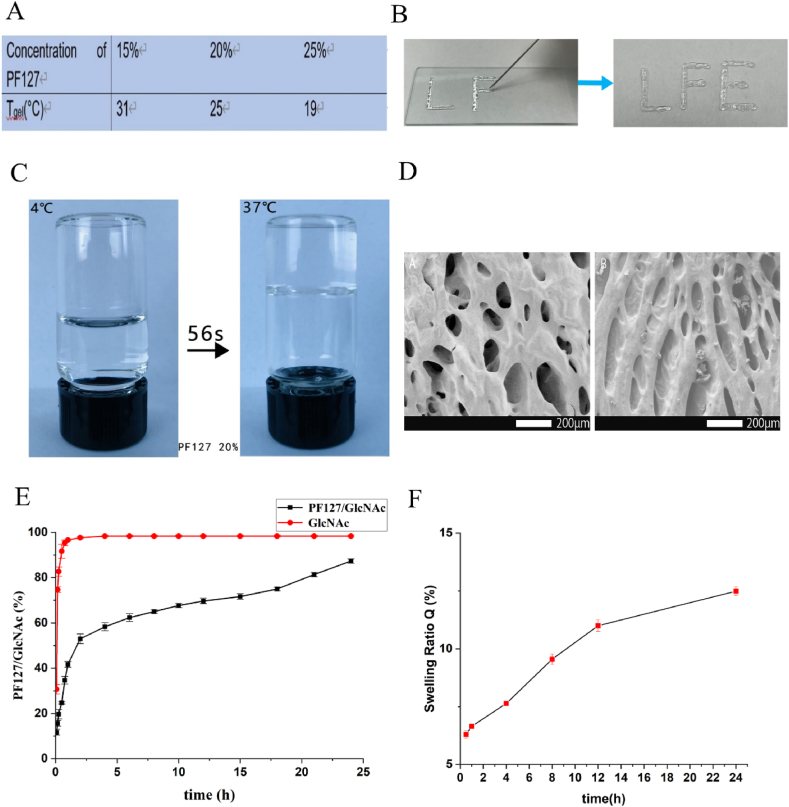
To select the most suitable gel concentration for injection, the gel transition temperatures of three commonly used concentrations were measured (Fig. 2A). At 25 °C, 20% PF127 converts into gel, which is more suitable for injection (Fig. 2B). We also tested the temperature sensitivity of the hydrogel. As shown in Figs. 2C and 20% PF127 can change into gel in 56 s at 37 °C. Scanning electron microscopy (SEM) indicated that PF127 hydrogel had a porous structure (Fig. 2D). To further test the ability of PF127 after drug loading, the drug-release ability of GlcNAc in normal saline and 20% PF127 hydrogel was tested in vitro. The release rate of GlcNAc in normal saline for approximately 1 h reached over 95%, and the drug-release rate was rapid. In contrast, GlcNAc in PF127 hydrogel promoted drug release evenly and slowly, thereby proving that PF127 hydrogel had a good sustained-release effect (Fig. 2E). In addition, the swelling rate experiment carried out on PF127/GlcNAc hydrogel indicated that the swelling rate increased slowly with time (Fig. 2F).
Fig. 2.
Property test of hydrogel (A) The trend of Tgel with the change of concentration of PF127. (B) Injectable properties of hydrogel. (C) Appearance of GlcNAc hydrogel at 4 °C and 37 °C. (D) The SEM images of PF127 hydrogel and PF127/GlcNAc hydrogel. (E) In vitro, the drug release curves of GlcNAc aqueous solution and GlcNAc/PF127 hydrogel in PBS (PH = 7. 4) (F) Within 24 h, at 37 °C, the swelling rate of PF127/GlcNAc hydrogel in PBS (pH = 7.4).
