Abstract
The moderate production of reactive oxidative species (ROS) is important because ROS act as second messengers. However, their depletion through the over-activity of the antioxidant system may lead to reductive stress (RS) which is characterized by an increase in reducing equivalents and an elevation of some components of the antioxidant system disturbing redox homeostasis. Hibiscus sabdariffa Linnaeus (HSL) is a plant with antioxidant properties that provides compounds that favor the antioxidant system. However, excess chronic consumption could lead to the over expression of the antioxidant enzymatic system, and this could contribute to decrease ROS. Therefore, the objective of this study was to evaluate the alteration of the vascular reactivity associated to excessive and chronic consumption of HSL infusions at different percentages. 40 male Wistar rats were divided into 4 groups. Group 1 control (drinking tap water), group 2, 3 and 4, drinking water supplemented with 15, 30 and 60 g/L of HSL calyxes respectively. The systolic blood pressure (SBP), vascular reactivity, morphological changes, and different components of the enzymatic antioxidant system were evaluated in the thoracic aorta by spectrophotometry. We also determined glucose-6-phosphate dehydrogenase (G6PD), glutathione-S-transferase (GST), thioredoxin-reductase (TrxR), glutathione peroxidase (GPx) and glutathione reductase (GR) and some markers of the non-enzimatic system such as the NO3−/NO2−ratio, glutathione (GSH), selenium, thiols, lipoperoxidation (LPO), and 3-nitrityrosine (3-NT). Vasoconstriction was increased and vasorelaxation was decreased. These alterations were reversed by O2− and H2O2. There was an increase in the wall thickness and elastic fibers (p = 0.004 and p = 0.02, respectively) and in G6PD, GPX, TrxR (p = 0.02, p = 0.03, and p = 0.01 respectively). LPO, GSH (p = 0.01), and selenium (p = 0.04) were decreased. There was a decrease in thiols (p < 0.001), 3-NT (p = 0.04) and GST (p = 0.0005) in rats that received the infusion at 3 and 6%. The excess antioxidants provided by the HSL infusions at 3% and 6% modified vascular reactivity, increasing the enzymatic antioxidant system, and depleting ROS.
Keywords: Hypertension, Vascular function, Aorta, Reductive stress, HSL, Antioxidant system, Reactive oxygen species, Redox homeostasis
1. Introduction
Redox balance is important for cellular homeostasis and a moderate production of reactive oxygen species (ROS) leads to beneficial effects [1,2]. ROS, such as nitric oxide (NO), anion superoxide (O2−) and peroxide hydrogen (H2O2) in small amounts act as second messengers that contribute to aortic vasodilation [3]. In this sense, H2O2 modulates signal transduction by the reversible oxidation of redox-active cysteines [4] and it elevates the expression of at least 40 gene products [5]. In contrast, overproduction of ROS has deleterious effects. Therefore, enzymatic and non-enzymatic antioxidant systems play an important role in maintaining the non-cytotoxic level of ROS (at picomolar concentrations) [6]. Among the enzymes that constitute the antioxidant enzymatic system, thioredoxin reductase (TrxR), superoxide dismutase (SOD) isoforms, the glutathione peroxidase (GPx) family, glucose-6-phosphate dehydrogenase (G6PD) and glutathione-S-transferase (GST) can be mentioned [7]. On the other hand, molecules that participate in the non-enzymatic antioxidant system include vitamins C, E, A, glutathione (GSH), and Selenium (Se), among others. The proper functioning of the enzymatic- and non-enzymatic antioxidant systems contributes to the balance of redox homeostasis [8]. In addition, much attention has been paid on the effects of excessive ROS and oxidative stress (OS), acting upon complex metabolic pathways in multiple organs and systems and on their involvement in physiology and pathology [1]; however, less emphasis has been placed on the effects of reduced levels of ROS by over expressions of the enzymatic or non-enzymatic antioxidant system that could result in reductive stress (RS).
RS is the counterpart of OS, and it consists of an abnormal increase in reducing equivalents in the presence of intact oxidation and reduction systems [9]. Depletion of ROS and/or overproduction of the enzymatic and non-enzymatic antioxidant systems lead to pathological consequences [9]. In this sense, the excess reducing equivalents in the form of GSH/GSSG, NADPH/NADP+ and NAD/NADH+ redox couples or overexpression of antioxidant enzymatic systems can deplete ROS [9,10]. Excess reducing equivalents may reduce mitochondrial function, cell growth responses, and cellular metabolism. They may induce alterations in the formation of protein disulfide bonds, and contribute to the development of some diseases that are closely associated with inflammatory conditions such as muscular dystrophy, protein deficiency, hypertrophic cardiomyopathy, pulmonary hypertension, rheumatoid arthritis, cancer, and metabolic syndrome (SM); Furthermore, chronic RS may induce OS by a positive feedback regulation [[9], [10], [11]]. However, the metabolic pathways or the ROS that participate in the regulation of RS remain unknown.
