Abstract
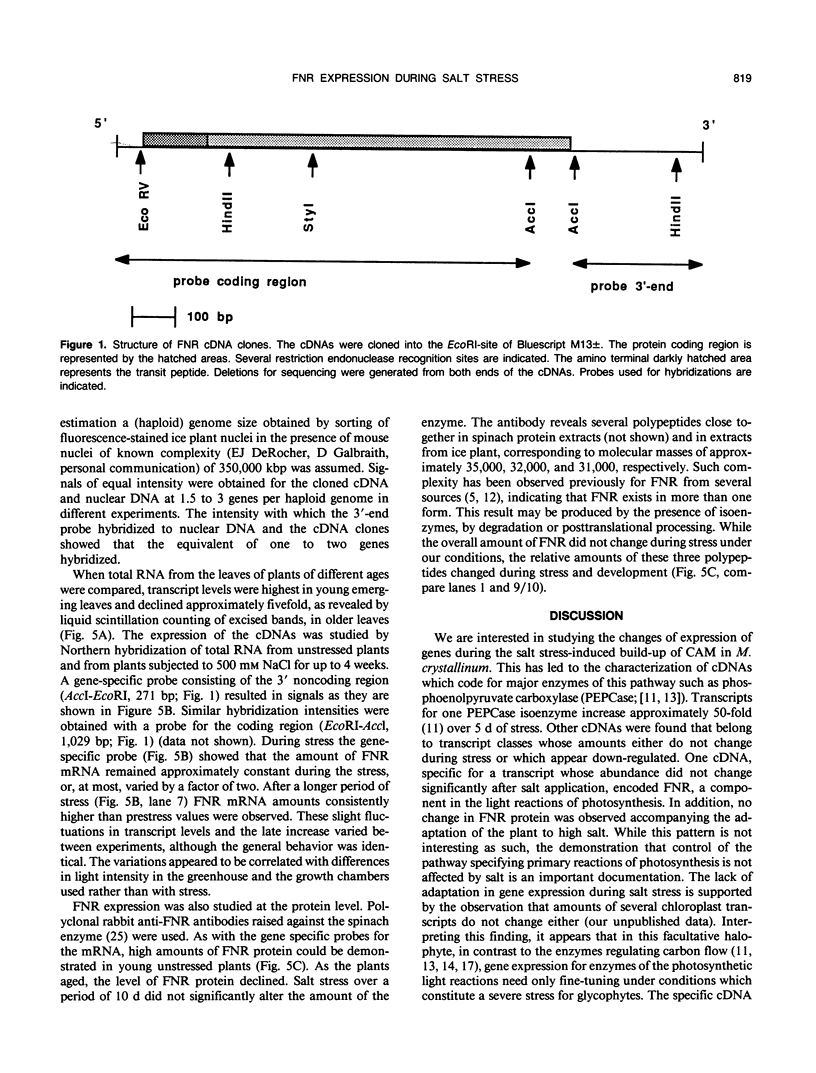
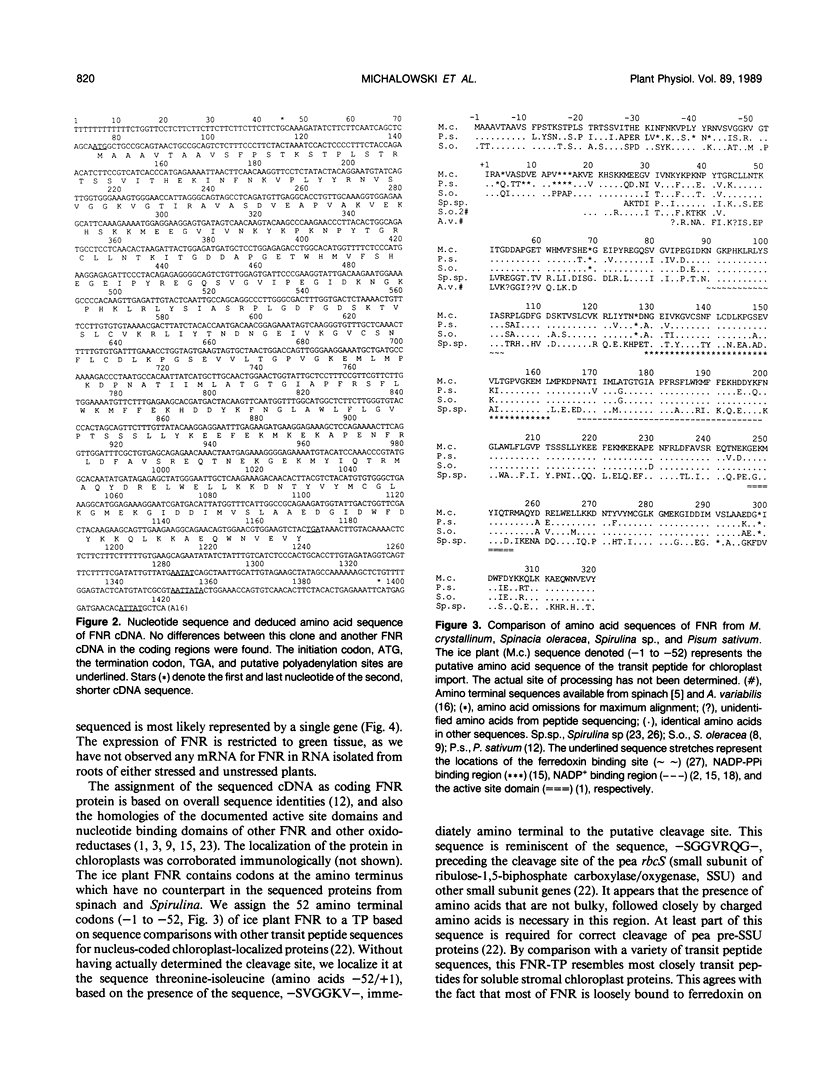
In the facultative halophyte Mesembryanthemum crystallinum (common ice plant) the enzyme ferredoxin-NADP+-reductase (FNR) is coded for by a small family of 2 to 3 genes. We have determined the expression characteristics as the plants adapt to high salt and the nucleotide sequence of a full-length cDNA coding for the precursor of this chloroplast-located enzyme. On a developmental scale amounts of FNR transcripts and protein are highest in young emerging leaves. The FNR cDNA is a member of a class of genes whose expression is only slightly affected by salt stress. Even less pronounced than mRNA fluctuations, the amount of FNR protein is unaffected by salt stress. The longest FNR cDNA found was 1,419 nucleotides in size. It consisted of 74 nucleotides 5′-leader sequence, 1,095 nucleotides of protein coding sequence encoding 365 amino acids, and 247 nucleotides 3-region excluding a short poly(A+) tail. As expected for a nucleus-coded chloroplast protein an amino terminal transit peptide (52 amino acids in length) was found. The mature FNR protein is predicted to contain 313 amino acids corresponding to a protein of Mr 35,713. The deduced amino acid sequence of the mature FNR protein is 93.2 and 85.9% identical to those of spinach and pea. The transit peptide of pea and spinach have 55.8 and 69.2% identity with that from ice plant.
Full text
PDF

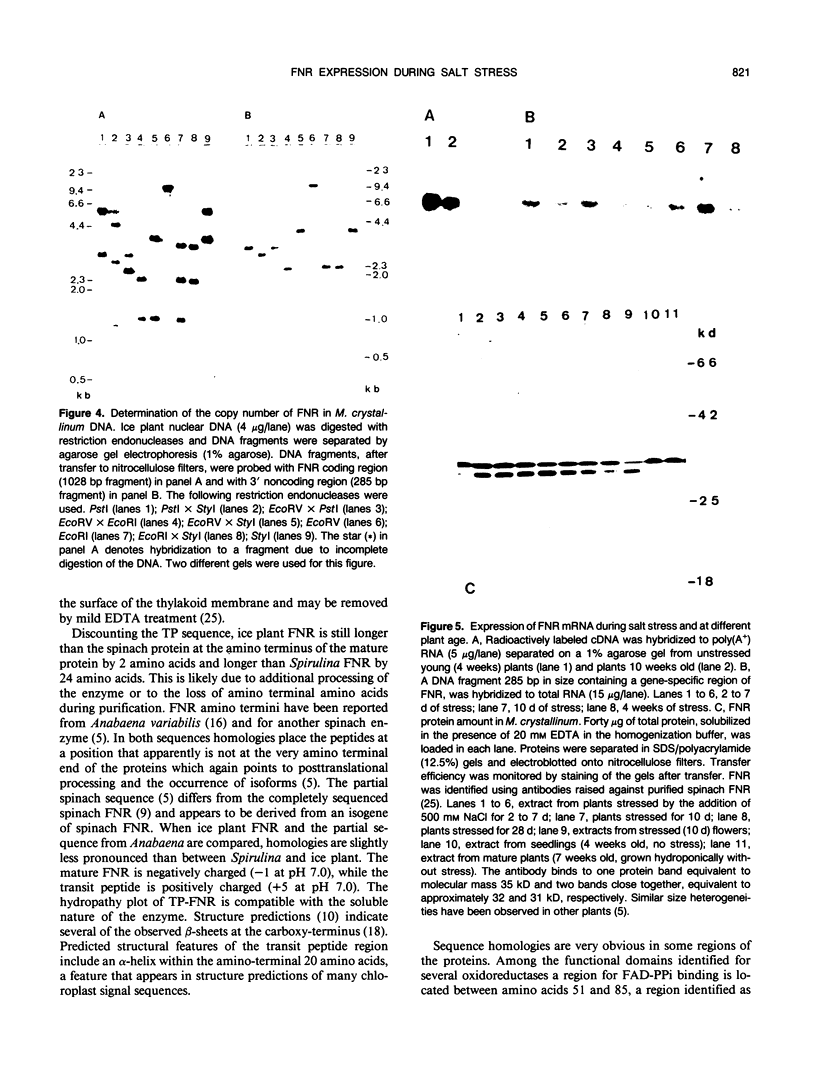

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan R. L., Carrillo N., Vallejos R. H. Isolation and sequencing of an active-site peptide from spinach ferredoxin-NADP+ oxidoreductase after affinity labeling with periodate-oxidized NADP+. Arch Biochem Biophys. 1985 Jul;240(1):172–177. doi: 10.1016/0003-9861(85)90020-7. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cidaria D., Biondi P. A., Zanetti G., Ronchi S. The NADP+-binding site of ferredoxin-NADP+ reductase. Sequence of the peptide containing the essential lysine residue. Eur J Biochem. 1985 Jan 15;146(2):295–299. doi: 10.1111/j.1432-1033.1985.tb08652.x. [DOI] [PubMed] [Google Scholar]