3.2. GlcNAc hydrogel improves locomotion recovery of rats after cartilage damage
To explore the effect of GlcNAc hydrogel on the motor ability of rats after cartilage injury, a rotarod test was carried out on the rats (Fig. 3A). It can be seen that the sham group (4 weeks: 8.67 ± 1.26 s, 8 weeks: 9.17 ± 1.62 s) falls easily owing to fatigue during the exercise. The motor ability of the GlcNAc group (4 weeks: 10.62 ± 2.57 s, 8 weeks: 12.49 ± 1.66 s) improved at 4 and 8 weeks, but this was not apparent. However, the PF127/GlcNAc group (4 weeks: 16.06 ± 3.52 s, 8 weeks: 25.46 ± 2.98 s) recovered their motor function well (Fig. 3B). Further, the movement ability of each group was examined through gait analysis (Fig. 3C). The step lengths (Fig. 3D) of each group's hind paws and the foot angles (Fig. 3E) of the hind paws were analyzed. Normal rats had longer step lengths (4 weeks: 14.57 ± 1.13 cm, 8 weeks: 14.75 ± 1.21 cm) and smaller foot angles (4 weeks: 17.13 ± 5.76°, 8 weeks: 13.17 ± 4.67°), which were even and symmetrical. After cartilage injury, the step length had shortened (4 weeks: 10.95 ± 1.10 cm, 8 weeks: 10.67 ± 0.40 cm) and the foot angle had evidently increased (4 weeks: 31.68 ± 5.60°, 8 weeks: 38.74 ± 7.11°). The behavior of the GlcNAc group was not very different in terms of the recovery of the step lengths (4 weeks: 12.70 ± 0.76 cm, 8 weeks: 12.44 ± 0.31 cm) and the reduction of the foot angles (4 weeks: 21.73 ± 3.26°, 8 weeks: 31.49 ± 12.01°). However, the gait of the PF127/GlcNAc group had clearly improved; the step lengths were increased (4 weeks: 13.57 ± 0.66 cm, 8 weeks: 14.77 ± 1.13 cm) and the foot angles were significantly reduced (4 weeks: 12.19 ± 2.52°, 8 weeks: 20.44 ± 2.27°). This indicates that treatment with PF127/GlcNAc has a good effect on the recovery of exercise gait in rats. The gross images of each group were examined at 4 and 8 weeks (Fig. 3F) and scored based on the Wayne scoring system (Table 2) [19]. Compared with the sham group (4 weeks: 2.63 ± 0.70, 8 weeks: 5.00 ± 1.00), the inflammation infiltration of the cartilage in the GlcNAc group (4 weeks: 5.25 ± 0.97, 8 weeks: 7.63 ± 0.70) had decreased, and a small part of the cartilage had regenerated, but this was not noticeable. However, in the PF127/GlcNAc group (4 weeks: 11.00 ± 0.71, 8 weeks: 13.12 ± 0.78), the transparent cartilage close to the normal cartilage was almost regenerated to fill the defect. The cartilage was transparent and had almost no inflammatory infiltration. When compared with the 4-week PF127/GlcNAc group (11.00 ± 0.71), the 8-week PF127/GlcNAc group (13.12 ± 0.78) had received adequate treatment (Fig. 2G).
Fig. 3.
(A) State of rats when they exercise on the roller. (B) Statistics of the fall time of rats on the roller in each group (n = 4), **P < 0.01. (C) Footprint of rats. (D) Step length of the hindfoot of rats. (n = 4), *P < 0.05, **P < 0.01 (E) Foot angle of rats (n = 4), *P < 0.05, **P < 0.01. (F) Gross image of knee cartilage injury after 4 and 8 weeks of treatment. (G) Scores obtained by various researchers based on the Wayne scoring scale, with a total score of 16 (n = 8), *P < 0.05, **P < 0.01.