The aorta and other vessels are susceptible to the harmful effects of RS [9,11]. This vessel is the largest artery in the organism of animals and humans and any alteration in its structure and/or function disturbs the cardiovascular system [12]. The aortic wall has three layers: Intima (endothelium), media (smooth muscle cells, collagen, and elastic fibers), and adventitia. Both collagen and elastin determine the resistance to traction forces and stiffness of the aorta. The mechanical properties of the aorta depend on the amounts of the main components of these layers, and on the spatial organization and the mechanical interactions between these components [13,14]. In addition, endothelial cells, located in the intima, are metabolically active and release various vasoactive mediators such as NO, prostaglandins, and endoperoxides [15,16] and may stimulate the synthesis metalloproteinase, growth factors and heparin [15]. Homeostasis of the production of these substances is atheroprotective, since an imbalance generates endothelial dysfunction, which predicts the development of atherosclerosis and hypertension [16]. Likewise, endothelial cells are constantly exposed to various stimuli, among which oxidized lipoproteins, frictional forces, inflammatory agents, cytokines, and ROS can be counted [17]. The alteration in the structure and/or function of endothelial cells leads to various pathologies such as cardiomyopathies [15].
On the other hand, the high consumption by the population of antioxidants from plants such as Hibiscus sabdariffa Linnaeus (HSL) or antioxidant synthetic products is increasing without a proper assessment of the physiological impact that may be caused in the long-term, including RS. HSL is known as Jamaica flower in México and Karkade or Zoborodo in Sudan and Nigeria respectively [18,19]. It is used in the preparation of different drinks. In traditional medicine, it has been used as treatment for hypercholesterolemia, hypertension, liver damage, kidney stones, pyrexia, choleretic, diuretics, and hypotensive effects and for stimulating intestinal peristalsis [[19], [20], [21]]. It has been described that the HSL calyxes contain many chemical components including flavonoids, polyphenols, quercetin, vitamin C, selenium (Se) and protocatechuic, gallic, tannic acids that have been used against the OS present in pathologies such as the MS and hypertension [22]. Also, various chemical compounds present in the HSL calyxes are water soluble and can be extracted. Infusion of this plant contain considerable concentrations of these antioxidants. In this sense, polyphenols, may protect cellular components from oxidative damage, such as lipoperoxidation (LPO) [23], increase the activities of catalase (CAT), GPx and SOD isoforms, inhibit the activities of xanthine-oxidase, angiotensin converting enzyme, cyclooxygenase 2 and the inducible nitric oxide synthase (iNOS), and contribute to regeneration of vitamin C and E. They increase the concentration of GSH and the formation of thiol bridges between the cysteines of the proteins [24]. They may also prevent liver cell apoptosis by inhibiting the activation of p-JNK and p38 MAPK, which are transcription factors activated during inflammation [25]. However, polyphenols can show pro-oxidant activity when they are consumed at high doses [26]. The pro-oxidant activities of several polyphenols, such as quercetin, catechins, and gallic acid, have been reported [27]. Several studies have shown that cell survival and viability, thiol content, total antioxidant capacity (TAC), and activities of SOD isoforms, CAT, and glutathione-S-transferase (GST) are reduced by quercetin at 50 μM [28]. High levels of flavonoids (50–250 μM) produce cytotoxicity, DNA damage, apoptosis, and the presence of ROS by auto-oxidation [29]. In addition, polyphenolic acids at high concentrations exhibit pro-oxidant activities when transition metal ions are present, such as copper and iron, forming chelator agents and reducing the TAC [30]. The phenolic antioxidants are converted to phenoxyl radicals which can be the basis of a cascade of pro-oxidant events that are characterized by self-oxidation of diphenol or polyphenols concomitant with univalent reduction of molecular oxygen, followed by dismutation of O2− formation and subsequent OH• formation in a Fenton-Weiss type reaction [31].
There is little experimental evidence to suggest that chronic consumption of high concentrations of antioxidants may lead to the development of aortic alterations. Therefore, the objective of this study was to evaluate the changes in the vascular reactivity associated to excessive and chronic consumption of HSL infusions at different percentages. Our hypothesis was that the excess of antioxidant agents supplied by different percentages of HSL in infusion could modify vascular function by generating the overexpression of reducing equivalents and/or antioxidant systems that eliminate ROS, thus favoring RS.
2. Materials and methods
2.1. Animals
The study was designed and carried out in compliance with the Laboratory Animal Care Committee of the National Institute of Cardiology “Ignacio Chávez” in Mexico (SICUAE.DC.2019/3–4) approved for experiments in animals. Experiments were conducted in compliance with the Guide for the Care and use of laboratory animals of the National Institutes of Health (NIH). Exclusion criteria: Rats weighing less or more than required, and animals that presented clinical diseases such as respiratory problems, digestive problems, loss of appetite, aggressiveness, among others. 40 male Wistar rats were used to form 4 groups with 10 animals each, as follows: Group 1, control (water); Group 2, HSL at 1.5%; Group 3, HSL at 3%; Group 4 HSL at 6%. The animals were kept for 4 weeks under the following conditions: 12-h light/12-dark cycle, environment temperature, and relative humidity between the ranges of 18–26 °C and 40–70%, respectively. Commercial rodent feed that contained 6% crude fiber, 4.5% crude fat, 23% crude protein, 2.5% minerals and 8% ash,(Labdiet 5008; PMI Nutrition International, Richmond, IN.) was provided freely. The HSL infusion at different percentages was administered orally (ad libitum) in drinking water. The systolic blood pressure (SBP) determinations were taken at the end of the experimental period of 4 weeks and it was measured using a tail cuff attached to a pneumatic pulse transducer method [32], (Narco Bio-Systems Inc., Houston, TX, USA).
