Abstract
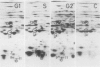
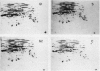
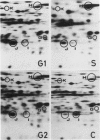
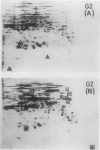
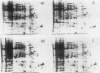
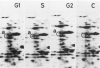
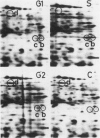
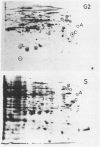
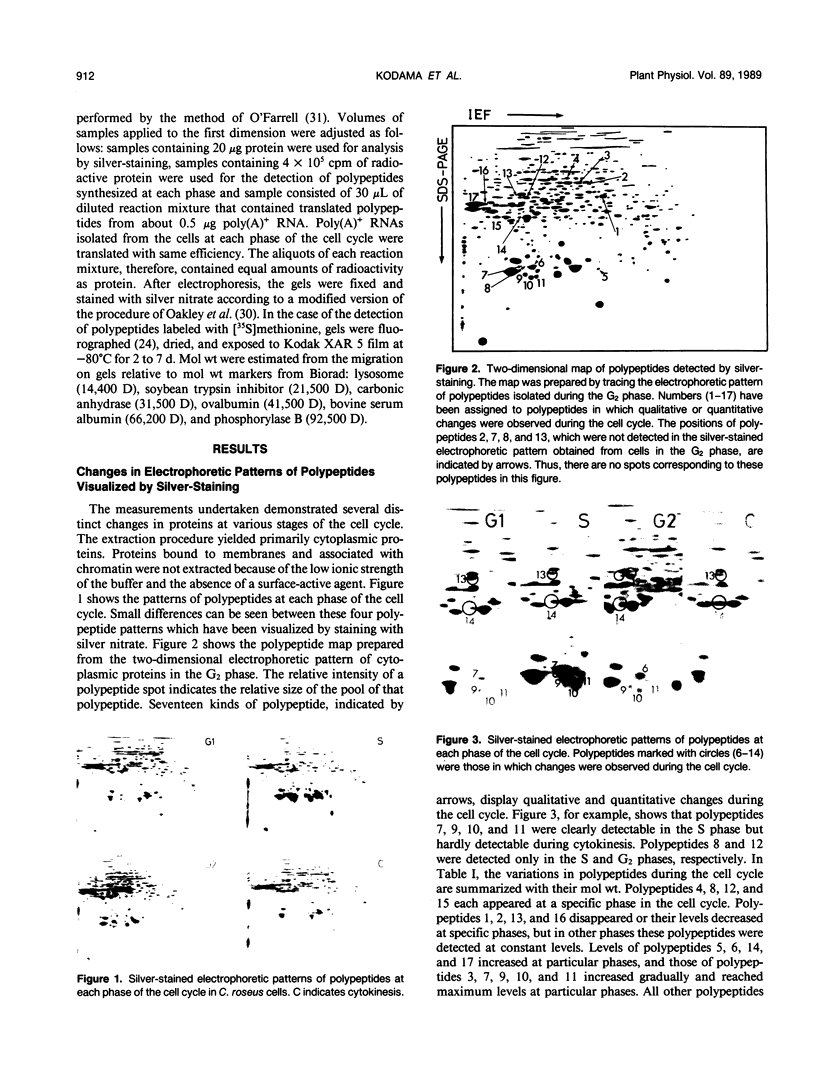
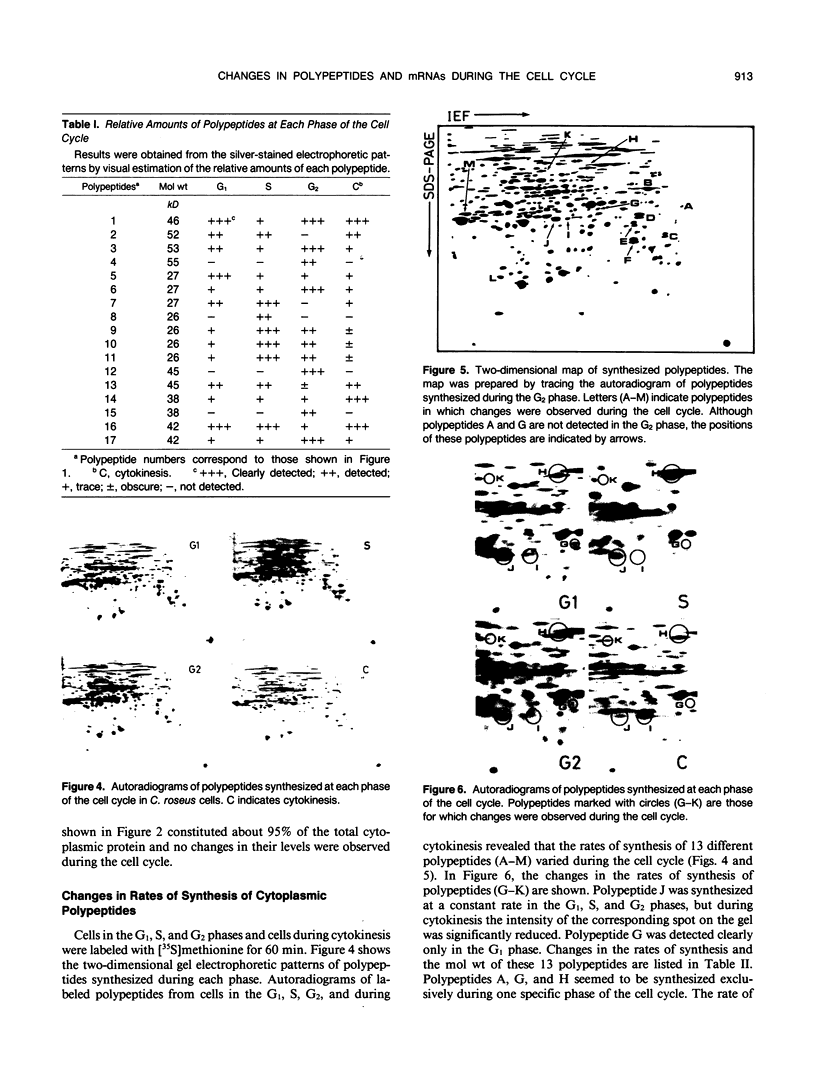
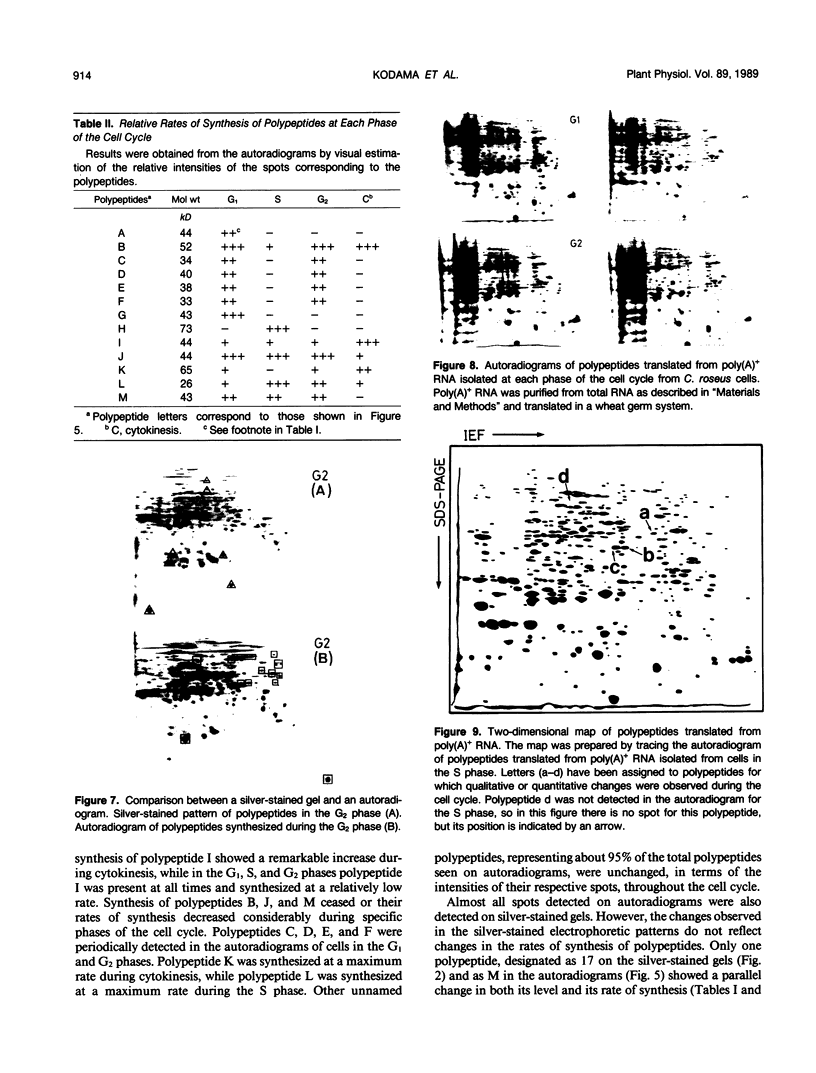
This study shows an overall analysis of gene expression during the cell cycle in synchronous suspension cultures of Catharanthus roseus cells. First, the cellular cytoplasmic proteins were fractionated by two-dimensional gel electrophoresis and visualized by staining with silver. Seventeen polypeptides showed qualitative or quantitative changes during the cell cycle. Second, the rates of synthesis of cytoplasmic proteins were also investigated by autoradiography by labeling cells with [35S]methionine at each phase of the cell cycle. The rates of synthesis of 13 polypeptides were found to vary during the cell cycle. The silverstained electrophoretic pattern of proteins in the G2 phase in particular showed characteristic changes in levels of polypeptides, while the rates of synthesis of polypeptides synthesized during the G2 phase did not show such phase-specific changes. This result suggests that posttranslational processing of polypeptides occurs during or prior to the G2 phase. In the G1 and S phases and during cytokinesis, several other polypeptides were specifically synthesized. Finally, the variation of mRNAs was analyzed from the autoradiograms of in vitro translation products of poly(A)+ RNA isolated at each phase. Three poly(A)+ RNAs