Table 2.
A modified Wayne's grading scale scoring system for gross appearance.
| Gross appearance | Grade |
|---|---|
| Coverage | |
| >75% fill | 4 |
| 50–75% fill | 3 |
| 25–50% fill | 2 |
| <25% fill | 1 |
| No fill | 0 |
| Neocartilage color | |
| Normal | 4 |
| 25% yellow/brown | 3 |
| 50% yellow/brown | 2 |
| 75% yellow/brown | 1 |
| 100% yellow/brown | 0 |
| Defect margins | |
| Invisible | 4 |
| 25% circumference visible | 3 |
| 50% circumference visible | 2 |
| 75% circumference visible | 1 |
| Entire circumference visible | 0 |
| Surface | |
| Smooth/level with normal | 4 |
| Smooth but raised | 3 |
| Irregular 25–50% | 2 |
| Irregular 50–75% | 1 |
| Irregular >75% | 0 |
3.3. GlcNAc hydrogel promotes cartilage regeneration
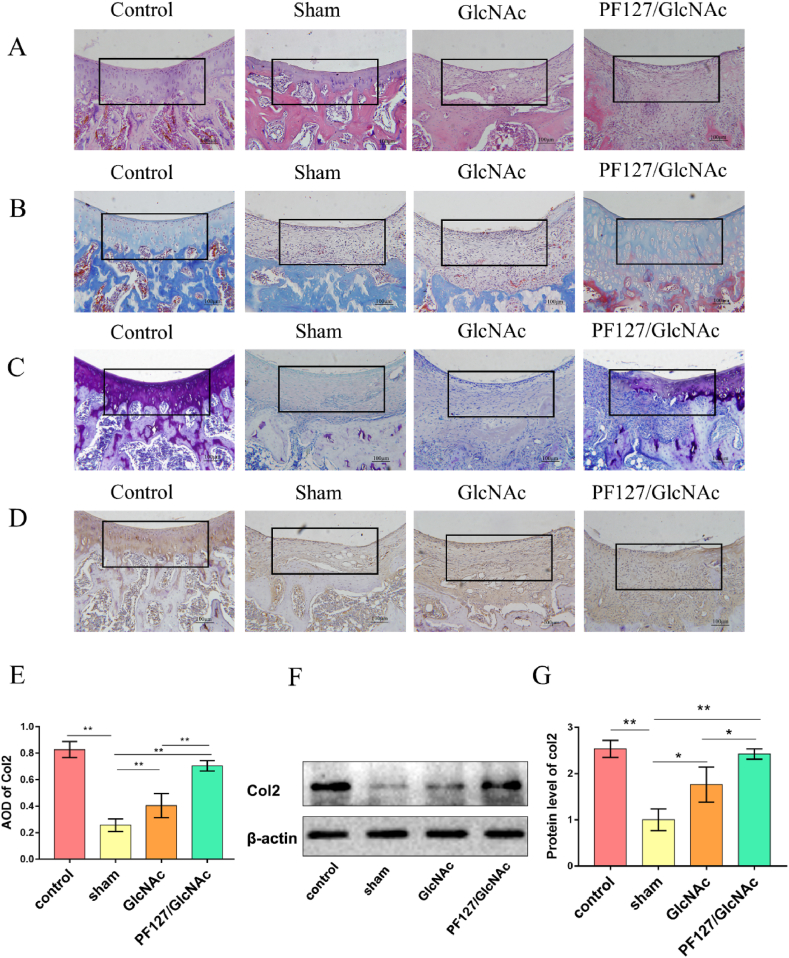
Histological staining was performed at 8 weeks, and the pathological changes effected in each group were studied to further explore the regeneration ability of GlcNAc hydrogel on the cartilage. HE staining indicated that the cartilage layer in the sham group was thinner, and its surface was uneven. The surface of the cartilage in the PF127/GlcNAc group was smooth, and the cartilage layer was thicker (Fig. 4A). Masson staining was used to observed the distribution of collagen. As shown in Fig. 4B, the contents of collagen in the sham and GlcNAc groups are very low, whereas a large amount of collagen was present in the PF127/GlcNAc group. Toluidine blue staining is a common method to observe cartilage matrix. The content of cartilage matrix decreased in sham group, but increased in GlcNAc group and PF127/GlcNAc group. Moreover, the matrix content in the PF127/GlcNAc group increased more significantly (Fig. 4C). Type-II collagen (col2) is the most abundant collagen type in the cartilage matrix. To further confirm the content of col2 in regenerated tissues, immunohistochemical staining (Fig. 4D and E) and Western blot analysis of col2 were performed (Fig. 4F and G). It was seen that the expression of col2 in the PF127/GlcNAc group (0.71 ± 0.04) were higher than those in the sham group (0.26 ± 0.04). The Western blot analysis also indicated that the protein content of col2 in the PF127/GlcNAc group (2.42 ± 0.09) was higher when compared with the sham (1.00 ± 0.19) and GlcNAc groups (1.76 ± 0.09).
Fig. 4.
GlcNAc hydrogel promotes cartilage regeneration. (A) HE staining of rat cartilage tissue in each group. (B) Masson staining of rat cartilage tissue in each group. (C) Toluidine blue staining of rat cartilage tissue in each group. (D) Immunohistochemical staining of type-II collagen. (E) Statistics of the average optical density of type-II collagen. (F, G) Western blotting analysis of type-II collagen. *P < 0.05, **P < 0.01.