2.2. Preparation of the HSL infusion
The HSL calyces were acquired in Chilapa de Alvarez, México. To one liter of boiling water, 15, 30, and 60 g of HSL calyxes were added. The solution was kept boiling for 10 min, and was then left to cool, filtered and stored at 4 °C until consumption.
2.3. Determinations of polyphenols, total flavonoids, and total anthocyanins in the HSL infusion
The determination of total flavonoids was done by the Jia method [33]. This technique determines total flavones and flavonols that react with aluminum trichloride forming stable complexes such as apigenin, chrysin, and luteonin, as the flavonols morin, quercetin, myricetin, kaempferol, and galangin, which can be analyzed spectrophotometrically. The calibration curve was achieved using quercetin as a standard and the absorbance was read at 510 nm. Determination of total anthocyanins was performed by Lee's method [[34], [35]]. This technique determines the total anthocyanins which have various monomeric pigments in their structure that manifest when the pH changes. At acidic pH, this molecule is in a stable form known as oxonium, which is of a deep red color, while at basic pH; the molecule loses a proton and adds water to its structure, giving rise to its unstable form known as hemicetal, which has no color. The absorbance was measured at 520 nm and 700 nm and compared with a blank cell containing distilled water. The absorbance difference was performed to calculate cyaniding-3-glucoside.
2.4. Vascular reactivity
The thoracic aorta (TA) was dissected and sectioned in rings of 2 mm that were suspended by metallic hooks in 5 ml glass chambers for isolated organ register according to the method described by Pérez-Torres et al. [17]. Concentration-response curves to acetylcholine (Ach) were obtained; the contraction of the rings was induced initially with 2 × 10−7 M, norepinephrine (NE) and then Ach was added in concentrations from 2 × 10−9 to 10−5 M accumulatively. For the contraction curves, NE was added at increasing concentrations; 2 × 10−9 to 2 × 10−5 M; when the maximal contraction response curve was reached for each concentration, the next concentration was added. After obtaining each series of contraction or relaxation curves, the aortic rings were washed three times with Krebs solution and allowed to recuperate for 30 min. Dose-response curves to assess the participation of ROS were obtained in the presence of potassium superoxide (KO2). The aortic rings were incubated for 10 min with KO2 at a concentration of 2 × 10−7 M (Half maximal effective concentration, EC50). After this time, the relaxation curve was made by adding 2 × 10−7 M of NE (EC50). Once the contraction curve reached its maximum, increasing and cumulative concentrations of Ach; 2 × 10−9 M to 2 × 10−5 M were added. After obtaining the relaxation and contraction curves in the presence of KO2, dose-response curves were obtained with H2O2 at a concentration of 2 × 10−6 M (EC50), in the same way as for the KO2.
2.5. Histology of the aortic rings
The aortic rings of each of the rats were preserved in 10% formalin in a 1:20 ratio. Histological sections were made and stained with the Masson trichrome and Jones methylamine silver techniques. The histological sections were analyzed with a Carl Zeiss light microscope (66300 Model) equipped with a 9-megapixel Cool SNAP-Pro digital camera and analyzed with the Sigma Scan Pro 5® program.
2.6. Homogenization of the thoracic aorta
After obtaining the aortic rings, the rest of the TA was frozen in liquid nitrogen and homogenized, according to the methods that were previously described by Soto et al. [36]. The determination of total proteins from the homogenate was carried out according to the method described by Bradford.
2.7. NO3−/NO2− ratio
NO2− reacts in acid media with sulfanilic acid to form a diazonium cation which is then coupled to the aromatic amine 1-naphthylamine to produce a red-violet coloration that can be read by the spectrophotometer at 540 nm. The NO3− was reduced to NO2− by the nitrate reductase enzyme reaction (5 units), of 50 μg protein from the TA homogenate previously deproteinized with 0.5 N NaOH. The absorbance was measured at 540 nm, and detected by the Griess method [37].
2.8. Glutathione concentration
This test consists of the oxidation of GSH by means of Ellman's reagent to form the yellow chromophore 5′-thio-2-nitrobenzoic (TNB), which can be quantified by using the spectrophotometer. The rate of TNB formation is proportional to the concentration of GSH in the sample. 100 μg of protein from the TA homogenate were used to make the determination according to a previous method by Ellman, and the absorbance was read at 412 nm [38].
2.9. Selenium determination
200 μg of protein from the TA homogenate were used to make this determination according to the method described by Soto et al. [37], and the absorbance was read at 600 nm.
2.10. Determination of total thiol groups
This test consists of the reaction of Ellman's reagent with a thiol, commonly a thiolate, producing thoil-nitrobenzoate. The technique used was previously described by Erel and Neselioglu [39], with some modifications carried out in our laboratory as previously reported [37]. 50 μg of protein from the TA homogenate were used for the determination and the absorbance was measured at 415 nm.
