Abstract
Background
Metabolic syndrome (MetS) is accompanied by chronic low-grade inflammation, and inflammatory markers like high-sensitivity C-reactive protein(hs-CRP), interleukin-6(IL-6), and homocysteine(Hcy) contribute to inflammation, obesity, and insulin resistance. Adiponectin(AdipoQ) and interleukin-10(IL-10) are anti-inflammatory markers that play protective roles in MetS. This study aimed to investigate the association between these biochemical marker changes and MetS in a sample of the Tehranian population during six years of follow-up.
Methods
In this longitudinal study, 340 adults at baseline and after a six-year follow-up, aged ≥18 years, were selected randomly from the Tehran Lipid and Glucose Study (TLGS). MetS was defined according to the Joint Interim Statement (JIS) criteria. Individuals were categorized into four groups based on their MetS status at baseline and follow-up: 1) non-MetS: participants who did not have MetS at both baseline and follow-up; 2) incident MetS: participants who did not have MetS at baseline but developed MetS during the follow-up ; 3) recovery MetS: participants who had MetS at baseline but no longer had MetS during the follow-up; 4) persistent MetS: participants who had MetS both at baseline and follow-up.
Results
The mean follow-up time was 6.1 years. There were 176 subjects in the non-MetS group, 35 in the incident MetS group, 41 in the recovery MetS group, and 88 in the persistent MetS group. Increases in the levels of both hs-CRP 1.40 (95% CI: 1.15, 1.71, p = 0.001) and IL-6 1.09 (95% CI: 1.03, 1.17, p = 0.004) significantly increased the odds of the incident and persistent MetS, respectively. The area under the ROC curve (AUC) was more than 0.69 (p < 0.000) for hs-CRP in predicting MetS incidence and more than 0.86 (p < 0.000) for IL-6 in predicting MetS persistence.
Conclusion
After a six-year average follow-up, hs-CRP and IL-6 levels were deemed more reliable predictors of MetS incidence and persistence, respectively.
Keywords: Inflammation, Cytokine, Longitudinal study, Metabolic syndrome, TLGS
1. Introduction
Metabolic syndrome (MetS) is a common clinical disorder defined as the aggregation of cardiovascular risk factors such as central obesity, impaired glucose tolerance, hypertension, and dyslipidemia [1]. MetS is recognized as a target for preventing cardiovascular diseases (CVD) and is associated with an increased risk of coronary heart disease (CHD) [2]. The inflammatory condition associated with MetS is known as 'low-grade' chronic inflammation and does not appear to be caused by tissue damage, infection, or a sign of autoimmune disease. Many reports have suggested that markers of low-grade inflammation such as acute-phase reactants (e.g., high-sensitivity C-reactive protein (hs-CRP)), abnormal cytokine production (e.g., interleukin-6 (IL-6)), and the activation of inflammatory signaling pathways might play an important role [[3], [4], [5]]. Such markers contribute to inflammation, obesity, and insulin resistance. They are correlated with MetS regardless of obesity status and are higher when metabolic alterations are prevalent [6]. Studies have revealed that inflammation is associated with CVD and that inflammatory markers predict cardiovascular events [7].
High-sensitivity C-reactive protein as a sensitive marker for systemic inflammation is a product of hepatic inflammation [8]. The association between high hs-CRP levels and an increased risk of MetS development has been documented [9,10]. Elevated hs-CRP levels are also strongly associated with increased adipose tissue IL-6 expression and release [11]. IL-6 is a pro-inflammatory cytokine associated with obesity, MetS, and other MetS-related disorders and predicts the risk of acquiring insulin resistance and type 2 diabetes (T2D) [12,13]. Another indicator introduced in recent decades that is associated with incident CHD events is homocysteine (Hcy), a non-essential amino acid formed by the breakdown of methionine and known as a marker of endothelial injury [14,15]. Hyper-homocysteinemia, pro-inflammatory cytokines, and acute phase proteins are considered risk factors for developing MetS and atherosclerosis [16,17].
Adiponectin, an adipokine, is predominantly released by adipose tissue. It has anti-inflammatory and insulin-sensitizing properties [18]. This biomarker appears to have protective effects on MetS and T2D, unlike other adipokines [19,20]. Adiponectin deficiency has also been suggested as an independent risk factor for CVD [21]. Studies have shown that AdipoQ can induce interleukin-10 (IL-10) synthesis, and the anti-inflammatory properties of AdipoQ may be partially mediated through the stimulation of IL-10 [22]. Interleukin-10 is also an important anti-inflammatory cytokine, a major immune system regulator, and induces down-regulation of pro-inflammatory cytokines [23]. Its important protective effect on atherosclerotic lesions in experimental animals has been revealed [24]. Elevated levels of IL-10 were observed in obese Caucasian women, and its low levels have been associated with MetS [25]. Anti-inflammatory cytokine, IL-10 reduces IL-6 and other pro-inflammatory cytokines' inflammatory processes [22]. Studies have reported that elevated levels of IL-6 and IL-10 are related to the increase in the prevalence of CVD [26,27].
Importantly, the prevalence of MetS in Iran has been estimated at more than 30%, which is higher than that of some Western countries [[28], [29], [30]] and based on the increasing prevalence of obesity in children and adolescents in Iran [31], the prevalence of MetS is expected to increase in the future. Despite various preventive modalities for CVD, its prevalence is still increasing rapidly [32]. However, there is a paucity of studies investigating the correlation of inflammatory markers with MetS over time. We selected markers with both pro-inflammatory and anti-inflammatory properties (CRP and Hcy), pro-inflammatory (IL-6), and anti-inflammatory (AdipoQ and IL-10) properties, which have well-established associations with both MetS and CVD. Therefore, this study aimed to investigate the association between these biochemical marker changes and MetS in a sample of the Tehranian population during six years of follow-up.
2. Material and methods
2.1. Study population
In this longitudinal study, the participants were selected from the Tehran Lipid and Glucose Study (TLGS) population. The TLGS is a large, long-term, integrated, and community-based study conducted in Tehran, Iran. Inclusion criteria for our study were as follows: adults over the age of 18 who took part in phases I (baseline, conducted from 1999 to 2001) and III (follow-up, conducted from 2006 to 2008) of the TLGS, and information on age, sex, demographic, biochemical and serum were completed. Three hundred forty of these participants were randomly selected. TLGS is a large, long-term, integrated, and community-based study aiming to evaluate the prevalence of non-communicable diseases and all-cause mortality in the urban population of Tehran, the capital of Iran. Tehran's District 13 population was randomly sampled using a multistage stratified cluster random sampling approach, yielding a total of 15005 persons aged three and above. The district is situated in central Tehran, and its population age structure mirrors that of the city as a whole. The TLGS encompasses two primary parts: a cross-sectional prevalence research examining cardiovascular disease and its related risk factors, a prospective 20-year follow-up in several phases at approximately 3.6-year intervals [[33], [34], [35]]. The research protocol obtained approval of the Research Ethics Committee of the Research Institute for Endocrine Sciences (RIES), which is affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran. The code assigned to this approval is "IR.SBMU.ENDOCRINE.REC.1398.104".
2.2. Anthropometric and biochemical measurements
Information on age, sex, demographic, and medication usage for diabetes, hypertension, and lipid disorders were collected with a standardized questionnaire. Anthropometric measures, encompassing height, weight, and waist circumference (WC) were taken for all the subjects as described previously [5]. By dividing weight (kg) by height (m2), the body mass index (BMI) was computed. After at least 10 min of repose, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were determined using a standard sphygmomanometer. All the assays, including fasting blood glucose (FBS), triglyceride (TG), and high-density lipoprotein-cholesterol (HDL-C) levels, were done on the day of sampling. FBS level was measured by the glucose oxidase method (Glucose kit; Pars Azmun, Tehran, Iran). TG level of the samples was determined by enzymatic colorimetric method (TG kit; Pars Azmun, Tehran, Iran). The HDL-C samples were determined by precipitation and enzymatic colorimetric method (HDL-C; Pars Azmun, Tehran, Iran). Plasma levels of hs-CRP, Hcy, and IL-6 were measured using Enzyme-Linked Immunosorbent Assay (ELISA) kits (dbc co. Canada, diazyme co. USA, and dia clone co. France, respectively). Using human ELISA kits created by Diaclone (France) and Mercodia Company (Sweden), it was possible to measure the plasma concentrations of IL-10 and AdipoQ, respectively. Intra- and inter-assay coefficients of variation (CVs) were <7.5% for all biochemical measurements. The reliability of all methods was described previously [36].
2.3. Definition of MetS
MetS was defined according to the Joint Interim Statement (JIS) criteria [37] as the presence of three or more of the following features: TG ≥ 150 mg/dl or on medication; HDL-C <40 mg/dl in men and <50 mg/dl in women or on medication; SBP ≥130 mmHg and/or DBP ≥85 mmHg or drug treatment; FBS ≥100 mg/dl or drug treatment, and WC ≥ 90 Cm for both gender [38].