3.4. GlcNAc hydrogel reduces apoptosis in rats after cartilage injury
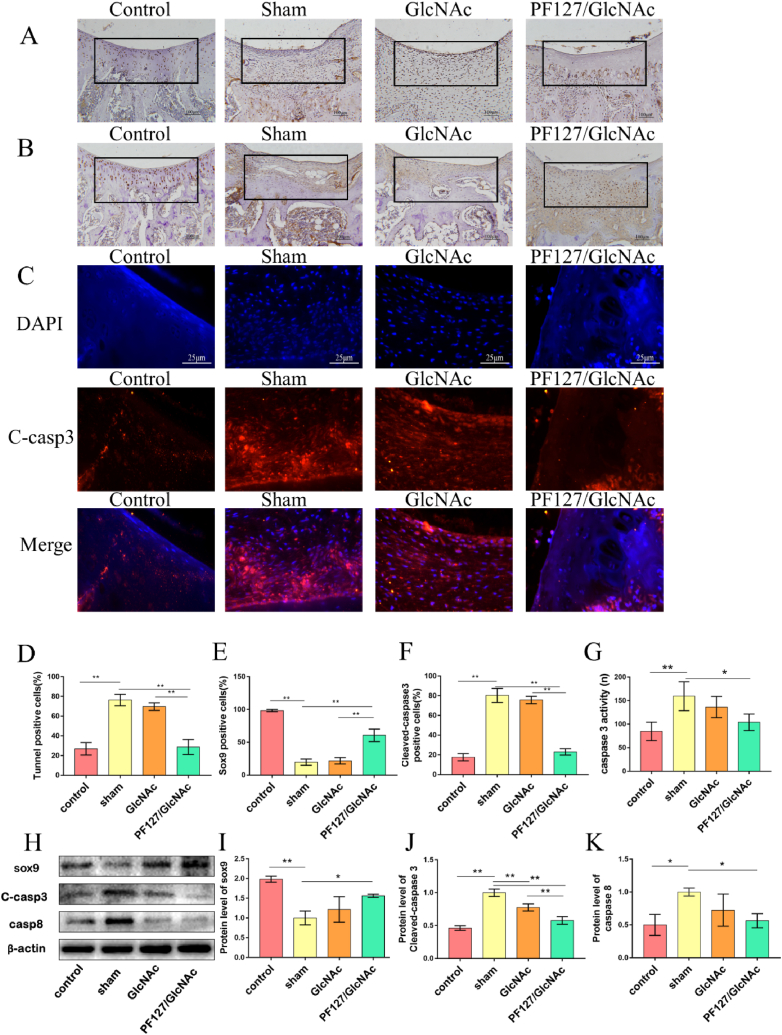
In order to explore the effect of GlcNAc hydrogel on chondrocyte apoptosis, TUNEL staining was used. As shown in Fig. 5A and D, the ratio of apoptotic cells was reduced in the PF127/GlcNAc group (28.66 ± 6.84%) compared with sham group (76.28 ± 5.24%). We also detected the expression of Sox9 through immunohistochemical staining and Western blot analysis. The results of immunohistochemical staining (Fig. 5B, E) indicated that the expression of Sox9 significantly reduced after cartilage injury (sham & control, 19.64 ± 4.27% & 98.32 ± 1.43%). The number of Sox9 positive cells increased obvious in the PF127/GlcNAc group (60.64 ± 8.46%), but the number of Sox9 positive cells is higher than GlcNAc group (21.72 ± 4.43%). The result of Western blot is consistent with immunohistochemical staining. As shown in Fig. 5H and I, GlcNAc hydrogel can increase the level of Sox9 (Control: 1.98 ± 0.06, Sham: 1.00 ± 0.14, GlcNAc: 1.22 ± 0.26, PF127/GlcNAc: 1.56 ± 0.03).
Fig. 5.
GlcNAc hydrogel reduces apoptosis in rats after cartilage injury. (A) TUNEL staining at the eighth week of each group. (B) Immunohistochemical staining of Sox9 in rat cartilage tissue of each group. (C) Immunofluorescence staining performed for cleaved-caspase3. (D) Statistics of TUNEL positive cells. (E) Statistics of immunohistochemical positive cells of Sox9. (F) Statistics of immunofluorescence positive cells of cleaved-caspase3. (G) Detection of caspase3 enzyme activity at the eighth week. (H, I, J, K) Western blotting analysis of Sox9, cleaved-capase3, and caspase8. One-way ANOVA, *P < 0.05, **P < 0.01.
To further prove the anti-apoptosis ability of GlcNAc hydrogel, the immunofluorescence staining of cleaved-caspase3 was performed (Fig. 5C, F). After merging, it was seen that there were many cleaved-caspase3 positive cells in the sham group (80.28 ± 6.33%), but a relatively fewer cells in the PF127/GlcNAc group (23.04 ± 2.93%). We also detected caspase3 enzyme activity in the cartilage. The activity of caspase3 in the PF127/GlcNAc group (103.90 ± 15.14 n) was the lowest among all groups (Sham:159.33 ± 26.58n, GlcNAc group:136.25 ± 19.44n) (Fig. 5G). Further, the Western blot analysis indicated that GlcNAc hydrogel reduced the levels of cleaved-caspase3 and caspase8 (Fig. 5H, J, K). The relative protein level of cleaved-caspase3 for Control, Sham, GlcNAc and PF127/GlcNAc group are 0.46 ± 0.03, 1.00 ± 0.04, 0.78 ± 0.04, 0.58 ± 0.05, respectively. The relative level of caspase8 are as follows: PF127/GlcNAc: 0.56 ± 0.09, GlcNAc: 0.72 ± 0.20, Sham: 1.00 ± 0.05, Control: 0.50 ± 0.13.