increased in amount from the G1 to the S phase and one poly (A)+ RNA increased preferentially from the G2 phase to cytokinesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bader A. A., Orengo A., Rao P. N. G2 phase-specific proteins of HeLa cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6064–6068. doi: 10.1073/pnas.75.12.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayusawa D., Shimizu K., Koyama H., Kaneda S., Takeishi K., Seno T. Cell-cycle-directed regulation of thymidylate synthase messenger RNA in human diploid fibroblasts stimulated to proliferate. J Mol Biol. 1986 Aug 20;190(4):559–567. doi: 10.1016/0022-2836(86)90241-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Nielsen S. Proliferation-sensitive nuclear phosphoprotein "dividin" is synthesized almost exclusively during S phase of the cell cycle in human AMA cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8187–8190. doi: 10.1073/pnas.83.21.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Rate of macromolecular synthesis through the cell cycle of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4384–4388. doi: 10.1073/pnas.75.9.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger C., Lehle L. Auxin induces rapid changes in phosphatidylinositol metabolites. Nature. 1988 Jan 14;331(6152):176–178. doi: 10.1038/331176a0. [DOI] [PubMed] [Google Scholar]
- Fosket D. E., Volk M. J., Goldsmith M. R. Polyribosome Formation in Relation to Cytokinin-induced Cell Division in Suspension Cultures of Glycine max [L.] Merr. Plant Physiol. 1977 Oct;60(4):554–562. doi: 10.1104/pp.60.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco A., Ittmann M., Basilico C. Molecular cloning of a gene that is necessary for G1 progression in mammalian cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1565–1569. doi: 10.1073/pnas.84.