Participants were classified into four distinct groups based on their MetS status: (1) those who had no MetS at baseline and follow-up (non-MetS), (2) those who had no MetS at baseline and had MetS in follow-up (incident MetS), (3) those who had MetS at baseline and had no MetS in follow-up (recovery MetS) and (4) those who had MetS at baseline and follow-up (persistent MetS).
2.4. Statistical analysis
The Kolmogorov-Smirnov goodness-of-fit test assessed the normal distribution of continuous data. For continuous variables that exhibited a normal distribution, the mean ± standard deviation was reported, while variables that displayed a skewed distribution were reported using the median value with an interquartile range (IQR) of 25–75%. Categorical variables were expressed as counts and percentages. To compare different variables between the baseline and follow-up of the study, we utilized the paired t-test (normal variables), the Wilcoxon signed-rank test (skewed variables), and the McNemar test (categorical variables). The difference between the two phases of measuring markers was presented as a delta (Δ). The level of the Δ inflammatory markers with skewed distributions was rank normalized. Analyses of the covariance (ANCOVA) test were used to compare the mean of this delta value in four MetS status groups, adjusting for age, gender, BMI, and baseline value for each inflammatory marker. So that the markers' delta values were included as a dependent variable, Mets status as a factor, and other variables (sex and baseline values of age, BMI, and the level of each marker) were included in the analysis as covariates. Post hoc pairwise comparisons were performed using Bonferroni correction.
Multiple regression analysis was performed to estimate the association between delta values of markers and MetS in the two adjusted models: model 1 was adjusted for age (baseline), gender, and the baseline value of each biochemical marker, and model 2 included all factors in model 1 and BMI (baseline). The odds ratio (OR) and 95% confidence interval (CI) were reported. The association between the change in inflammatory markers and MetS components was measured via multiple linear regression models in which changes in MetS components were considered dependent variables and the covariates were gender, age, BMI, the value of each marker at baseline, and changes in the markers (delta (Δ)).
Receiver-operator curves (ROC) were generated to predict the performance of Δ hs-CRP and hs-CRP levels at baseline and follow-up on the "incident MetS" group, as well as Δ IL-6 and IL-6 levels at baseline and follow-up on the "persistent MetS" group. The ROC was constructed using a binary logistic regression model (adjusted for age, gender, BMI, and the baseline value of each biochemical marker). The area under the curve (AUC) and the 95% CI were calculated using the predicted probabilities from the prediction models. The diagnostic performance of the markers was assessed using sensitivity, specificity, and AUC. A model's goodness of fit (GOF) was evaluated using the Hosmer-Lemeshow goodness of fit test.
The SPSS software version 22.0 (SPSS Inc., Chicago, IL, USA) was utilized for conducting all statistical analysis. Two-sided statistical tests were employed, and any differences with probability values less than 0.05 were deemed to be statistically significant.
3. Results
Table 1 compares the characteristics of participants at baseline and follow-up. The mean age of participants was 42.2 ± 14.65 years at baseline and 48.4 ± 14.76 at follow-up; 56.9% were women. The mean follow-up time was 6.1 years. At baseline, the prevalence of MetS was 37.9%; by follow-up, it had reduced to 36.2%. WC, FBS, BMI, and hs-CRP levels were significantly elevated in the follow-up compared to baseline. Also, an increase in the use of diabetic and lipid-controlling medications was observed. Antihypertensive medication use and levels of SBP, DBP, TG, AdipoQ, IL-6, and IL-10 were significantly reduced. The changes in HDL-C and Hcy levels were insignificant between the two phases.
Table1.
Characteristics of participants at baseline and follow-up
| Variables | Baseline (n = 340) | Follow-up (n = 340) | p-value |
|---|---|---|---|
| Male | 127 (37.4) | ||
| Female | 213 (62.6) | ||
| MetS | 129 (37.9) | 123 (36.2) | 0.560 |
| Age (years) | 42.21 ± 14.65 | 48.36 ± 14.76 | <0.001 |
| BMI (kg/m2) | 26.78 ± 4.63 | 28.03 ± 4.48 | <0.001 |
| MetS components | |||
| WC (cm) | 87.23 ± 11.50 | 92.41 ± 11.49 | <0.001 |
| FBS (mg/dl) | 89 (83,97) | 91 (85,99) | 0.001 |
| SBP (mmHg) | 119.71 ± 19.12 | 117.55 ± 17.23 | 0.017 |
| DBP (mmHg) | 77.58 ± 10.17 | 73.00 ± 8.99 | <0.001 |
| TG (mg/dl) | 146.5 (97,207) | 131.5 (95,188) | 0.002 |
| HDL-C (mg/dl) | 42 (35,49) | 41 (35,47) | 0.130 |
| Biochemical Markers | |||
| AdipoQ (mg/l) | 13.14 (6.8,23.2) | 11.84 (5.8,21.3) | <0.001 |
| Hcy (μmol/l) | 11.62 (7.7,18.4) | 11.38 (7.7,18.2) | 0.710 |
| hs-CRP (mg/l) | 0.94 (0.47,2.4) | 1.11 (0.52,2.9) | 0.008 |
| IL-6 (pg/ml) | 4.41 (1.92,12.36) | 3.1 (1.32,6.3) | <0.001 |
| IL-10 (pg/ml) | 4.54 (2.5,7.76) | 4.22 (2.17,6.6) | <0.001 |
| Medication use | |||
| Antidiabetic medication (%) | 20 (5.9) | 34 (10) | 0.004 |
| Lipid-lowering medication (%) | 15 (4.4) | 29 (8.5) | 0.030 |
| Antihypertensive medication (%) | 82 (24.1) | 19 (5.6) | <0.001 |
Abbreviations: MetS, metabolic syndrome; BMI, body mass index; WC, waist circumference; FBS fasting blood sugar; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; HDL-C, high-density lipoprotein; AdipoQ, adiponectin; Hcy, homocysteine; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; IL-10, interleukin-10.
Data are shown as numbers (%) or median (IQR).
The p-values are for paired t-test (normal variables), Wilcoxon signed-rank test (skewed variables), and the McNemar test (categorical variables).
There were 176 subjects in the non-MetS group (51.8%), 35 in the incident MetS group (10.3%), 41 in the recovery MetS group (12%), and 88 in the persistent MetS group (25.9%). The BMI and WC were increased, and the levels of DBP, AdipoQ, IL-6, and IL-10 were significantly decreased in the follow-up compared to baseline in the "non-MetS" group. Moreover, as predicted, in the "incident MetS" group, except for DBP, all MetS components and BMI and hs-CRP levels shifted in favor of MetS incidence. However, in the "recovery MetS" group, the SBP, DBP, TG, and IL-6 values decreased significantly over the follow-up period. Besides, the WC and BMI were elevated, and the DBP, TG, and IL-10 were diminished in the "persistent MetS" group (Table 2).
Table2.
Characteristics of participants by categories of MetS status at baseline and follow-up
| Variables | Non-MetS (n = 176) |
Incident MetS (n = 35) |
Recovery MetS (n = 41) |
Persistent MetS (n = 88) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | P | Baseline | Follow-up | P | Baseline | Follow-up | P | Baseline | Follow-up | P | |
| WC | 81.24 ± 8.83 | 86.49 ± 9.38 | <0.001 | 86.4 ± 11.68 | 97.61 ± 8.91 | <0.001 | 91.25 ± 10.61 | 93.27 ± 11.86 | 0.167 | 97.66 ± 7.93 | 101.8 ± 8.41 | <0.001 |
| BMI | 25 ± 3.86 | 26.41 ± 3.63 | <0.001 | 26.47 ± 4.37 | 29.47 ± 4.27 | <0.001 | 27.42 ± 4.29 | 27.36 ± 4.67 | 0.881 | 30.17 ± 4.43 | 30.99 ± 4.4 | <0.001 |
| FBS | 87 (81,92) | 88 (83,92) | 0.173 | 87 (79,96) | 94 (86,107) | 0.002 | 89 (83,100) | 92 (86.5,97) | 0.141 | 99.5 (89,111) | 100.5 (91,119) | 0.283 |
| SBP | 111 (104,119) | 110 (102,120) | 0.110 | 115 (111,127) | 123 (115,132) | 0.015 | 121 (114,135) | 114 (104.5126.5) | 0.001 | 128.5 (115.5143.75) | 126 (116.25,138.75) | 0.161 |
| DBP | 73.82 ± 8.52 | 70.54 ± 8.15 | <0.001 | 76.26 ± 5.34 | 74.66 ± 7.83 | 0.226 | 81.81 ± 11.15 | 73.46 ± 9.28 | <0.001 | 83.64 ± 10.68 | 77.03 ± 9.36 | <0.001 |
| TG | 104 (77.25,146.75) | 105 (78.75,138.75) | 0.364 | 135 (102,177) | 170 (112,209) | 0.037 | 180 (161.5228) | 128 (107,179) | <0.001 | 210.5 (151.25,266.75) | 191.5 (142.5259) | 0.015 |
| HDL-C | 42 (39,49) | 43 (36.25,49) | 0.160 | 42 (35,53) | 39 (32,46) | 0.002 | 39 (35,49) | 43 (37,47.5) | 0.056 | 39 (32,46) | 38 (33,43) | 0.786 |
| AdipoQ | 13.24 (6.59,25.13) | 12.11 (5.77,22.8) | 0.004 | 12.48 (7.06,19.19) | 9.32 (5.67,15) | 0.010 | 10.78 (7.34,21.15) | 11.04 (6.32,19.17) | 0.056 | 11.87 (6.32,21.83) | 11.84 (5.75,21.35) | 0.980 |
| Hcy | 11.62 (7.72,18.09) | 11.34 (7.73,17.19) | 0.716 | 9.48 (7.15,19.14) | 10.48 (7.64,16.13) | 0.166 | 12.01 (7.88,23.47) | 12.24 (7.86,20.24) | 0.957 | 12.04 (8.15,18.29) | 12.26 (7.74,19.75) | 0.779 |
| hs-CRP | 0.86 (0.35,1.97) | 0.85 (0.42,2.04) | 0.335 | 0.74 (0.42,1.77) | 1.9 (0.68,3.21) | <0.001 | 1.7 (0.91,2.73) | 1.39 (0.51,3.16) | 0.510 | 1.18 (0.74,3.47) | 1.97 (0.93,3.43) | 0.083 |
| IL-6 | 3.98 (1.83,10.73) | 2.08 (1.01,4.6) | <0.001 | 5.48 (2.16,10.62) | 3.57 (2.42,7.5) | 0.256 | 6.02 (2.12,19.09) | 4.02 (1.37,7.61) | 0.006 | 4.59 (2.08,11.95) | 3.87 (1.76,8.21) | 0.067 |
| IL-10 | 4.46 (2.58,7.57) | 3.87 (2.43,6.58) | 0.001 | 3.96 (1.97,8.22) | 4.19 (1.97,6.32) | 0.158 | 4.28 (2.6,7.76) | 4.69 (2.02,7.75) | 0.706 | 4.38 (2.43,7.74) | 4.22 (2.11,6.48) | 0.037 |
Abbreviations: MetS, metabolic syndrome; BMI, body mass index; WC, waist circumference; FBS fasting blood sugar; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; HDL-C, high-density lipoprotein; AdipoQ, adiponectin; Hcy, homocysteine; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; IL-10, interleukin-10.