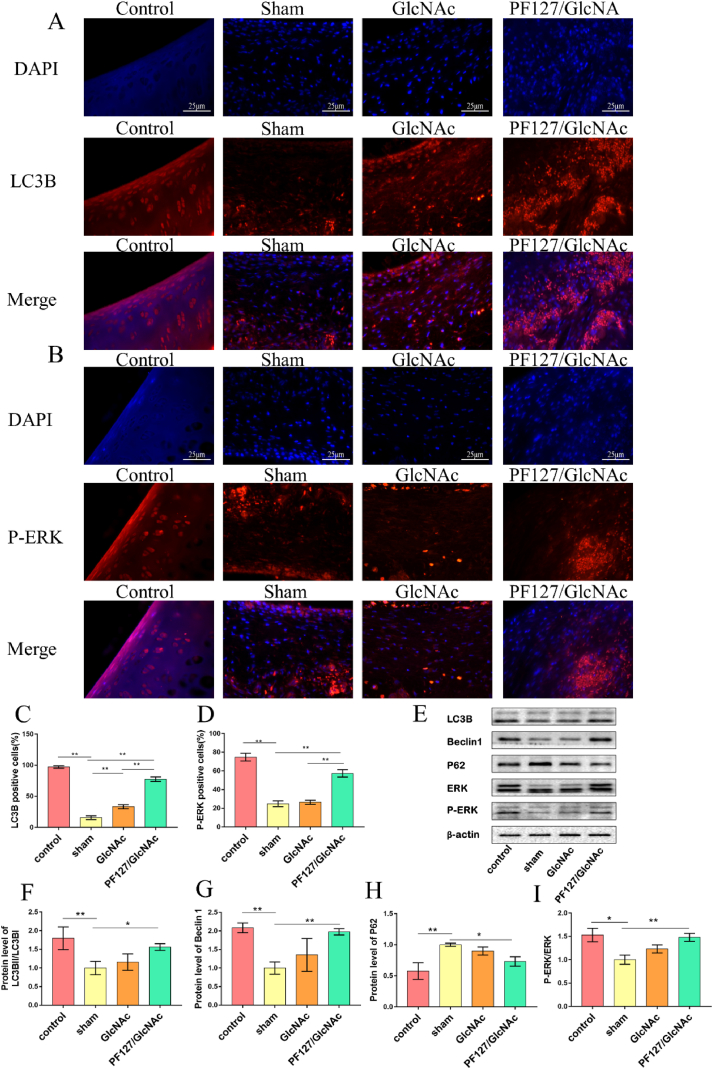
3.5. PF127/GlcNAc hydrogel activates autophagy through ERK signal pathway
To further explore how hydrogels can reduce chondrocyte apoptosis and matrix degradation, we found that GlcNAc hydrogel can activate autophagy through ERK. It can be seen from the immunofluorescence staining of LC3B that there are more LC3B positive cells in the PF127/GlcNAc group (77.50 ± 3.30%) than in the sham (15.58 ± 2.64%) and GlcNAc groups (33.30 ± 2.93%) (Fig. 6A, C). Three vital autophagy proteins were analyzed through Western blotting, among which the concentrations of LC3B and Becline1 were higher in the PF127/GlcNAc group than in the sham group (LC3B, PF127/GlcNAc group & sham group: 1.56 ± 0.07 & 1.00 ± 0.14) (Becline1, PF127/GlcNAc group & sham group: 1.98 ± 0.07 & 1.00 ± 0.13) (Fig. 6 E, F, G). Whereas, the content of P62 reduced after GlcNAc hydrogel treatment, the relative protein expression level in sham group and PF127/GlcNAc group are 1.00 ± 0.02 and 0.73 ± 0.06 (Fig. 6 E, H). To further explore the mechanism, we verified whether autophagy could be activated through the activation of the ERK pathway. The immunofluorescence staining of P-ERK revealed that the number of P-ERK positive cells in the PF127/GlcNAc group (57.36 ± 3.56%) was higher when compared with the sham (24.82 ± 2.72%) and GlcNAc groups (26.42 ± 1.99%) (Fig. 6 B, D). The Western blot analysis indicated that PF127/GlcNAc activated the ERK pathway and the ratio of P-ERK and ERK was higher than that in the sham group (PF127/GlcNAc group: 1.48 ± 0.07, Sham:1.00 ± 0.08) (Fig. 6 E, I).
Fig. 6.
