Abstract
We have developed a rapid procedure for isolating a fraction enriched in plasma membrane from Dunaliella salina using an aqueous two-phase system (dextran/polyethylene glycol, 6.7%/6.7%). An enriched plasma membrane fraction, free of chloroplast and mitochondrial contamination, could be obtained in 2.5 hours. Plasma membrane proteins, which accounted for approximately 1% of the total membrane protein, contained a number of unique proteins compared with the other cell fractions, as shown by gel electrophoresis. The lipids of the plasma membrane fraction from 1.7 molar NaCl-grown cells were extracted and characterized. Phosphatidylethanolamine and phosphatidylcholine were the two most prevalent phospholipids, at 20.6% and 6.0% of the total lipid, respectively. In addition, inositol phospholipids were a significant component of the D. salina plasma membrane fraction. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate accounted for 5.2% and 1.5% of the plasma membrane phospholipid, respectively. Diacylglyceryltrimethylhomoserine accounted for 7.9% of the plasma membrane total lipid. Free sterols were the major component of the plasma membrane fraction, at 55% of the total lipid, and consisted of ergosterol and 7-dehydroporiferasterol. Sterol peroxides were not present in the plasma membrane fraction. The lipid composition of enriched plasma membrane fractions from cells grown at 0.85 molar NaCl and 3.4 molar NaCl were compared with those grown at 1.7 molar NaCl. The concentration of diacylglyceryltrimethylhomoserine and the degree of plasma membrane fatty acid saturation increased in 3.4 molar plasma membranes. The relative concentration of sterols in the plasma membrane fraction was similar in all three NaCl concentrations tested.
Full text
PDF