Data are n (%), median (IQR), or mean±SD.
The p-values are for the paired t-test (normal variables) and Wilcoxon signed-rank test (skewed variables).
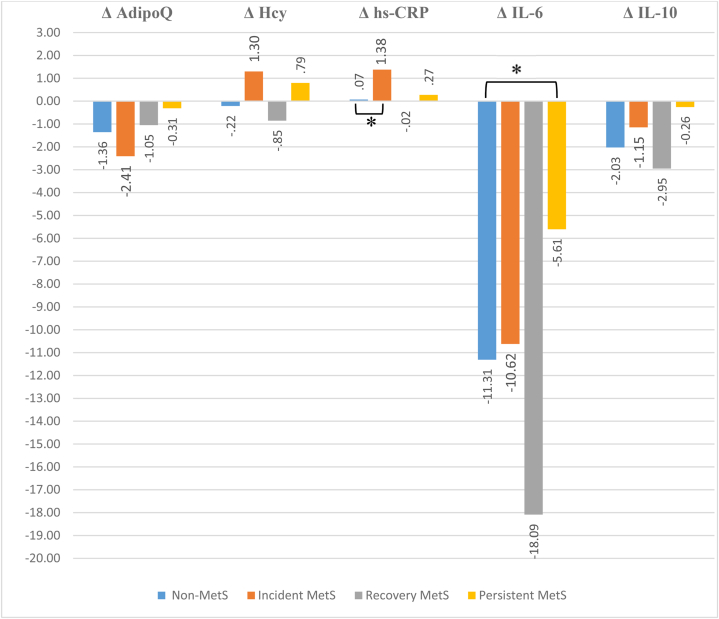
Fig. 1 displays the results of the ANCOVA analysis. The levels of AdipoQ, IL-6, and IL-10 markers in all groups declined between the two phases of the study. The groups "non-MetS" and "recovery MetS" had decreases in hs-CRP and Hcy levels, whereas the "incident MetS" and "persistent MetS" groups showed increases. However, only the higher rise in hs-CRP level in the "incident MetS" versus the "non-MetS" group and the lower decrease in IL-6 level in the "persistent MetS" over the "non-MetS" group were statistically significant (Fig. 1).
Fig. 1.
Mean difference of biochemical markers according MetS status during the follow-up period * Bonferroni correction p-value <0.05, based on ANCOVA adjusted for baseline age, gender, baseline value of each inflammation marker, and baseline body mass index. Abbreviations: Δ, initial level - follow-up level; MetS, metabolic syndrome; AdipoQ, adiponectin; Hcy, homocysteine; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; IL-10, interleukin-10.
The correlation between alterations in inflammatory markers and MetS status, with the "non-MetS" group as a reference, was shown in Table 3. Following the findings of the ANCOVA analysis, increases in the levels of both hs-CRP and IL-6 significantly increased odds of the incident and persistent MetS, respectively, in two adjusted multiple regression analysis models. For each unit change in hs-CRP and each unit change in IL-6 in model 2, the ORs for MetS incidence and persistence were 1.40 (95% CI: 1.15, 1.71) and 1.09 (95% CI: 1.03, 1.17), respectively. None of the other correlations between marker changes and other MetS statuses were statistically significant. The findings of the correlation between changes in markers and MetS components are displayed in Supplementary Table 1. Of the investigated biomarkers, AdipoQ alterations were most strongly associated with MetS component changes. Thus, in the "incident MetS" group, which was associated with a significant decrease in the level of this adipokine, the changes in AdipoQ levels were associated with alterations in WC (β = −0.6, p = 0.000), serum HDL-C (β = 0.35, p = 0.019), SBP (β = −0.6, p = 0.002), and DBP (β = −0.41, p = 0.039). There was a correlation between the changes in hs-CRP levels and the changes in the three MetS components, including FBS, TG, and WC. This association was restricted to the two components of TG (β = 0.18, p = 0.018) and WC (β = 0.16, p = 0.046) in the "non-MetS" group. In the "recovery MetS" group, the positive correlation between hs-CRP and WC changes was stronger (β = 0.51, P = 0.001). In contrast, in the "persistent MetS" group, the correlation between the inflammatory marker and TG changes was reversed (β = −0.23, p = 0.042), and there was a correlation between the changes of this marker and FBS (β = 0.32, p = 0.005). Interestingly, hs-CRP level changes were not independently associated with any component in the "incident MetS" group; additionally, no correlation was found between alterations in IL-6 and IL-10 levels and changes in any of the MetS components among all four groups (Supplementary Table 1).
Table 3.
Association between changes in the levels of inflammation markers and MetS status
| Markers | Models | Non-MetS | Incident MetS |
Recovery MetS |
Persistent MetS |
|||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |||
| Δ AdipoQ | Model 1 | Ref | 0.934 (0.869,1.00) | 0.066 | 0.986 (0.924,1.05) | 0.681 | 0.999 (0.948,1.05) | 0.960 |
| Model 2 | Ref | 0.931 (0.865,1.00) | 0.059 | 0.983 (0.919,1.05) | 0.617 | 0.994 (0.938,1.05) | 0.846 | |
| Δ Hcy | Model 1 | Ref | 1.021 (0.967,1.08) | 0.457 | 1.003 (0.956,1.05) | 0.900 | 1.021 (0.982,1.06) | 0.294 |
| Model 2 | Ref | 1.022 (0.968,1.08) | 0.430 | 1.008 (0.960,1.06) | 0.755 | 1.032 (0.989,1.08) | 0.143 | |
| Δ hs-CRP | Model 1 | Ref | 1.447 (1.18,1.77) | <0.001 | 1.137 (0.912,1.42) | 0.254 | 1.221 (1.02,1.46) | 0.030 |
| Model 2 | Ref | 1.399 (1.15,1.71) | 0.001 | 1.109 (0.887,1.39) | 0.364 | 1.176 (0.967,1.43) | 0.105 | |
| Δ IL-6 | Model 1 | Ref | 1.067 (0.995,1.15) | 0.068 | 1.054 (0.984,1.13) | 0.133 | 1.109 (1.047,1.17) | <0.001 |
| Model 2 | Ref | 1.06 (0.989,1.14) | 0.100 | 1.045 (0.976,1.12) | 0.207 | 1.096 (1.03,1.17) | 0.004 | |
| Δ IL-10 | Model 1 | Ref | 0.962 (0.860,1.08) | 0.493 | 0.996 (0.914,1.09) | 0.921 | 1.004 (0.933,1.08) | 0.907 |
| Model 2 | Ref | 0.957 (0.854,1.07) | 0.444 | 0.995 (0.912,1.09) | 0.904 | 1.000 (0.925,1.08) | 0.990 | |
Abbreviations: Δ, initial level - follow-up level; MetS, metabolic syndrome; AdipoQ, adiponectin; Hcy, homocysteine; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; IL-10, interleukin-10.