PF127/GlcNAc hydrogel activates autophagy through ERK signal pathway. (A) Immunofluorescence staining was performed for LC3B. (B) Immunofluorescence staining was performed for P-ERK. (C) Statistics of immunofluorescence positive cells of LC3B. (D) Statistics of immunofluorescence positive cells of P-ERK. (E) The immunoblotting bands of LC3B, Becline1, P62, ERK, and P-ERK. (F) The relative protein content of LC3B. (G) The relative protein content of Becline1. (H) The relative protein content of P62. (I) The protein content ratio of P-ERK and ERK. One-way ANOVA, *P < 0.05, **P < 0.01.
4. Discussion
In the present study, we synthesized a thermosensitive hydrogel loaded with GlcNAc, and confirmed that this hydrogel promoted cartilage regeneration in a rat cartilage injury model. Although GlcNAc has been demonstrated to have therapeutic effect on cartilage damage in the previous studies, but the potency is not significant due to rapid metabolism [20]. Hydrogel has been widely used in the treatment of cartilage injury owing to its biocompatibility, porosity, and sustained release ability [21,22]. So, we prepared GlcNAc hydrogel based on PF127, which has injectability, proper porosity and slow release performance. Through the application in the rat cartilage injury model, we found that GlcNAc hydrogel significantly promoted the recovery of motor ability (Fig. 3A–E). Although this GlcNAc hydrogel has a good curative effect on cartilage injury, but due to the limitations of experimental conditions and time, we did not detect other characteristics of the hydrogel. Moreover, this hydrogel lacks targeting, and more precise targeting should be added in the future.
Morphological staining was used to detected the curative effect of GlcNAc hydrogel. As shown in Fig. 4, the injured cartilage tissue of rats treated with GlcNAc hydrogel had a relatively complete structure, increasing of Col2 and matrix content. GlcNAc hydrogel could significantly promote the regeneration of injured cartilage tissue. At present, research on the application of GlcNAc in the treatment of cartilage injury mainly focuses on anti-inflammatory aspects [23], and there are few studies related to chondrocyte apoptosis. But, apoptosis and matrix degradation are the main pathological processes of cartilage injury [[24], [25], [26]]. In this study, we confirmed the anti-apoptotic ability of GlcNAc hydrogel. Through TUNEL staining, immunohistochemistry and Western blot analysis, it was proven that GlcNAc hydrogel inhibited chondrocyte apoptosis and upregulated the expression of Sox9 (Fig. 5). We also demonstrated that the expression of Cleaved-caspase3 and Caspase-8 significantly reduced, and the activity of Caspase3 kinase significantly weakened after treated with GlcNAc hydrogel. These results indicated that GlcNAc hydrogel promote cartilage regeneration maybe by inhibiting chondrocyte apoptosis. Further studies are needed to clarify this point.
Previous studies have indicated autophagy plays a vital role in the formation of articular cartilage [27]. Chen et al. [28] confirmed that enhancing autophagy could inhibit chondrocyte apoptosis and cartilage degradation. We found that GlcNAc hydrogel upregulated the expression of LC3B and Becline1, but downregulated the level of P62 (Fig. 6). These results indicate that the GlcNAc hydrogel significantly promote autophagy, which maybe the reason of inhibiting chondrocyte apoptosis. The ERK pathway is significant for autophagy. Previous studies have proved that activating ERK pathway can enhance the proliferation ability of chondrocytes [29]. Immunofluorescence staining and Western blot analysis were performed, and we found that GlcNAc hydrogel increased the P-ERK/ERK ratio, which indicates that the GlcNAc hydrogel may activate autophagy through the ERK pathway. However, the mechanism of signaling pathways is very complex, involving multiple cytokines. We only tested ERK, this cannot fully demonstrate that the effect of GlcNAc hydrogel is achieved through this pathway.
In summary, we synthesized a thermosensitive hydrogel loaded with GlcNAc. The GlcNAc hydrogel attenuates cartilage injury. Although in order to reduce result errors, we used many methods such as double blind scoring. But, this experiment has many shortcomings due to the factors including insufficient sample size and limited experimental conditions etc. We only observed the effect after 8 weeks of cartilage injury, and we need to extend the testing time in the subsequent experiments. The targeting of hydrogel, dosage form and administration method need to be improved.
5. Conclusion
Temperature-sensitive hydrogels based on PF127/GlcNAc have been developed and explored in this study. PF127/GlcNAc has excellent temperature-sensitive and slow-release properties. The slow release of GlcNAc in the gel into the articular cavity has a good therapeutic effect on cartilage injury. Autophagy is activated through the ERK pathway to reduce chondrocyte apoptosis and matrix degradation, and the therapeutic effect of thermosensitive hydrogel is better than that of the same dose of hydrogel in aqueous solution.
Author contribution statement
Yijin Chang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yaguang Wang, Jiansheng Liu: Analyzed and interpreted the data.