- DeRocher E. J., Ramage R. T., Michalowski C. B., Bohnert H. J. Nucleotide sequence of a cDNA encoding rbcS from the desert plant Mesembryanthemum crystallinum. Nucleic Acids Res. 1987 Aug 11;15(15):6301–6301. doi: 10.1093/nar/15.15.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasumi H., Nagata E., Nakamura S. Molecular heterogeneity of ferredoxin-NADP+ reductase from spinach leaves. Biochem Biophys Res Commun. 1983 Jan 14;110(1):280–286. doi: 10.1016/0006-291x(83)91292-5. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Höfner R., Vazquez-Moreno L., Winter K., Bohnert H. J., Schmitt J. M. Induction of Crassulacean Acid Metabolism in Mesembryanthemum crystallinum by High Salinity: Mass Increase and de Novo Synthesis of PEP-Carboxylase. Plant Physiol. 1987 Apr;83(4):915–919. doi: 10.1104/pp.83.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen T., Reiländer H., Steppuhn J., Herrmann R. G. Analysis of cDNA clones encoding the entire precursor-polypeptide for ferredoxin:NADP+ oxidoreductase from spinach. Curr Genet. 1988 Jun;13(6):517–522. doi: 10.1007/BF02427758. [DOI] [PubMed] [Google Scholar]
- Karplus P. A., Walsh K. A., Herriott J. R. Amino acid sequence of spinach ferredoxin:NADP+ oxidoreductase. Biochemistry. 1984 Dec 18;23(26):6576–6583. doi: 10.1021/bi00321a046. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
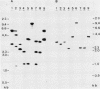
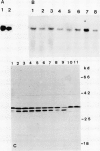
- Michalowski C. B., Olson S. W., Piepenbrock M., Schmitt J. M., Bohnert H. J. Time Course of mRNA Induction Elicited by Salt Stress in the Common Ice Plant (Mesembryanthemum crystallinum). Plant Physiol. 1989 Mar;89(3):811–816. doi: 10.1104/pp.89.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem J. A., Olson S. W., Schmitt J. M., Bohnert H. J. Salt Stress Increases the Level of Translatable mRNA for Phosphoenolpyruvate Carboxylase in Mesembryanthemum crystallinum. Plant Physiol. 