Results of multivariate logistic regression analyses with MetS status as a dependent variable.
Ref: The non-MetS group was used as the reference in logistic regression analysis.
Model 1: adjustment for baseline value of each inflammation marker, baseline age, and gender; model 2: model 1+ baseline BMI.
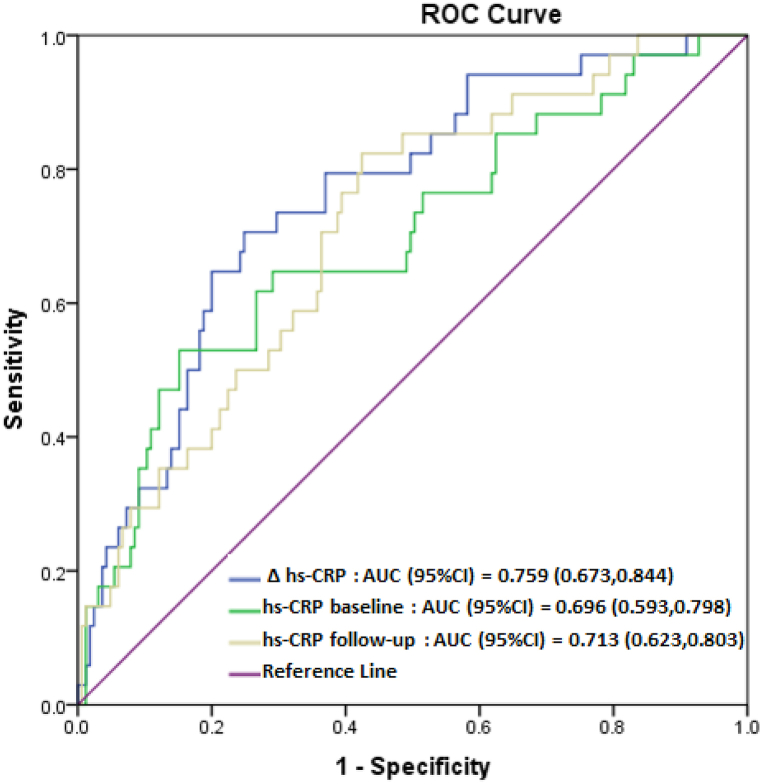
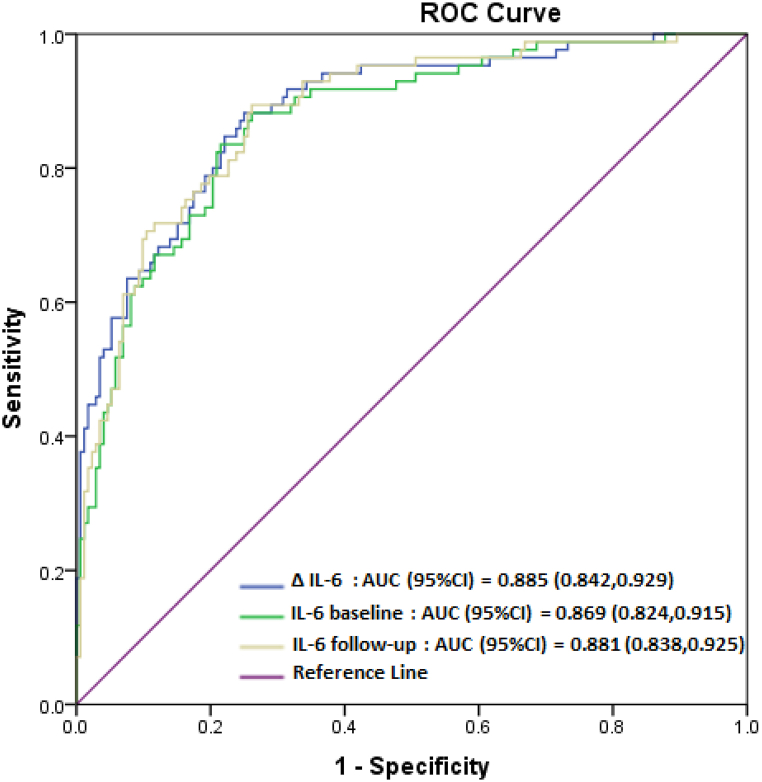
The predictive power of hs-CRP and IL-6 was evaluated using ROC curve analyses, as shown in Fig. 2, Fig. 3. The area under the ROC curve (AUC) was more than 0.69 (p < 0.000) for hs-CRP in predicting MetS incidence and more than 0.86 (p < 0.000) for IL-6 in predicting MetS persistence.
Fig. 2.
Receiver operating characteristic (ROC) curves of hs-CRP levels in predicting MetS incidence.
Fig. 3.
Receiver operating characteristic (ROC) curves of IL-6 levels in predicting MetS persistent.
4. Discussion
In this longitudinal study of Iranian adults, we investigated the correlation between MetS status and inflammatory biomarker levels and the changes in these markers during the six-year period. The findings indicated that during the follow-up period, the levels of AdipoQ, IL-6, and IL-10 were significantly decreased in the three status categories of incident, recovery, and persistent MetS, respectively. This decrease was also significant for the three markers in the "non-MetS" group. The only significant increase in hs-CRP in the "incident MetS" group was not accompanied by a significant change in the "non-MetS" group. By examining changes in the levels of these inflammatory markers and after adjusting for baseline values of markers, BMI, age, and gender, the correlation between the levels of AdipoQ and IL-10 and MetS status disappeared. By referring to the "non-MetS" group, it was determined that further increases in hs-CRP and IL-6 levels enhanced the incidence of MetS and persistent MetS, respectively, after the six-year follow-up. In addition, considering age, gender, BMI, and baseline levels of each marker as covariates, IL-6 levels were highly predictive of persistent MetS. Moreover, the predictive powers of hs-CRP and IL-6 for the risk and persistence of MetS were 76% and 88%, respectively.
Central obesity and insulin resistance are considered vital contributors to the development of MetS, despite its complex etiology [39]. Adipose tissue secretes cytokines as an endocrine organ with metabolic activity [40]. Hence, visceral obesity induces subclinical inflammation, MetS, and increases the risk of cardiovascular disease by elevating inflammatory cytokines [41]. In comparison to individuals without MetS, patients with MetS have a simultaneous elevation in pro-inflammatory cytokines (such as IL-6 and TNF) and a reduction in anti-inflammatory cytokines (such as IL-10 and AdipoQ) [42]. Therefore, an imbalance between pro-inflammatory and anti-inflammatory cytokines contributes to the pathophysiology of MetS [43]. On the other hand, it has been demonstrated that patients with MetS have a distinct inflammatory pattern, and MetS affects the expression of pro-inflammatory genes in adipose tissue and the amount of circulating inflammatory cytokines [44]. Hence, the question remains open for discussion as to whether MetS is the causal factor or the consequence of alterations in inflammatory marker levels.
Hs-CRP is a well-investigated inflammatory indicator associated with MetS. Most of these investigations were cross-sectional; hs-CRP levels were assessed in quartiles, and the results demonstrated a positive association between hs-CRP and MetS in Iranian and other populations [[45], [46], [47], [48], [49]]. In fewer longitudinal studies, this positive and significant relationship has also been observed with MetS incidence [[50], [51], [52]]. The relationship between longitudinal changes in hs-CRP levels and the incidence of MetS has also been studied in a limited number of studies conducted on Asian populations [53,54]. In a cohort of 3748 healthy Korean men with a follow-up of seven years, the risk of MetS incidence was significantly higher in the group with elevated hs-CRP levels (OR = 1.44), even after adjusting for confounding variables [53]. In addition to age, the study's confounding factors included smoking, alcohol use, and exercise, but not BMI or hs-CRP baseline levels. A one-unit increase in CRP level elevated the risk of MetS incidence by 23% (OR = 1.23) in a population of 4116 Chinese individuals after adjusting for covariates [54]. This study was adjusted for the influence of many confounding factors, including education level, place of residence, smoking habits, alcohol consumption, hypertension, dyslipidemia, diabetes, heart disease, and stroke. Similarly, in the present study, one unit (1 pg/ml) elevation in hs-CRP levels was correlated with a 40% increased risk of incident MetS (OR = 1.40) after adjusting for baseline age, sex, BMI, and hs-CRP level. Therefore, the observed variations in the outcomes may be attributed, at least in part, to differences in confounding factors.
The relatively high AUC value of hs-CRP for MetS incidence confirms its outstanding diagnostic capability. One study examined the predictive ability of different levels of the hs-CRP marker for developing MetS based on AUC values [55]. In this study, Japanese men and women had similar hs-CRP AUCs at baseline and follow-up, and the average of the two tests indicated early MetS. This investigation revealed that AUCs ranging from 0.71 to 0.75 are consistent with our findings. Interestingly, in the present study, the change in hs-CRP levels had slightly more predictive power than the baseline and follow-up levels. Therefore, consistent with prior research [53,54], the results of our study indicate that elevations in hs-CRP levels within the normal range and a progressive rise in hs-CRP levels over time may serve as diagnostic indicators for MetS. Consequently, it is essential to monitor individuals for the early prevention and diagnosis of MetS, even if they appear healthy.