Xu Chen, Xuejing Ma, Yu Hu: Performed the experiments.
He Tian: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xiaomei Wang, Changzheng Mu: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by grants from Natural Science Foundation of Liaoning Province {20170540341, 20180550649, 2022-MS-390}
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19879.
Contributor Information
He Tian, Email: tianhe@jzmu.edu.cn.
Xiaomei Wang, Email: wxm631229@163.com.
Changzheng Mu, Email: muchangzheng2008@sohu.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Zhu W., Niu T., Wei Z., Yang B., Weng X. Advances in biomaterial-mediated gene therapy for articular cartilage repair. Bioengineering (Basel) 2022;9(10) doi: 10.3390/bioengineering9100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon T.M., Jackson D.W. Articular cartilage: injury pathways and treatment options. Sports Med. Arthrosc. Rev. 2018;26(1):31–39. doi: 10.1097/jsa.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 3.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Correa D., Lietman S.A. Articular cartilage repair: current needs, methods and research directions. Semin. Cell Dev. Biol. 2017;62:67–77. doi: 10.1016/j.semcdb.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Ahn M.Y., Yoon H.J., Hwang J.S., Jin J.M., Park K.K. The role of noble bumblebee (Bombus terrestris) queen glycosaminoglycan in aged rat and gene expression profile based on DNA microarray) Toxicol. Res. 2021;37(1):85–98. doi: 10.1007/s43188-020-00065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H.C., Lin Y.T., Lin T.H., Chang N.J., Lin C.C., Hsu H.C., Yeh M.L. Intra-articular injection of N-acetylglucosamine and hyaluronic acid combined with PLGA scaffolds for osteochondral repair in rabbits. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0209747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kianičková K., Pažitná L., Kundalia P.H., Pakanová Z., Nemčovič M., Baráth P., Katrlíková E., Šuba J., Trebatická J., Katrlík J. Alterations in the glycan composition of serum glycoproteins in attention-deficit hyperactivity disorder. Int. J. Mol. Sci. 2023;24(10) doi: 10.3390/ijms24108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Qin Z., Wang C., Jiang Z. N-acetyl-d-glucosamine-based oligosaccharides from chitin: enzymatic production, characterization and biological activities. Carbohydr. Polym. 2023;315 doi: 10.1016/j.carbpol.2023.121019. [DOI] [PubMed] [Google Scholar]
- 9.Yang J., Zhang Y.S., Yue K., Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25. doi: 10.1016/j.actbio.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Q., Huang S., Wen J., Jiao Y., Su X., Shi G., Huang J. PF-127 hydrogel plus sodium ascorbyl phosphate improves Wharton's jelly mesenchymal stem cell-mediated skin wound healing in mice. Stem Cell Res. Ther. 2020;11(1):143. doi: 10.1186/s13287-020-01638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picheth G.F., Marini T.C., Taladriz-Blanco P., Shimamoto G.G., Dos Santos G., Meneau F., de Oliveira M.G. Influence of Pluronic F127 microenvironments on the photochemical nitric oxide release from S-nitrosoglutathione. J. Colloid Interface Sci. 2019;544:217–229. doi: 10.1016/j.jcis.2019.02.087. [DOI] [PubMed] [Google Scholar]
- 12.Wang P., Wang Q., Ren T., Gong H., Gou J., Zhang Y., Cai C., Tang X. Effects of Pluronic F127-PEG multi-gel-core on the release profile and pharmacodynamics of Exenatide loaded in PLGA microspheres. Colloids Surf. B Biointerfaces. 2016;147:360–367. doi: 10.1016/j.colsurfb.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Kim C., Shores L., Guo Q., Aly A., Jeon O.H., Kim D.H., Bernstein N., Bhattacharya R., Chae J.J., Yarema K.J., Elisseeff J.H. Electrospun microfiber scaffolds with anti-inflammatory tributanoylated N-Acetyl-d-Glucosamine promote cartilage regeneration. Tissue Eng Part A. 2016;22(7–8):689–697. doi: 10.1089/ten.TEA.2015.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riegger J., Baumert J., Zaucke F., Brenner R.E. The hexosamine biosynthetic pathway as a therapeutic target after cartilage trauma: modification of chondrocyte survival and metabolism by glucosamine derivatives and PUGNAc in an ex vivo model. Int. J. Mol. Sci. 2021;22(14) doi: 10.3390/ijms22147247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenig T.J., Dart A.J., McIlwraith C.W., Horadagoda N., Bell R.J., Perkins N., Dart C., Krockenberger M., Jeffcott L.B., Little C.B. Treatment of experimentally induced osteoarthritis in horses using an intravenous combination of sodium pentosan polysulfate, N-acetyl glucosamine, and sodium hyaluronan. Vet. Surg. 2014;43(5):612–622. doi: 10.1111/j.1532-950X.2014.12203.