1987 Aug;84(4):1270–1275. doi: 10.1104/pp.84.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter T. D., Kasper C. B. NADPH-cytochrome P-450 oxidoreductase: flavin mononucleotide and flavin adenine dinucleotide domains evolved from different flavoproteins. Biochemistry. 1986 Apr 8;25(7):1682–1687. doi: 10.1021/bi00355a036. [DOI] [PubMed] [Google Scholar]
- Sancho J., Peleato M. L., Gomez-Moreno C., Edmondson D. E. Purification and properties of ferredoxin-NADP+ oxidoreductase from the nitrogen-fixing cyanobacteria Anabaena variabilis. Arch Biochem Biophys. 1988 Jan;260(1):200–207. doi: 10.1016/0003-9861(88)90441-9. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Herriott J. R. Structure of ferredoxin-NADP oxidoreductase and the location on the NADP binding site. Results at 3-7 A resolution. J Mol Biol. 1981 Jan 15;145(2):441–451. doi: 10.1016/0022-2836(81)90214-x. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon D. M., Ostrem J. A., Schmitt J. M., Bohnert H. J. PEPCase Transcript Levels in Mesembryanthemum crystallinum Decline Rapidly upon Relief from Salt Stress. Plant Physiol. 1988 Apr;86(4):1002–1004. doi: 10.1104/pp.86.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmann C. C., Reiss B., Bohnert H. J. Complete processing of a small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase from pea requires the amino acid sequence Ile-Thr-Ser. J Biol Chem. 1988 Jan 15;263(2):617–619. [PubMed] [Google Scholar]
- Winter K., Foster J. G., Edwards G. E., Holtum J. A. Intracellular Localization of Enzymes of Carbon Metabolism in Mesembryanthemum crystallinum Exhibiting C(3) Photosynthetic Characteristics or Performing Crassulacean Acid Metabolism. Plant Physiol. 1982 Feb;69(2):300–307. doi: 10.1104/pp.69.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Tamura T., Wada K., Matsubara H., Kodo K. Spirulina ferredoxin-NADP+ reductase. The complete amino acid sequence. J Biochem. 1984 May;95(5):1513–1516. doi: 10.1093/oxfordjournals.jbchem.a134759. [DOI] [PubMed] [Google Scholar]