2.11. Determination of LPO levels and 3-nitrotyrosin
This test is based on the reaction of malondialdehyde, a secondary product of the oxidation of fatty acids with three or more bonds, with thiobarbituric acid in an acid medium and at high temperature, generating a pink-colored product [40]. 100 μl of protein from the TA homogenate were used for this determination and the absorbance was measured at 532 nm. The determination of 3-nitrotyrosin (3-NT) was made with a kit provided by LifeSpan BioSciencies, Seattle, WA, USA kit No. LS-40120). This assay is based on the competitive ELISA principle and is measured at a wavelength of 450 nm, using a visible light micro plate reader (Stat Fax 3200 Awareness Technology Palm City, FL, USA).
2.12. Determinations of the activities of TrxR, GPx, GST and GR
The activity of TrxR was determined using 100 μg of protein from the TA homogenate according to the method described by Soto et al. [38]. The sample was incubated and monitored at 412 nm for 6 min at 37 °C. To evaluate the GPx, GST and GR activities, 100 μg of protein from the TA homogenate were used as previously described [22]. The sample was incubated and monitored at 340 nm for 8 min at 37 °C.
2.13. Activity of glucose 6 phosphate dehydrogenase
To evaluate G6PD activity, 2.9 ml of TRIS-HCl buffer (0.1 M, pH 7.5) was used, plus 2 μl of G6P (2 mM) and 10 μl of NADP+ (5 mM). The mixture was read in a spectrophotometer at 340 nm and allowed to stabilize for 1 min at 37 °C, after which 100 μg of protein from the TA homogenate were added and the absorbance was monitored for 8 min at 340 nm [41].
2.14. Statistical analysis
The Sigma Plot program (SigmaPlot® version 14.5, Jandel Corporation) was used for statistical analysis. The GraphPad-Prism 8 Software. Inc. (San Diego, CA, USA), 1995–2023, was used to generate the graphs. The data are presented as mean ± standard error. Statistical significance was determined with one-way ANOVA and Tukey's post hoc test. A p ≤ 0.05 was considered as significant.
3. Results
3.1. Subsection guaranteed analysis
Table 1 shows the amounts anthocyanins, flavonoids and polyphenols contributed by the drinking water at the different percentages of HSL infusions. These infusions were administered to the rats from the different experimental groups.
Table 1.
Analysis of the components and the amounts of anthocyanins, flavonoids and polyphenols, contributed by the drinking water at the different percentages of the HSL infusions.
| Variables | HSL (%) |
||
|---|---|---|---|
| 1.5 | 3 | 6 | |
| Cyanidin-3-glucoside (mg/L) | 105.8 ± 19.48 | 551.44 ± 9.64 | 603.94 ± 10.03 |
| Quercetin (mg/day) | 0.29 ± 0.01 | 0.97 ± 0.00 | 1.24 ± 0.01 |
| Polyphenols (mmol/day) | 0.38 ± 0.00 | 0.70 ± 0.01 | 0.78 ± 0.00 |
| Corresponds equivalence the waters consumption and of the antioxidants per rat/day | |||
| Water consumption (mL/day) | 50.6 ± 1.9 | 48.2 ± 1.3 | 46.4 ± 0.5 |
| Cyaniding-3-glucoside (mg/day) | 0 | 5.09 ± 2 × 10−2 | 25.57 ± 4 × 10−3 |
| Quercetin (mg/day) | 0 | 0.01 ± 1 × 10−5 | 0.04 ± 9 × 10−6 |
| Polyphenols (mmol/day) | 0 | 0.01 ± 2 × 10−5 | 0.03 ± 4 × 10−6 |
3.2. Systolic blood pressure
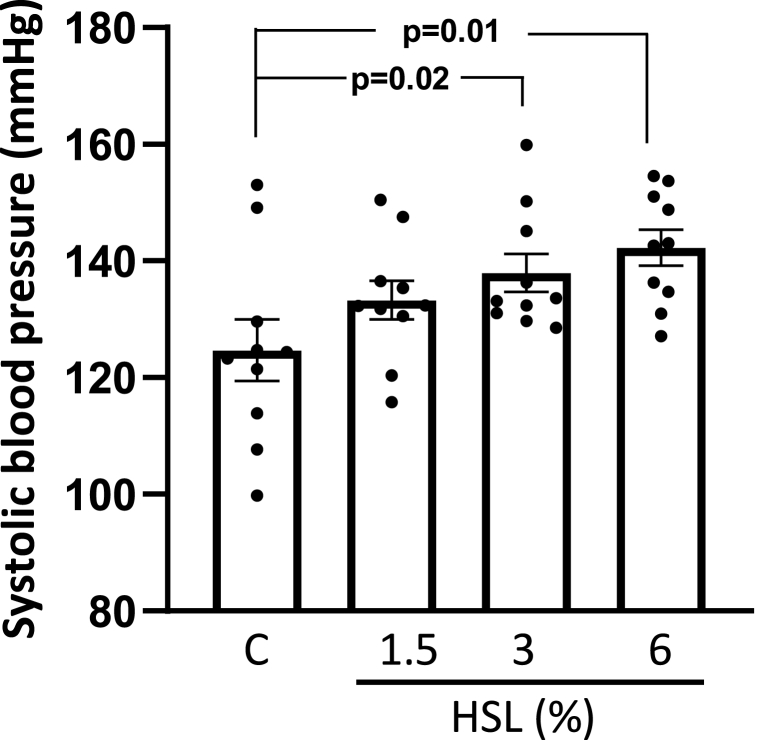
Fig. 1 Shows the SBP of the experimental groups. The rats that consumed the HSL infusion at 3% and 6% showed a significant increase (p = 0.02 and p = 0.01 respectively) compared to the control group.