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang N.J., Lin Y.T., Lin C.C., Wang H.C., Hsu H.C., Yeh M.L. The repair of full-thickness articular cartilage defect using intra-articular administration of N-acetyl-D-glucosamine in the rabbit knee: randomized controlled trial. Biomed. Eng. Online. 2015;14:105. doi: 10.1186/s12938-015-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T., Chen S., Dou H., Liu Q., Shu G., Lin J., Zhang W., Peng G., Zhong Z., Fu H. Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater. Sci. Eng., C. 2021;118 doi: 10.1016/j.msec.2020.111352. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Wayne J.S., McDowell C.L., Shields K.J., Tuan R.S. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005;11(5–6):953–963. doi: 10.1089/ten.2005.11.953. [DOI] [PubMed] [Google Scholar]
- 20.Terry D.E., Rees-Milton K., Pruss C., Hopwood J., Carran J., Anastassiades T.P. Modulation of articular chondrocyte proliferation and anionic glycoconjugate synthesis by glucosamine (GlcN), N-acetyl GlcN (GlcNAc) GlcN sulfate salt (GlcN.S) and covalent glucosamine sulfates (GlcN-SO4) Osteoarthritis Cartilage. 2007;15(8):946–956. doi: 10.1016/j.joca.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Gao F., Xu Z., Liang Q., Li H., Peng L., Wu M., Zhao X., Cui X., Ruan C., Liu W. Osteochondral regeneration with 3D-printed biodegradable high-strength supramolecular polymer reinforced-gelatin hydrogel scaffolds. Adv. Sci. 2019;6(15) doi: 10.1002/advs.201900867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Udomluck N., Kim S.H., Cho H., Park J.Y., Park H. Three-dimensional cartilage tissue regeneration system harnessing goblet-shaped microwells containing biocompatible hydrogel. Biofabrication. 2019;12(1) doi: 10.1088/1758-5090/ab5d3e. [DOI] [PubMed] [Google Scholar]
- 23.Kubomura D., Ueno T., Yamada M., Nagaoka I. Evaluation of the chondroprotective action of N-acetylglucosamine in a rat experimental osteoarthritis model. Exp. Ther. Med. 2017;14(4):3137–3144. doi: 10.3892/etm.2017.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shkhyan R., Van Handel B., Bogdanov J., Lee S., Yu Y., Scheinberg M., Banks N.W., Limfat S., Chernostrik A., Franciozi C.E., Alam M.P., John V., Wu L., Ferguson G.B., Nsair A., Petrigliano F.A., Vangsness C.T., Vadivel K., Bajaj P., Wang L., Liu N.Q., Evseenko D. Drug-induced modulation of gp130 signalling prevents articular cartilage degeneration and promotes repair. Ann. Rheum. Dis. 2018;77(5):760–769. doi: 10.1136/annrheumdis-2017-212037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papathanasiou I., Michalitsis S., Hantes M.E., Vlychou M., Anastasopoulou L., Malizos K.N., Tsezou A. Molecular changes indicative of cartilage degeneration and osteoarthritis development in patients with anterior cruciate ligament injury. BMC Musculoskelet Disord. 2016;17:21. doi: 10.1186/s12891-016-0871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang Y.H., Park S., Ahn C., Song J., Kim D., Jin E.J. Beneficial reward-to-risk action of glucosamine during pathogenesis of osteoarthritis. Eur. J. Med. Res. 2015;20:89. doi: 10.1186/s40001-015-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang X., Yang W., Feng D., Jin X., Ma Z., Qian Z., Xie T., Li H., Liu J., Wang R., Li F., Li D., Sun H., Wu S. Cartilage-specific autophagy deficiency promotes ER stress and impairs chondrogenesis in PERK-ATF4-CHOP-dependent manner. J. Bone Miner. Res. 2017;32(10):2128–2141. doi: 10.1002/jbmr.3134. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z., Jin T., Lu Y. AntimiR-30b inhibits TNF-α mediated apoptosis and attenuated cartilage degradation through enhancing autophagy. Cell. Physiol. Biochem. 2016;40(5):883–894. doi: 10.1159/000453147. [DOI] [PubMed] [Google Scholar]
- 29.Wei L., Qin S., Ye Y., Hu J., Luo D., Li Y., Gao Y., Jiang L., Zhou Q., Xie X., Li N. Chondrogenic potential of manganese-loaded composite scaffold combined with chondrocytes for articular cartilage defect. J. Mater. Sci. Mater. Med. 2022;33(10):74. doi: 10.1007/s10856-022-06695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.