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hendrickson S. L., Scher C. D. Platelet-derived growth factor-modulated translatable mRNAs. Mol Cell Biol. 1983 Aug;3(8):1478–1487. doi: 10.1128/mcb.3.8.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Chartier Y. Hormonal Control of Mitotic Development in Tobacco Protoplasts: TWO-DIMENSIONAL DISTRIBUTION OF NEWLY-SYNTHESIZED PROTEINS. Plant Physiol. 1981 Dec;68(6):1273–1278. doi: 10.1104/pp.68.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Zahn K. The synthesis of ninety proteins including actin throughout the HeLa cell cycle. J Cell Biol. 1978 Dec;79(3):833–838. doi: 10.1083/jcb.79.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Bartlett S. G., Grossman A. R., Cashmore A. R., Chua N. H. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981 Nov;91(2 Pt 1):468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara P. S., Wright D. A., Rao P. N. Mitotic factors from mammalian cells induce germinal vesicle breakdown and chromosome condensation in amphibian oocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2799–2802. doi: 10.1073/pnas.76.6.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Price C. A. Translation of mRNAs for subunits of chloroplast coupling factor 1 in spinach. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6304–6308. doi: 10.1073/pnas.79.20.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood J. T., Wagenaar E. B., Church R. B. Synthesis of unique low molecular weight proteins during late G2 and mitosis. J Biol Chem. 1985 Jan 25;260(2):695–698. [PubMed] [Google Scholar]