Fig. 1.
Systolic blood pressure of the experimental groups. Values are expressed as mean ± standard error; (n = 10). Abbreviations: HSL= Hibiscus Sabdariffa Linnaeus.
3.3. Vascular reactivity
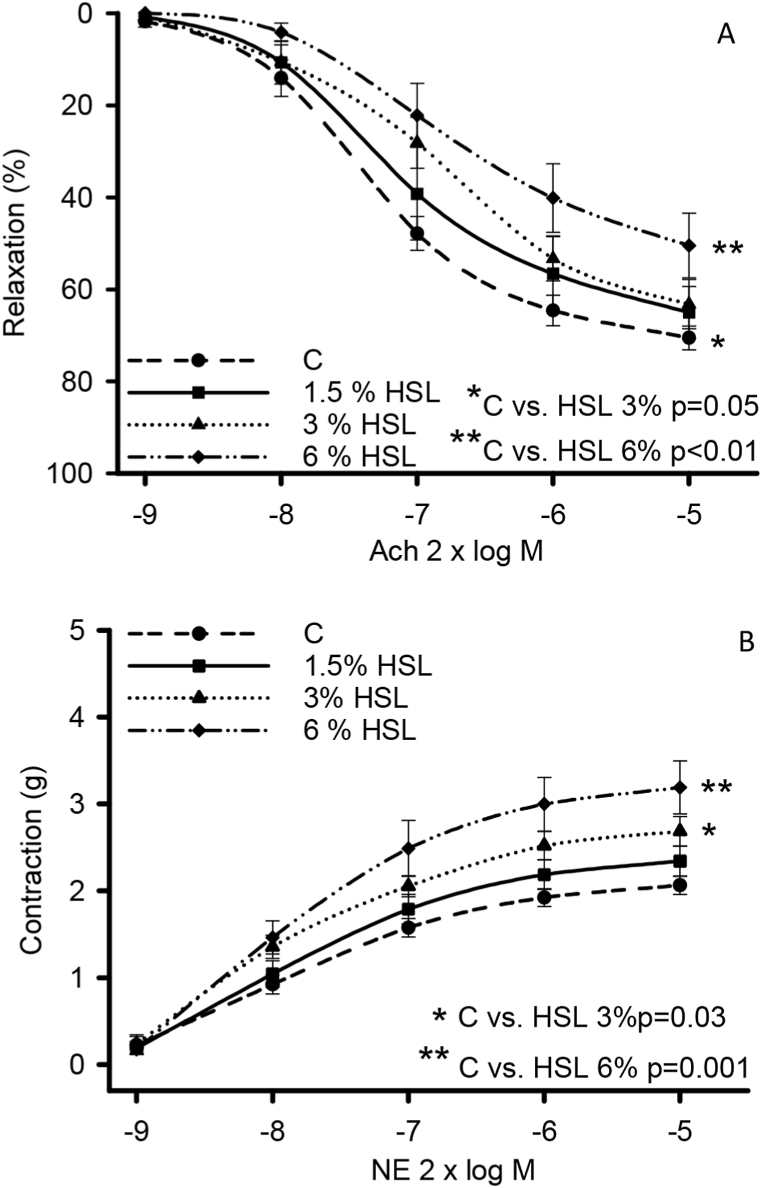
Fig. 2 (A) shows the vasodilation response in the aortic rings of the experimental groups. There is a decrease in the vasodilation response compared to the control group in the rats that consumed the HSL infusion at 3% (p = 0.05) and 6% (p < 0.01). In Fig. 2B the vasoconstriction in the aortic rings of the rats that consumed the HSL infusion at 3% (p = 0.3) and 6%, p = 0.001) showed an increase when compared to the control group.
Fig. 2.
Vasorelaxation (A) and vasoconstriction (B) responses in the aortic rings of the experimental groups of the rats that consumed the HSL infusion at different percentages. Values are expressed as mean ± standard error; (n = 10). Abbreviations: HSL= Hibiscus Sabdariffa Linnaeus, NE = norepinephrine, Ach = acetylcholine.