In the current study, multivariate logistic regression analyses showed that a unit increase in IL-6 levels elevated the risk of MetS by 9% in the "persistent MetS" group. Previous cross-sectional studies found a positive relationship between IL-6 levels and the prevalence of MetS in the Iranian population [[56], [57], [58]], including the TLGS community [59], and in other populations [[60], [61], [62], [63], [64], [65]]. The convergence of the pathological mechanisms involved in the pathogenesis of MetS leads to a pro-inflammatory state, which explains the increased levels of several inflammatory biomarkers, including IL-6 and CRP, in individuals with MetS. The major mechanisms underlying the pathophysiology of MetS, insulin resistance, and systemic oxidative stress induced by obesity lead to the activation of downstream inflammatory cascades [66]. The precise physiological role of IL-6 signaling in the development of MetS is unclear, despite the involvement of this marker in low-grade chronic inflammation [67]. The origin of IL-6 can influence its response to inflammation. IL-6 is produced by various cell types within adipose tissue, including adipocytes, adipose tissue macrophages, and other cell types present in adipose tissue. In mice, it has been observed that adipocyte-derived IL-6 significantly enhances the accumulation of macrophages in adipose tissue, even without substantial alterations in glucose or insulin tolerance. Conversely, IL-6 derived from myeloid cells suppresses the polarization of M1 macrophages, reduces the accumulation of macrophages in adipose tissue, and improves glucose and insulin tolerance [67]. These results indicate the complexity of the physiology of the metabolic function of IL6. Possibly because of this complexity, in line with the results obtained in two other Iranian populations [58,68], the positive relationship between persistent MetS and IL-6 changes proven in our study was also independent of MetS components. However, our study specifically examined the correlation between changes in MetS components and IL-6, a relationship that was not investigated in a previous study.
Few studies have monitored the changes in IL-6 levels over time. In one intervention study in an animal model, 28 rats were fed a high-fat or high-salt diet for seven weeks. A significant positive correlation was identified between IL-6 levels in adipose tissue and various risk factors for MetS, including fasting insulin, blood lipid profile, body weight, and visceral fat mass. Based on these findings, the authors concluded that IL-6 could be considered an early and typical marker in the pathogenesis and development of MetS and CVD [69]. The predictive power of IL-6 levels in the two phases of our study and the difference between these two levels were similar for persistent MetS. The AUC values of IL-6 obtained for persistent MetS in our investigation were higher than those reported in previous research on the prevalence of MetS [[70], [71], [72]]. Hence, based on the current investigation results, it can be inferred that elevated levels of IL-6, whether observed at a single time or as an ongoing increase over time, serve as a robust indicator of persistent MetS. To our knowledge, no previous study has investigated the relationship between IL-6 and the incidence of MetS in the long term. Further longitudinal investigations are recommended to study the predictive ability of the IL-6 marker for persistent MetS.
Many variables contribute to elevated hs-CRP and IL-6 levels. One potential aspect to consider is the development of MetS. As mentioned, it remains unclear whether the increase in inflammation-related markers results from a higher incidence of MetS or whether it is the cause. The results of Mendelian randomization analysis also did not favor the causality of inflammatory markers for MetS [73,74]. Obesity and insulin resistance, the two possible critical causes of MetS, are additional variables that raise these two markers [6,75]. Central obesity and insulin resistance stimulate IL-6 secretion by adipocytes. Adipocytes, particularly those from visceral adipose tissue, are the primary sources of IL-6, making it a cytokine with a cellular origin. IL-6 is correlated with other cytokines and inflammatory mediators, and one of its most vital functions is to stimulate liver cell production of CRP, the major mediator of the inflammatory response [6]. Dyslipidemia, characterized by increased TG and decreased HDL, was also positively correlated with hs-CRP and IL-6 levels [76,77]. Lifestyle variables, including dietary patterns, levels of physical activity, and smoking habits, can significantly impact inflammatory markers like hs-CRP and IL-6. Unhealthy diets high in processed foods and saturated fats, sedentary behavior, and smoking have been associated with increased levels of hs-CRP and IL-6, thereby promoting chronic inflammation and metabolic dysfunction [[78], [79], [80]]. The observed variations in hs-CRP and IL-6 levels may also be influenced by genetic and epigenetic factors [[81], [82], [83], [84]]. The current research demonstrated the association between the development of MetS and the increase in the levels of hs-CRP and IL-6, among the influencing factors in the serum. However, this association was not affected by the two additional factors of dyslipidemia and obesity (BMI), consistent with the findings of some prior investigations [57,58,85]. In this research, the impact of other factors was not examined.
Abnormal levels of the pro-inflammatory cytokines IL-6 and TNF-α may lead to dysregulation of the insulin signaling system. IL-10 can potentially restore normal insulin signaling by either inhibiting oxidative stress caused by NADPH oxidase or counteracting the effects of IL-6 and TNF-α [[86], [87]]. Since insulin resistance is one of the primary mechanisms underlying the development of MetS and a reverse association between IL-10 and other MetS components has been reported [88,89], it appears that this marker's protective effect against MetS is probable. In this regard, it has been found in some studies that a reduced IL-10 level is associated with an elevated risk of MetS. For example, obese and non-obese women with MetS showed significantly decreased levels of IL-10 [90]. Other studies [42,91] have shown that the IL-10 levels of both men and women with MetS have significantly decreased. Following most previous studies, we observed a significant decrease in IL-10 levels in the "persistent MetS" group. Still, this decrease was also observed in the "non-MetS" group throughout the follow-up period. Age was one of the main variables that changed in the "non-MetS" group during the follow-up period, and the decrease in the level of IL-10 in this healthy group can be attributed to the documented age-related declines in this marker [[92], [93], [94], [95]]. Nevertheless, no significant correlation between serum IL-10 and the incidence of MetS was found in 258 sera collected from older people, despite the negative correlation between IL-10 and BMI [96].
Adiponectin enhances target tissue insulin sensitivity and assumes a pivotal role in the development of MetS by affecting lipid and glucose metabolism [97]. On the other hand, evidence suggests that AdipoQ has a significant inverse relationship with obesity and hypertension, two other components of MetS [98]. In the current investigation, AdipoQ levels were significantly lower in those who developed MetS during follow-up. However, this association disappeared when these individuals were compared to the reference group (non-MetS). Consistent with our results, the incidence of MetS and baseline serum AdipoQ levels in three cohort studies were determined to have a negative association [20,99,100]. The changes in AdipoQ level were associated with changes in MetS components only in the "incident MetS" group, including WC, SBP, and DBP, positively and with HDL-C negatively. This correlation with MetS components was consistent with the results of another Iranian population [101], with the exception that in the latter study, correlations with other MetS components, such as FBS and TG, were also observed, and the demonstrated correlation was between AdipoQ values and MetS components, not between changes in their levels. So, in the present investigation, AdipoQ may play a role in the pathophysiology of MetS via adiposity, dyslipidemia, and blood pressure-related components. Monitoring and increasing AdipoQ levels can therefore be effective factors in preventing the development of this syndrome.
During the present study's follow-up period, the level of IL-6 in the "Recovery MetS" group decreased significantly. In this group, the levels of anti-inflammatory markers IL-10 and AdipoQ increased. In contrast, another inflammatory marker, Hs-CRP, decreased during the follow-up period. Still, these changes were not significant, possibly attributable to the limited size of the sample. Furthermore, changes in hs-CRP, IL-6, IL-10, and AdipoQ levels may serve as potential therapeutic targets for improving metabolic function in individuals with MetS. Monitoring these biomarkers can help evaluate the degree of inflammation, metabolic dysfunction, and cardiovascular risk associated with MetS.
Consequently, reducing IL-6 levels may be a viable therapeutic objective for treating MetS. Tocilizumab, an IL-6 inhibitor, was evaluated for its efficacy in treating MetS in rat models. Tocilizumab is a monoclonal antibody that functions as an antagonist of the IL-6 receptor. The results indicated that the drug was effective, which confirms the current research and previous study findings. This IL-6 inhibitor shows promise for this indication, but further human studies are required before it can be used clinically [102]. On the other hand, IL-6 levels were significantly reduced when dietary interventions were implemented [[103], [104]]. Nutritional interventions combined with anti-inflammatory drugs may thus become an innovative way of treating MetS in future approaches.
The potential of AdipoQ as a biotarget for the modulation of metabolic and cardiovascular disease is noteworthy. Nonetheless, the administration of recombinant AdipoQ from an external source is hindered by difficulties in generating stable isoforms and high endogenous levels with a short in vivo half-life. The achievement of therapeutic effectiveness is contingent upon the augmentation of endogenous expression and the precise targeting of AdipoQ signaling and its subsequent effector pathways. On the other hand, lifestyle treatments, including exercise, calorie restriction, pharmaceutical agents, and gastric bypass surgery, have consistently demonstrated favorable outcomes in relation to AdipoQ levels. Integrating lifestyle modifications and pharmaceutical therapies has demonstrated the ability to enhance AdipoQ levels in individuals diagnosed with MetS [105,106].
