Abstract
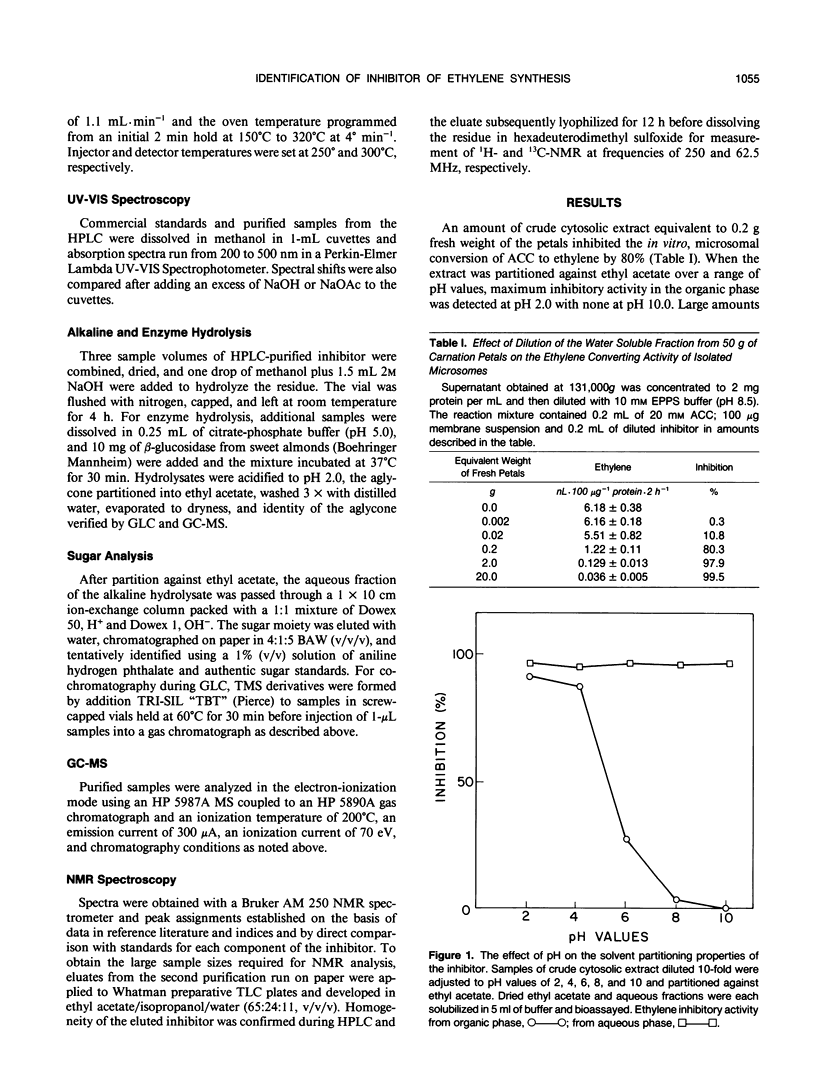
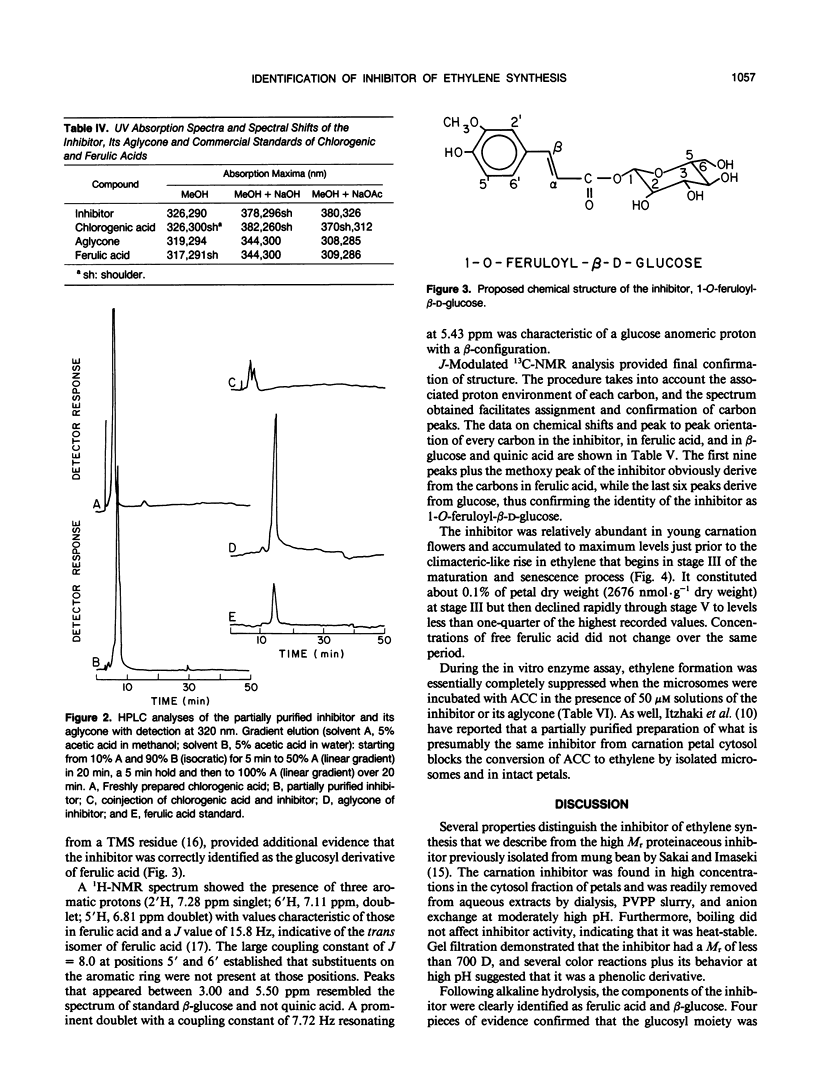
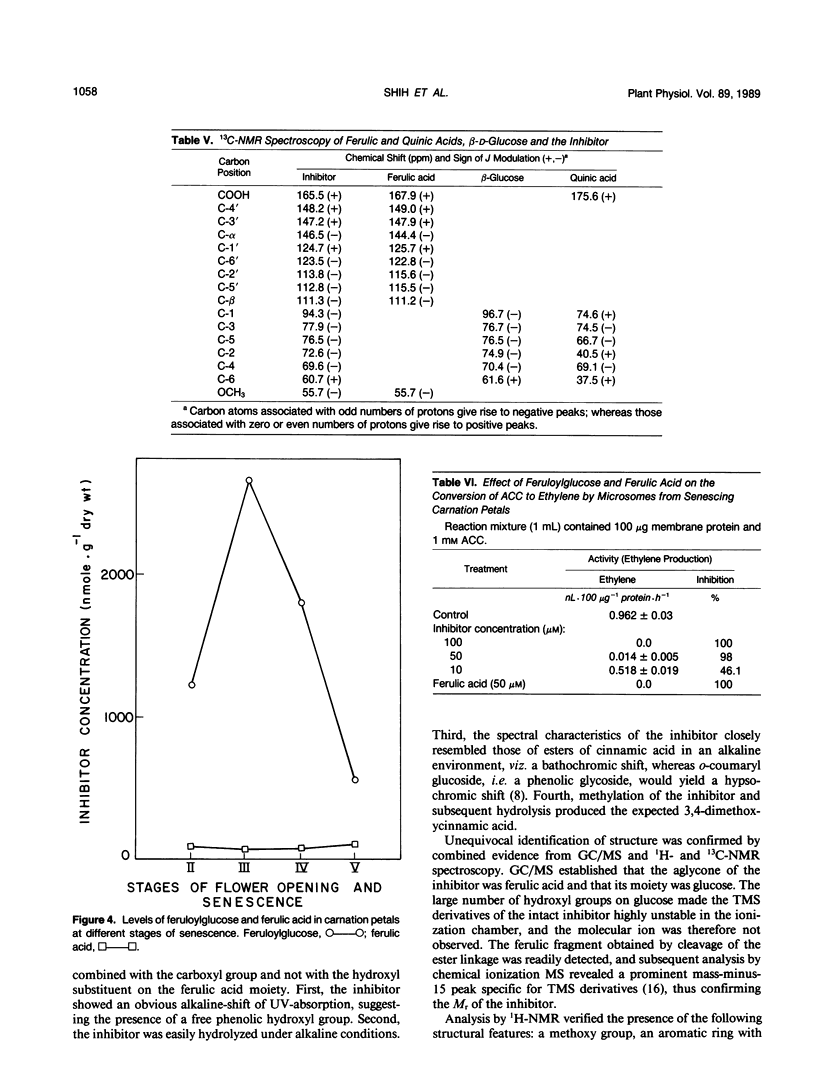
During cell-free experiments with membranes isolated from carnation petals (Dianthus caryophillus L. cv White Sim), the conversion of 1-aminocyclopropane-1-carboxylic acid into ethylene was blocked by a factor derived from the cytosol. Subsequent characterization of the inhibitor revealed that its effect was concentration dependent, that it was water soluble, and that it could be removed from solution by dialysis and addition of polyvinyl-polypyrrolidone. Activity profiles obtained after solvent partitioning over a range of pH values and after chromatography on silica gel, size exclusion gel, and ion exchange resins revealed that the inhibitor was a highly polar, low molecular weight species that was nonionic at low pH and anionic at pH values above 8. Use of selected solvent systems during paper and thin layer chromatography combined with specific spray reagents tentatively identified the compound as a hydroxycinnamic acid derivative. Base hydrolysis and subsequent comparison with known standards by high performance liquid