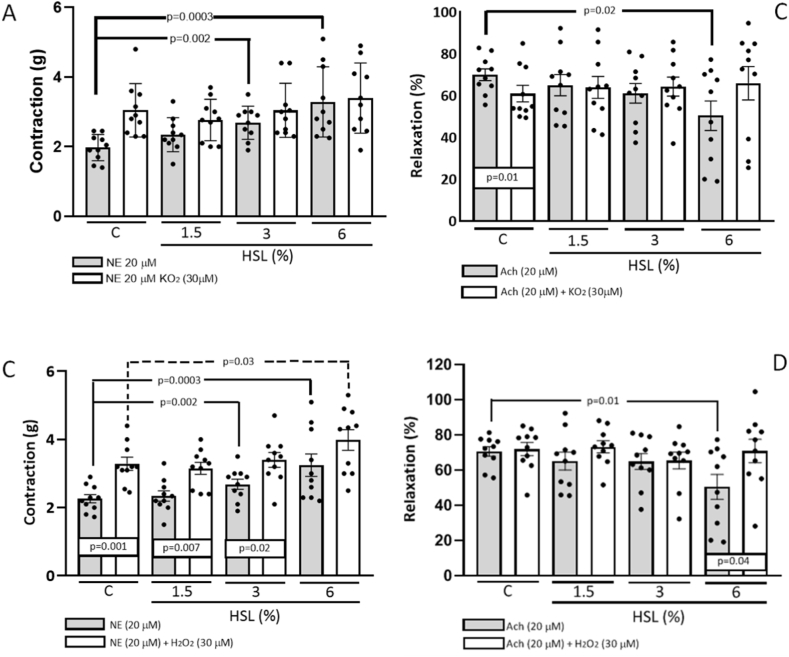
Fig. 3 (A) shows the O2− participation in the vasoconstriction and vasodilatation response in the thoracic aorta rings of the different experimental groups. The vasoconstriction response showed an increase in the control group in the presence of NE vs. NE plus KO2 (30 μM), (p < 0.001). In addition, an increase in the vasoconstriction response in the aortic rings was observed between the control group in the presence of NE and the 3% and 6% HSL infusion groups (p = 0.002 and p = 0.0003 respectively).
Fig. 3.
Vasoconstriction responses in presence of the KO2 (3 A) and H2O2 (3C) respectively. Vasorelaxation responses in presence of the KO2 (3 B) and H2O2 (3 D) respectively in the aortic rings of the experimental groups of the rats that consumed the HSL infusion at different percentages. Abbreviations: HSL= Hibiscus Sabdariffa Linnaeus, KO2= Potassium superoxide, H2O2=Hydrogen peroxide, NE = norepinephrine, Ach = acetylcholine. Values are expressed as mean ± standard error; (n = 10).
Fig. 3 (B) shows that the vasodilatation response in the control group was lower (p = 0.01) with a significant difference in the presence of Ach vs. Ach plus KO2 (30 μM). This same trend was present in the vasodilation response in the control group in the presence of Ach and in the group of rats that received the 6% HSL infusion (p = 0.02). Fig. 3 (C) shows there was a significant difference with the HSL infusion at 1.5%, 3% and 6% in the presence of NE vs. NE plus H2O2 (p = 0.001, p = 0.007 and p = 0.02 respectively). In addition, there was a significant increase in the vasoconstriction response in the presence of NE in the control group against the experimental groups with HSL infusion at 3% and 6% (p = 0.002 and p = 0.0003 respectively). There was also an increase in the vasoconstriction response in the presence of NE plus H2O2 between the groups with the HSL infusion at 6% (p = 0.03) against the control group. Fig. 3 (D) shows that the vasodilation response in the thoracic aorta ring was decreased significantly in the HSL group at 6% with Ach (p = 0.01) in comparison with the control group. In addition, when aortas were incubated with Ach plus H2O2, an increase was observed in the same group with the HSL infusion at 6% (p = 0.04).
3.4. Histology and densitophotometry analysis
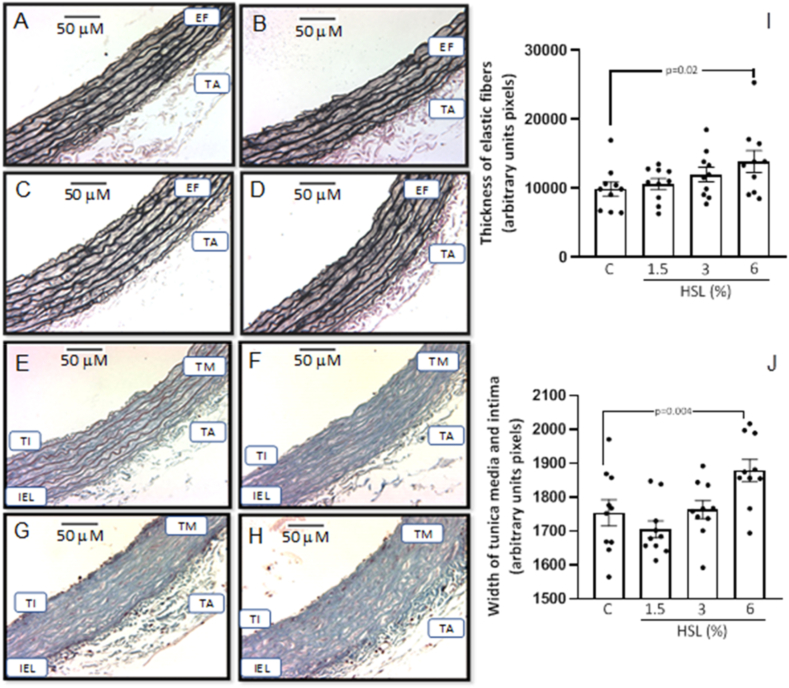
Fig. 4 (A, B, C, and D) shows the representative photomicrographs of the Jones methylamine silver stain in thoracic aortas of the experimental groups. It can be observed that the HSL infusion at a percentage of 6% increases in the thickness of the elastic fibers in the TA in comparison with the control group. This observation was corroborated with the analysis by densitophotometry of the photomicrographs. Fig. 4 (I), shows a greater thicknesses of the elastic fibers with a significant difference between the same group (p = 0.02). Fig. 4 (E, F, G, and H) shows the representative photomicrographs of Masson's trichrome stain. The percentage at 6% of the HSL infusion increased the thickness of the tunica media and intima of the TA with respect to the control group. This observation was corroborated with the analysis and the densitophotometry of the photomicrographs. Fig. 4 (J), shows a greater thickness of the tunica media and intima of the TA with a significant difference against the same group (p = 0.004).