Methionine metabolism results in the production of a mediator called homocysteine. Hyperhomocysteinemia has an extensive range of biological effects on various organs and is recognized to be associated with cardiovascular disease through different mechanisms, such as vascular dysfunction [107,108]. Furthermore, it has been suggested that an increased level of Hcy is pathophysiologically involved in the increased risk of MetS [109]. However, the mechanisms involved in Hcy-associated diseases have not been entirely clarified, and whether the relationship between MetS and Hcy level is causal remains obscure. However, recently, using the Mendelian randomization approach, a significant relationship was elucidated between Hcy and the risk of MetS in the Korean population [110]. On the other hand, Hcy increases inflammatory responses and correlates with IL-6, TNFα, and hs-CRP levels [111]. Although some previous studies demonstrated that Hcy was associated with the MetS or its components [112] in obese or overweight individuals [113] or hypertensive patients [17] with features of the MetS, in our research, the Hcy level was associated with neither the baseline nor the follow-up of the MetS status groups and its components. Consistent with our results, in Iranian populations, Fakhrzadeh et al. (2005) and Naderyan Fe'li et al. (2020) did not find a significant difference between the mean level of this marker in people with and without MetS [114,115]. Moreover, Nabipour et al. (2009) did not observe a significant association between the MetS and serum Hyc levels by multiple logistic regression analysis after adjusting for some confounders such as sex, age, smoking, fruit and vegetable intake pattern, BMI, and physical inactivity [116]. A similar lack of association has been documented in other populations: Budak et al. (2009) in Turkish individuals [117] and Garcin et al. (2006) in French people [118].
A prospective longitudinal design is one of the current study's strengths. According to our knowledge, this is the first study to investigate the relationship between changes in inflammatory markers, including AdipoQ, Hyc, IL-6, and IL-10, and MetS status and MetS components. However, the present study has some limitations, including the small sample size, the lack of data on some confounding factors such as dietary habits, smoking, and physical activity due to the unavailability of accurate data, and the lack of research on some pro-inflammatory cytokines, including TNF-α.
5. Conclusion
After an average six-year follow-up, the changes in hs-CRP and IL-6 levels were regarded as more reliable predictors of incidence and persistent MetS, respectively, compared to the changes in AdipoQ, IL-10, and Hcy. Interleukin-6 levels at baseline and follow-up were similarly predictive of the persistence of MetS. However, the predictive power of the changes in the hs-CRP level was marginally higher than the other two measurements over the two phases of the study. Further, larger and longer-term longitudinal studies are needed to determine the role of IL-6 in persistent MetS.
Author contribution statement
Asiyeh Sadat Zahedi: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Maryam Sadat Daneshpour: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mahdi Akbarzadeh: Analyzed and interpreted the data; Wrote the paper.
Mehdi Hedayati: Conceived and designed the experiments; Wrote the paper.
Fereidoun Azizi:Contributed reagents, materials, analysis tools or data.
Maryam Zarkesh: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
This study was supported by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences (Tehran, Iran), and received no specific funding or grant from the public and commercial agencies.
Data availability statement
Data included in article/supp. material/referenced in article.
Ethics approval and consent to participate
Written informed consent was obtained from all participants. The study protocol was approved by the Research Ethics Committee of the Research Institute for Endocrine Sciences (code of "IR.SBMU.ENDOCRINE.REC.1398.104″) affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e19911.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Keaney J.F., Jr., Curfman G.D., Jarcho J.A. A pragmatic view of the new cholesterol treatment guidelines. N. Engl. J. Med. 2014;370(3):275–278. doi: 10.1056/NEJMms1314569. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Hernández H., et al. 2013. Obesity and Inflammation: Epidemiology, Risk Factors, and Markers of Inflammation. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dallmeier D., et al. Metabolic syndrome and inflammatory biomarkers: a community-based cross-sectional study at the Framingham Heart Study. Diabetol Metab Syndr. 2012;4(1):28. doi: 10.1186/1758-5996-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faam B., et al. Association between abdominal obesity and hs-CRP, IL-6 and HCY in tehranian adults: TLGS. Iranian Journal of Diabetes and Metabolism. 2014;13(2):163–171. [Google Scholar]
- 5.Zarkesh M., et al. The relationship between metabolic syndrome, cardiometabolic risk factors and inflammatory markers in a Tehranian population: the Tehran Lipid and Glucose Study. Intern Med. 2012;51(24):3329–3335. doi: 10.2169/internalmedicine.51.8475. [DOI] [PubMed] [Google Scholar]
- 6.de F., Rocha A.R., et al. Inflammatory biomarkers and components of metabolic syndrome in adolescents. a systematic review. 2022;45(1):14–30. doi: 10.1007/s10753-021-01549-1. [DOI] [PubMed] [Google Scholar]
- 7.Tabrizi R., et al. The effects of statin use on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;141:85–103. doi: 10.1016/j.phrs.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Chen S.-J., et al. 2012. Relationships between Inflammation, Adiponectin, and Oxidative Stress in Metabolic Syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sah S.K., et al. Association of high-sensitivity C-reactive protein and uric acid with the metabolic syndrome components. SpringerPlus. 2016;5(1):269. doi: 10.1186/s40064-016-1933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong G.-b., et al. High-sensitivity C-reactive protein leads to increased incident metabolic syndrome in women but not in men: a five-year follow-up study in a Chinese population. Diabetes, Metab. Syndrome Obes. Targets Ther. 2020;13:581. doi: 10.2147/DMSO.S241774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellulu M.S., et al. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci.: AMS. 2017;13(4):851. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zatterale F., et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020;10:1607. doi: 10.3389/fphys.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingelsson E., et al. Inflammatory markers in relation to insulin resistance and the metabolic. syndrome. 2008;38(7):502–509. doi: 10.1111/j.1365-2362.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 14.Karger A.B., et al. Association between homocysteine and vascular calcification incidence, prevalence, and progression in the MESA cohort. J. Am. Heart Assoc. 2020;9(3) doi: 10.1161/JAHA.119.013934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Škovierová H., et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. 2016;17(10):1733. doi: 10.3390/ijms17101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catena C., et al. Elevated homocysteine levels are associated with the metabolic syndrome and cardiovascular events in hypertensive patients. Am. J. Hypertens. 2015;28(7):943–950. doi: 10.1093/ajh/hpu248. [DOI] [PubMed] [Google Scholar]
- 17.Catena C., et al. Elevated homocysteine levels are associated with the metabolic syndrome and cardiovascular events in hypertensive patients. 2015;28(7):943–950. doi: 10.1093/ajh/hpu248. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen T.M.D. Adiponectin: role in physiology and pathophysiology. Int. J. Prev. Med. 2020;11:136. doi: 10.4103/ijpvm.IJPVM_193_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy B., Palaniyandi S.S.J.C. Bioscience, Tissue-specific role and associated downstream signaling pathways of adiponectin. 2021;11(1):77. doi: 10.1186/s13578-021-00587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K.W., D.J.I.J.o.E.R. Shin. Health P. Prospective associations of serum adiponectin, leptin, and leptin-adiponectin ratio with incidence of metabolic syndrome: the Korean Genome and Epidemiology Study. 2020;17(9):3287. doi: 10.3390/ijerph17093287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui X., et al. Adiponectin and cardiovascular health: an update. Br. J. Pharmacol. 2012;165(3):574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang J.S., et al. Interleukin 10 and clustering of metabolic syndrome components in pediatrics. 2014;44(4):384–394. doi: 10.1111/eci.12247. [DOI] [PubMed] [Google Scholar]
- 23.Steen E.H., et al. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv. Wound Care. 2020;9(4):184–198. doi: 10.1089/wound.2019.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almer G., et al. Interleukin-10: an anti-inflammatory marker to target atherosclerotic lesions via PEGylated liposomes. Mol. Pharm. 2013;10(1):175–186. doi: 10.1021/mp300316n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esposito K., et al. Association of low Interleukin-10 levels with the metabolic syndrome in obese women. Obes. Metabol. 2005;2(1):43. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 26.Simon T.G., et al. Circulating Interleukin-6 is a biomarker for coronary atherosclerosis in nonalcoholic fatty liver disease: results from the Multi-Ethnic Study of Atherosclerosis. Int. J. Cardiol. 2018;259:198–204. doi: 10.1016/j.ijcard.2018.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz M.I., et al. The relationship between IL-10 levels and cardiovascular events in patients with CKD. Clin. J. Am. Soc. Nephrol. 2014;9(7):1207–1216. doi: 10.2215/CJN.08660813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadaegh F., et al. Incidence of metabolic syndrome over 9 Years follow-up; the importance of sex differences in the role of insulin resistance and other risk factors. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0076304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farmanfarma K.K., et al. Prevalence of metabolic syndrome in Iran. A meta-analysis of 69 studies. 2019;13(1):792–799. doi: 10.1016/j.dsx.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 30.Saberi-Karimian M., et al. A pilot study of the effects of crocin on high-density lipoprotein cholesterol uptake capacity in patients with metabolic syndrome. A randomized clinical trial. BioFactors (Oxford, England) 2021;47(6):1032–1041. doi: 10.1002/biof.1783. [DOI] [PubMed] [Google Scholar]