chromatography, gas-liquid chromatography, and ultraviolet light spectroscopy established that the inhibitor was a conjugate with a ferulic acid moiety. Release of ferulic acid following treatment with β-glucosidase also indicated the presence of a glucose moiety, and unequivocal identification of the inhibitor as 1-O-feruloyl-β-d-glucose was confirmed by gas chromatography-mass spectroscopy and by ultraviolet light, 1H-, and 13C- nuclear magnetic resonance spectroscopy. Feruloylglucose constituted about 0.1% of the dry weight of stage III (preclimacteric) carnation petals, but concentrations fell sharply during stage IV (climacteric), when ethylene production peaks and the flowers senesce. In a reaction mixture containing microsome-bound ethylene forming enzyme system, 98% of all ethylene production was abolished in the presence of 50 μm concentrations of the inhibitor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. D., Lieberman M., Stewart R. N. Ethylene production by apple protoplasts. Plant Physiol. 1979 May;63(5):931–935. doi: 10.1104/pp.63.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Burgoon A. C., Anderson J. D., Solomos T., Lieberman M. Some Characteristics of the System Converting 1-Aminocyclopropane-1-carboxylic Acid to Ethylene. Plant Physiol. 1981 Jan;67(1):80–84. doi: 10.1104/pp.67.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Wang S. Y., Burgoon A. C., Baker J. E., Lieberman M. Inhibition of the Conversion of 1-Aminocyclopropane-1-carboxylic Acid to Ethylene by Structural Analogs, Inhibitors of Electron Transfer, Uncouplers of Oxidative Phosphorylation, and Free Radical Scavengers. Plant Physiol. 1981 Jan;67(1):74–79. doi: 10.1104/pp.67.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARBORNE J. B., CORNER J. J. Plant polyphenols. 4. Hydroxycinnamic acid-sugar derivatives. Biochem J. 1961 Nov;81:242–250. doi: 10.1042/bj0810242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Adams D. O. Chilling-Induced Ethylene Production in Cucumbers (Cucumis sativus L.). Plant Physiol. 1982 Feb;69(2):424–427. doi: 10.1104/pp.69.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Inhibition of ethylene production by 2,4-dinitrophenol and high temperature. Plant Physiol. 1980 Aug;66(2):286–290. doi: 10.1104/pp.66.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]