Fig. 4.
Representative photomicrographs of the histology of the TA of rats that received the different percentages of the HSL infusion (A control, B, C, and D different percentages of (HSL). The tissue was processed according to conventional histological procedures, and histological sections were made and stained by Jones methylamine silver stain at 16x. In figuresE, F, G, and H the representative photomicrographs of the histology of the aorta rings stained with Masson's trichrome stain at 16x are shown. Likewise, figures I and J show the densitophotometric analysis for each stain. Abbreviations: TI = Tunica intima, IEL= Internal elastic lamina, TM = Tunica media, TA = Tunica adventitia, EF = Elastic fibers.Values are expressed as mean ± standard error; (n = 10).
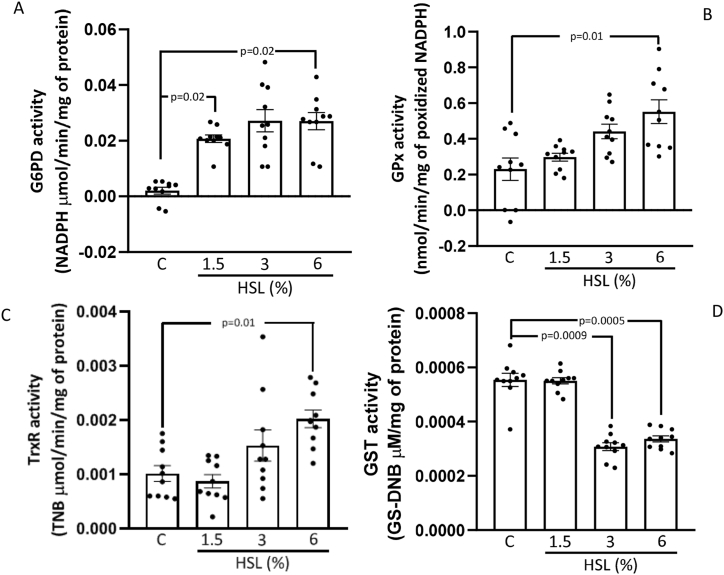
3.5. Enzymatic activities of the G6PH, GPx, TxrR, and GST
Fig. 5 (A, B, and C) shows the enzymatic activities of G6PD, GPX, and TxrR respectively in the TA homogenate. There were significant increases in the HSL group at 6% in comparison with the control group (p = 0.02, p = 0.03 and p = 0.01 respectively). Moreover, in the HSL group at 1.5% the G6PH activity showed an increase in comparison to the control group (p = 0.01). However, the GST activity showed a significant decrease in the HSL group at 3% (p = 0.0009), and 6% (p = 0.0005) with respect to the control group (Fig. 5 (D).
Fig. 5.
Enzymatic activities of the G6PD (A), GPx (B), TxrR (C), and GST (D) in the TA aorta homogenate. Values are expressed as mean ± standard error; (n = 10).
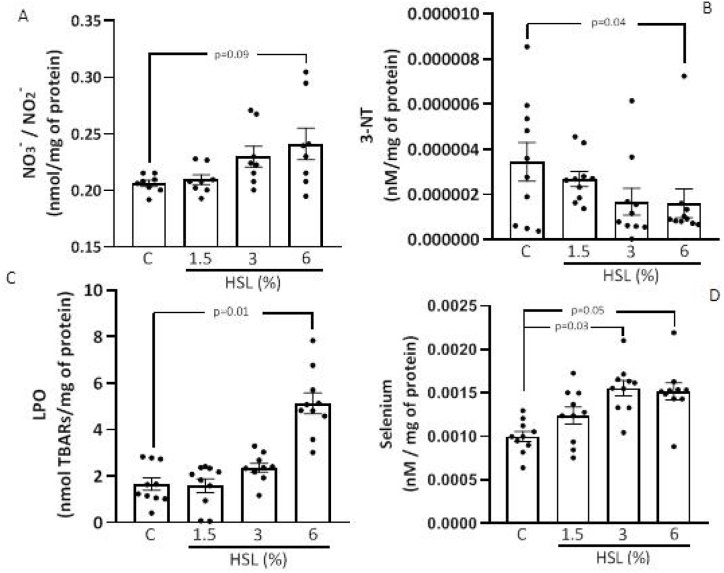
3.6. Oxidative stress markers
Fig. 6 (A, B, C, and D) shows the concentration of NO3−/NO2− ratio, LPO, Se and 3-NT in the TA, respectively. There was a tendency to increase in the NO3−/NO2− ratio without there being a significant difference (p = 0.09, Fig. 6 (A). The LPO levels showed an increase in the HSL group at 6% in comparison with the control group (p = 0.01, Fig. 6 (B). In the groups that received the HSL infusion at percentages of 3% and 6%, there was an increase in the Se concentration (p = 0.03 and p = 0.05 respectively, Fig. 6 (C)). However, the 3-NT in the group that consumed the HSL infusion at 6% showed a significant decrease in comparison with the control group (p = 0.04, Fig. 6 (D).