- 31.Esmaili H., et al. Prevalence of general and abdominal obesity in a nationally representative sample of Iranian children and adolescents: the CASPIAN-IV study. Iranian journal of pediatrics. 2015;25(3) doi: 10.5812/ijp.25(3)2015.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y., Benjamin E.J., MacMahon S. Prevention and control of cardiovascular disease in the rapidly changing economy of China. Circulation. 2016;133(24):2545–2560. doi: 10.1161/CIRCULATIONAHA.115.008728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azizi F., et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials. 2009;10:5. doi: 10.1186/1745-6215-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedayati M., et al. Biochemical assessment: findings from 20 years of the tehran lipid and glucose study. Int. J. Endocrinol. Metabol. 2018;16(4 Suppl) doi: 10.5812/ijem.84783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daneshpour M.S., et al. 2022. Cohort Profile Update: Tehran Cardiometabolic Genetic Study, a Path toward Precision Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azizi F., et al. Prevention of non-communicable disease in a population in nutrition transition. Tehran Lipid and Glucose Study phase II. 2009;10(1):1–15. doi: 10.1186/1745-6215-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberti K.G., et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood Institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 38.Azizi F., et al. 2010. Appropriate Waist Circumference Cut-Off Points Among Iranian Adults: the First Report of the Iranian National Committee of Obesity. [PubMed] [Google Scholar]
- 39.Grundy S.M., et al. Definition of metabolic syndrome: report of the national heart, lung. and Blood Institute/American Heart Association conference on scientific issues related to definition. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 40.Koster A., et al. Body Fat Distribution and Inflammation Among Obese Older Adults With and Without Metabolic Syndrome. 2010;18(12):2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteiro R., Azevedo I.J.M.o.i. 2010. Chronic Inflammation in Obesity and the Metabolic Syndrome. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi K., et al. Serum adiponectin. interleukin-10 levels and inflammatory markers in the metabolic syndrome. 2007;75(2):235–240. doi: 10.1016/j.diabres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 43.Barona J., et al. Grape consumption increases anti-inflammatory markers and upregulates peripheral nitric oxide synthase in the absence of dyslipidemias in men with metabolic syndrome. 2012;4(12):1945–1957. doi: 10.3390/nu4121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss T.W., Arnesen H., Seljeflot I.J.M. Components of the interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. 2013;62(7):1008–1013. doi: 10.1016/j.metabol.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 45.Lim S., et al. C-reactive protein level as an independent risk factor of metabolic syndrome in the Korean population. CRP as risk factor of metabolic syndrome. 2005;70(2):126–133. doi: 10.1016/j.diabres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Ahmadnezhad M., et al. Association between serum uric acid. high sensitive C-reactive protein and pro-oxidant-antioxidant balance in patients with metabolic syndrome. 2018;44(3):263–271. doi: 10.1002/biof.1424. [DOI] [PubMed] [Google Scholar]
- 47.Mojaz Sarbijani H., et al. The association between metabolic syndrome and serum levels of adiponectin and high sensitive C reactive protein in Gorgan. 2016;16(2):107–112. doi: 10.2174/1871530315666150608123614. [DOI] [PubMed] [Google Scholar]
- 48.Meng G., et al. Comparing the diagnostic ability of inflammatory markers in metabolic syndrome. 2017;475:1–6. doi: 10.1016/j.cca.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Fröhlich M., et al. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23(12):1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 50.Hong G.-b., et al. High-sensitivity C-reactive protein leads to increased incident metabolic syndrome in women but not in men: a five-year follow-up study in a Chinese population. 2020;13:581. doi: 10.2147/DMSO.S241774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musani S.K., et al. Aldosterone. C-reactive protein, and plasma B-type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: findings from the Jackson Heart Study. 2013;36(10):3084–3092. doi: 10.2337/dc12-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bo S., et al. What predicts the occurrence of the metabolic syndrome in a population‐based cohort of adult healthy subjects? 2009;25(1):76–82. doi: 10.1002/dmrr.910. [DOI] [PubMed] [Google Scholar]
- 53.Yoon K., et al. Higher and increased concentration of hs-CRP within normal range can predict the incidence of metabolic syndrome in healthy men. 2018;12(6):977–983. doi: 10.1016/j.dsx.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Xue Q., et al. Association between baseline and changes in high-sensitive C-reactive protein and metabolic syndrome: a nationwide cohort study and meta-analysis. 2022;19(1):1–12. doi: 10.1186/s12986-021-00632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oda E., Kawai R.J.C.J. Reproducibility of high-sensitivity C-reactive protein as an inflammatory component of metabolic syndrome in Japanese. 2010;74(7):1488–1493. doi: 10.1253/circj.cj-10-0156. [DOI] [PubMed] [Google Scholar]
- 56.Mirhafez S.R., et al. Cytokine and growth factor profiling in patients with the metabolic syndrome. 2015;113(12):1911–1919. doi: 10.1017/S0007114515001038. [DOI] [PubMed] [Google Scholar]
- 57.Mohammadi M., et al. Clinical significance of serum IL-6 and TNF-α levels in patients with metabolic syndrome. 2017;6(1):74. [PMC free article] [PubMed] [Google Scholar]
- 58.Sarbijani H.M., et al. The association between Metabolic Syndrome and serum levels of lipid peroxidation and interleukin-6 in Gorgan. 2016;10(1):S86–S89. doi: 10.1016/j.dsx.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Zarkesh M., et al. The relationship between metabolic syndrome, cardiometabolic risk factors and inflammatory markers in a Tehranian population: the Tehran Lipid and Glucose Study. 2012;51(24):3329–3335. doi: 10.2169/internalmedicine.51.8475. [DOI] [PubMed] [Google Scholar]
- 60.Adejumo E., Adejumo O. O.J.A.o.H.R. Ogundahunsi, Inflammatory Biomarkers Predictive of Metabolic Syndrome in a Nigerian Population: A Case-Control Study. 2020;6(4):382–390. [Google Scholar]
- 61.Siemińska L., et al. Associations between metabolic syndrome, serum thyrotropin, and thyroid antibodies status in postmenopausal women. and the role of interleukin-6. 2015;66(5):394–403. doi: 10.5603/EP.2015.0049. [DOI] [PubMed] [Google Scholar]
- 62.Monserrat-Mesquida M., et al. Metabolic syndrome is associated with oxidative stress and proinflammatory state. 2020;9(3):236. doi: 10.3390/antiox9030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christiana U.I., et al. Plasma levels of inflammatory cytokines in adult Nigerians with the metabolic syndrome. 2016;57(1):64. doi: 10.4103/0300-1652.180569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dallmeier D., et al. Metabolic syndrome and inflammatory biomarkers: a community-based cross-sectional study at the Framingham Heart Study. 2012;4(1):1–7. doi: 10.1186/1758-5996-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phosat C., et al. Elevated C-reactive protein, interleukin 6. tumor necrosis factor alpha and glycemic load associated with type 2 diabetes mellitus in rural Thais: a cross-sectional study. 2017;17(1):1–8. doi: 10.1186/s12902-017-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fahed G., et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022;23(2) doi: 10.3390/ijms23020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han M.S., et al. Regulation of adipose tissue inflammation by interleukin 6. Proc Natl Acad Sci U S A. 2020;117(6):2751–2760. doi: 10.1073/pnas.1920004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohammadi M., et al. Clinical significance of serum IL-6 and TNF-α levels in patients with metabolic syndrome. Rep Biochem Mol Biol. 2017;6(1):74–79. [PMC free article] [PubMed] [Google Scholar]
- 69.Bao P., Liu G., Wei Y. Association between IL-6 and related risk factors of metabolic syndrome and cardiovascular disease in young rats. Int. J. Clin. Exp. Med. 2015;8(8):13491–13499. [PMC free article] [PubMed] [Google Scholar]
- 70.Hassannejad R., et al. Diagnostic power of circulatory metabolic biomarkers as metabolic syndrome risk predictors in community-dwelling older adults in northwest of england (A feasibility study) Nutrients. 2021;13(7) doi: 10.3390/nu13072275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tylutka A., et al. Assessment of metabolic syndrome predictors in relation to inflammation and visceral fat tissue in older adults. Sci. Rep. 2023;13(1):89. doi: 10.1038/s41598-022-27269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hassannejad R., et al. Diagnostic power of circulatory metabolic biomarkers as metabolic syndrome risk predictors in community-dwelling older adults in northwest of England (A feasibility study) 2021;13(7):2275. doi: 10.3390/nu13072275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Timpson N.J., et al. C-reactive protein and its role in metabolic syndrome. mendelian randomisation study. 2005;366(9501):1954–1959. doi: 10.1016/S0140-6736(05)67786-0. [DOI] [PubMed] [Google Scholar]