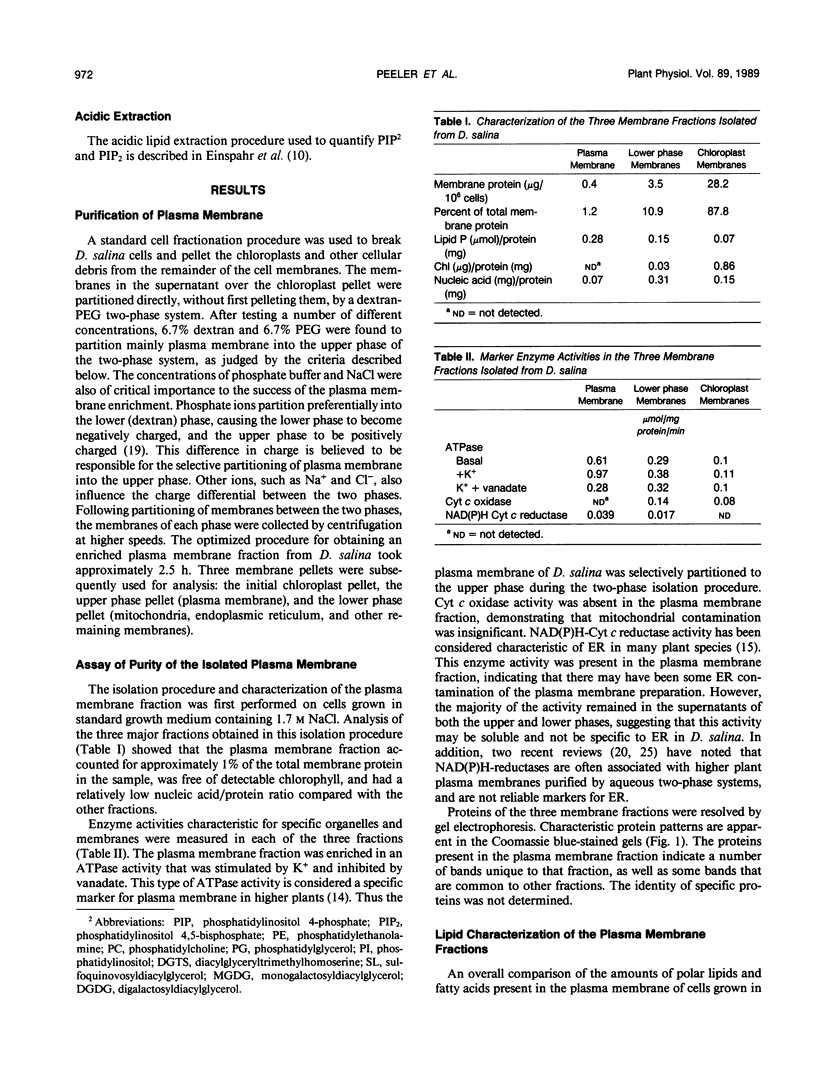

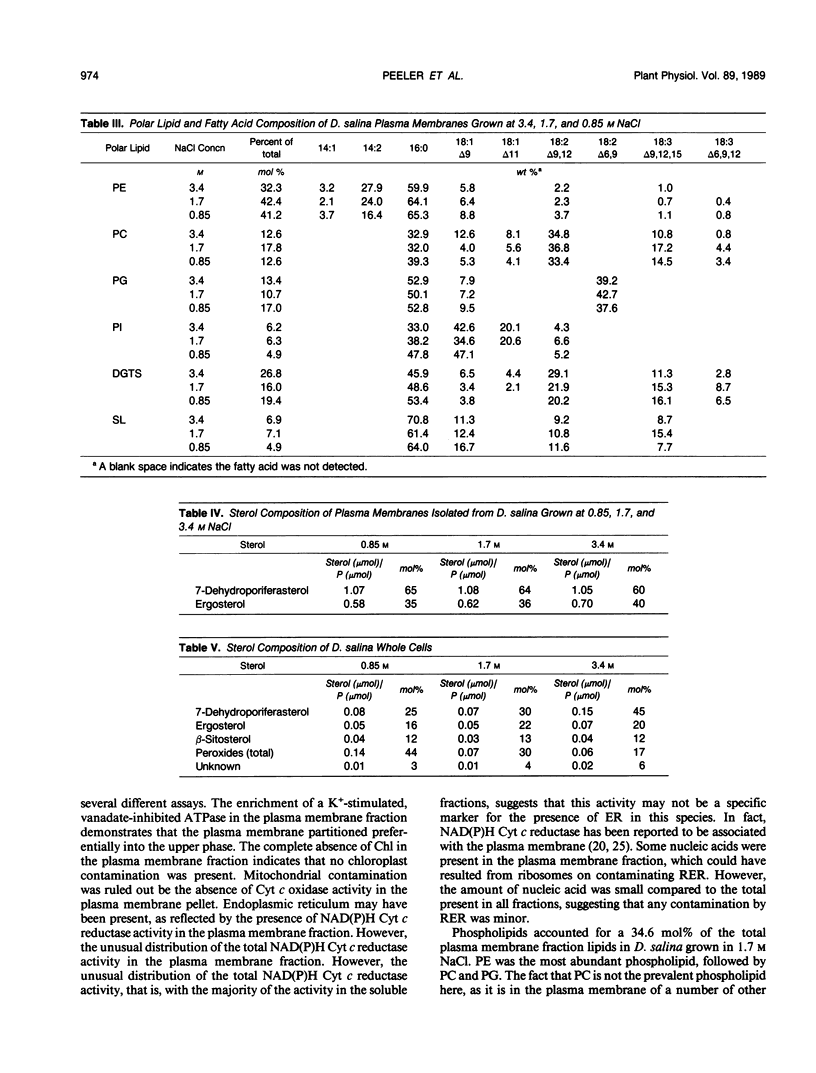


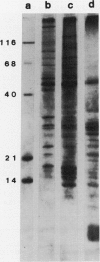
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. The Role of Glycerol in the Osmotic Regulation of the Halophilic Alga Dunaliella parva. Plant Physiol. 1973 May;51(5):875–878. doi: 10.1104/pp.51.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka L. J., Brown A. D. The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch Mikrobiol. 1974 Mar 1;96(1):37–52. doi: 10.1007/BF00590161. [DOI] [PubMed] [Google Scholar]
- Einspahr K. J., Maeda M., Thompson G. A., Jr Concurrent changes in Dunaliella salina ultrastructure and membrane phospholipid metabolism after hyperosmotic shock. J Cell Biol. 1988 Aug;107(2):529–538. doi: 10.1083/jcb.107.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K. J., Peeler T. C., Thompson G. A., Jr Rapid changes in polyphosphoinositide metabolism associated with the response of Dunaliella salina to hypoosmotic shock. J Biol Chem. 1988 Apr 25;263(12):5775–5779. [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Katz A., Avron M. Determination of intracellular osmotic volume and sodium concentration in dunaliella. Plant Physiol. 1985 Aug;78(4):817–820. doi: 10.1104/pp.78.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lynch D. V., Steponkus P. L. Plasma Membrane Lipid Alterations Associated with Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1987 Apr;83(4):761–767. doi: 10.1104/pp.83.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. V., Thompson G. A. Low Temperature-Induced Alterations in the Chloroplast and Microsomal Membranes of Dunaliella salina. Plant Physiol. 1982 Jun;69(6):1369–1375. doi: 10.1104/pp.69.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. V., Thompson G. A. Microsomal Phospholipid Molecular Species Alterations during Low Temperature Acclimation in Dunaliella. Plant Physiol. 1984 Feb;74(2):193–197. doi: 10.1104/pp.74.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester C. P., Kjellbom P., Andersson B., Larsson C. Lipid composition of plasma membranes isolated from light-grown barley (Hordeum vulgare) leaves: identification of cerebroside as a major component. Arch Biochem Biophys. 1987 Jun;255(2):385–391. doi: 10.1016/0003-9861(87)90406-1. [DOI] [PubMed] [Google Scholar]
- Sandstrom R. P., Deboer A. H., Lomax T. L., Cleland R. E. Latency of Plasma Membrane H-ATPase in Vesicles Isolated by Aqueous Phase Partitioning : Increased substrate Accessibility or Enzyme Activation. Plant Physiol. 1987 Nov;85(3):693–698. doi: 10.1104/pp.85.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Wheeler J. J., Boss W. F. Polyphosphoinositides are present in plasma membranes isolated from fusogenic carrot cells. Plant Physiol. 1987 Oct;85(2):389–392. doi: 10.1104/pp.85.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]