- 74.Brunner E.J., et al. Inflammation, insulin resistance, and diabetes—mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5(8):e155. doi: 10.1371/journal.pmed.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McLaughlin T., et al. Differentiation between obesity and insulin resistance in the association with C-reactive protein. 2002;106(23):2908–2912. doi: 10.1161/01.cir.0000041046.32962.86. [DOI] [PubMed] [Google Scholar]
- 76.Okopień B., et al. A new immunological marker of atherosclerotic injury of arterial wall. Research communications in molecular pathology and pharmacology. 2001;109(3–4):241–248. [PubMed] [Google Scholar]
- 77.Swastini D.A., et al. Atherosclerosis prediction with high sensitivity C-reactive protein (hs-CRP) and related risk factor in patient with dyslipidemia. Open Access Maced J Med Sci. 2019;7(22):3887–3890. doi: 10.3889/oamjms.2019.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galland, L.J.N.i.C.P Diet and inflammation. 2010;25(6):634–640. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 79.Fischer C., et al. Plasma levels of interleukin‐6 and C‐reactive protein are associated with physical inactivity independent of obesity. 2007;17(5):580–587. doi: 10.1111/j.1600-0838.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 80.Elisia I., et al. The effect of smoking on chronic inflammation, immune function and blood cell composition. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-76556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qi L., Rifai N., Hu F.B. Interleukin-6 receptor gene, plasma C-reactive protein, and diabetes risk in women. Diabetes. 2009;58(1):275–278. doi: 10.2337/db08-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ognjanovic S., et al. Serum CRP and IL-6, genetic variants and risk of colorectal adenoma in a multiethnic population. Cancer Causes & Control. 2010;21(7):1131–1138. doi: 10.1007/s10552-010-9540-7. [DOI] [PubMed] [Google Scholar]
- 83.Wörns M.A., et al. Genetic and environmental contributions to plasma C-reactive protein and interleukin-6 levels--a study in twins. Genes Immun. 2006;7(7):600–605. doi: 10.1038/sj.gene.6364330. [DOI] [PubMed] [Google Scholar]
- 84.Uddin M., et al. Epigenetic and inflammatory marker profiles associated with depression in a community-based epidemiologic sample. Psychol. Med. 2011;41(5):997–1007. doi: 10.1017/S0033291710001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shih Y.L., Lin Y., Chen J.Y. The association between high-sensitivity C-reactive protein and metabolic syndrome in an elderly population aged 50 and older in a community receiving primary health care in taiwan. Int J Environ Res Public Health. 2022;19(20) doi: 10.3390/ijerph192013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Srikanthan K., et al. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the west virginian population. Int. J. Med. Sci. 2016;13(1):25–38. doi: 10.7150/ijms.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hedayati M., et al. Association between TNF-α promoter G-308A and G-238A polymorphisms and obesity. Mol Biol Rep. 2012;39(2):825–829. doi: 10.1007/s11033-011-0804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Exel E., et al. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes. the Leiden 85-Plus Study. 2002;51(4):1088–1092. doi: 10.2337/diabetes.51.4.1088. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y., et al. IL-10/STAT3 is reduced in childhood obesity with hypertriglyceridemia and is related to triglyceride level in diet-induced obese rats. BMC Endocr. Disord. 2018;18(1):39. doi: 10.1186/s12902-018-0265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Esposito K., et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. 2003;88(3):1055–1058. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 91.Chen L., et al. Association of metabolic syndrome with serum interleukin-10 and high sensitive C reactive protein (hs-CRP) in old men. 2008;33(10):970–974. [PubMed] [Google Scholar]
- 92.Zhang B., et al. Age decreases macrophage IL-10 expression: implications for functional recovery and tissue repair in spinal cord injury. Exp. Neurol. 2015;273:83–91. doi: 10.1016/j.expneurol.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frank M.G., et al. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. 2006;27(5):717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 94.Ye S.-M., Johnson R.W.J.N. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. 2001;9(4):183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 95.Dagdeviren S., et al. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. Faseb j. 2017;31(2):701–710. doi: 10.1096/fj.201600832R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nematollahi H.R., et al. Interleukin 10, lipid profile, vitamin D, selenium, metabolic syndrome, and serum antioxidant capacity in elderly people with and without cardiovascular disease: amirkola health and ageing project cohort-based study. ARYA Atheroscler. 2019;15(5):233–240. doi: 10.22122/arya.v15i5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buchmann N., et al. Muscle mass and inflammation in older adults. Impact of the metabolic syndrome. 2022;68(9):989–998. doi: 10.1159/000520096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hong X., et al. Association between adiponectin and newly diagnosed type 2 diabetes in population with the clustering of obesity, dyslipidaemia and hypertension. a cross-sectional study. 2023;13(2) doi: 10.1136/bmjopen-2021-060377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim J.-Y., et al. Prospective study of serum adiponectin and incident metabolic syndrome: the ARIRANG study. 2013;36(6):1547–1553. doi: 10.2337/dc12-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahonen T., et al. The association of adiponectin and low-grade inflammation with the course of metabolic syndrome. 2012;22(3):285–291. doi: 10.1016/j.numecd.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Yosaee S., et al. Adiponectin: an indicator for metabolic syndrome. Iran. J. Public Health. 2019;48(6):1106–1115. [PMC free article] [PubMed] [Google Scholar]
- 102.Yahia H., et al. IL-6/STAT3 and adipokine modulation using tocilizumab in rats with fructose-induced metabolic syndrome. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393(12):2279–2292. doi: 10.1007/s00210-020-01940-z. [DOI] [PubMed] [Google Scholar]
- 103.Wang M., et al. Effects of dietary intervention on inflammatory markers in metabolic syndrome. A Systematic Review and Meta-Analysis. 2022;9 doi: 10.3389/fnut.2022.846591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hosseini-Esfahani F., et al. Western dietary pattern interaction with APOC3 polymorphism in the risk of metabolic syndrome: Tehran Lipid and Glucose Study. J Nutrigenet Nutrigenomics. 2014;7(2):105–117. doi: 10.1159/000365445. [DOI] [PubMed] [Google Scholar]
- 105.Ghadge A.A., Khaire A.A., Kuvalekar A.A. Adiponectin: a potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018;39:151–158. doi: 10.1016/j.cytogfr.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y., Vu V., Sweeney G. Examining the potential of developing and implementing use of adiponectin-targeted therapeutics for metabolic and cardiovascular diseases. Front. Endocrinol. 2019;10:842. doi: 10.3389/fendo.2019.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dubchenko E.A., et al. [Hyperhomocysteinemia and endothelial dysfunction in patients with cerebral vascular and autoimmune diseases] Zh. Nevrol. Psikhiatr. Im. S S Korsakova. 2019;119(11):133–138. doi: 10.17116/jnevro2019119111133. [DOI] [PubMed] [Google Scholar]
- 108.Price B.R., Wilcock D.M., Weekman E.M. Hyperhomocysteinemia as a risk factor for vascular contributions to cognitive impairment and dementia. Front. Aging Neurosci. 2018;10:350. doi: 10.3389/fnagi.2018.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sreckovic B., et al. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab Syndr. 2017;11(3):179–182. doi: 10.1016/j.dsx.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 110.Lee H.S.S., Park T. The homocysteine and metabolic syndrome. A Mendelian Randomization Study. Nutrients. 2021;13(7) doi: 10.3390/nu13072440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oudi M.E., et al. Homocysteine and markers of inflammation in acute coronary syndrome. 2010;15(2):e25. [PMC free article] [PubMed] [Google Scholar]
- 112.Sreckovic B., et al. Homocysteine is a marker for metabolic syndrome and atherosclerosis. 2017;11(3):179–182. doi: 10.1016/j.dsx.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 113.Sreckovic B., et al. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metabol. Syndr.: Clin. Res. Rev. 2017;11(3):179–182. doi: 10.1016/j.dsx.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 114.Fakhrzadeh H., et al. Homocysteine levels and its correlation to metabolic syndrome in 25-64 years old residents of the tehran medical university population lab % J Iranian Journal of Diabetes and Lipid Disorders. 2004;4(2):71–78. [Google Scholar]
- 115.Fe’li Sh. N., et al. Relationship between serum homocysteine and metabolic syndrome among patients with schizophrenia and bipolar disorder, a cross sectional. study. 2020;15(4) doi: 10.18502/ijps.v15i4.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nabipour I., et al. The metabolic syndrome is not associated with homocysteinemia: the Persian Gulf Healthy Heart Study. J. Endocrinol. Invest. 2009;32(5):406–410. doi: 10.1007/BF03346476. [DOI] [PubMed] [Google Scholar]
- 117.Budak N., et al. Is plasma homocysteine level associated with metabolic syndrome components in adolescents? 2009;7(4):357–362. doi: 10.1089/met.2008.0037. [DOI] [PubMed] [Google Scholar]
- 118.Garcin J.-M., et al. Is hyperhomocysteinemia an additional risk factor of the metabolic syndrome? 2006;4(3):185–195. doi: 10.1089/met.2006.4.185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.