Fig. 6.
Oxidative stress markers. Concentration of NO3–/NO2− ratio (A), LPO (B), Se (C) and 3-NT (D) in the TA of the rats. Values are expressed as mean ± standard error; (n = 10).
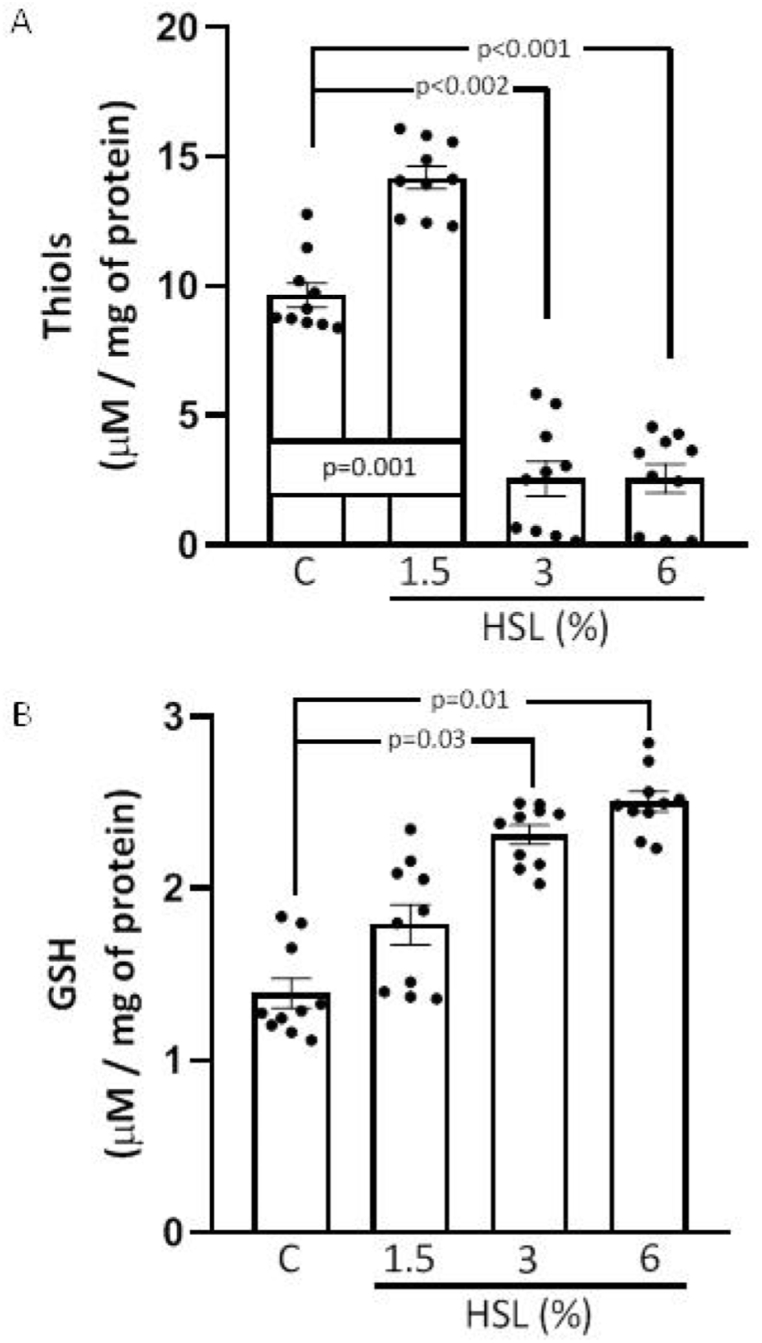
Fig. 7 (A and B) shows the concentration of total thiols and the concentration of GSH in the TA homogenate. There was an increase in the total thiols in the HSL group that received the infusion at 1.5% (p = 0.005, Fig. 7A). However, in the HSL groups at 3% and 6% there were significant decreases (p = 0.002 and p < 0.001) in comparison with the control group. The GSH concentration showed an increase in the HSL group that received the percentages at 3% and 6% (p = 0.03 and p = 0.01, respectively, in comparison with the control group Fig. 7 (B).
Fig. 7.
Concentration of total thiols (A) and GSH (B) in the TA homogenate in rats that received different percentages of the HSL infusion. Values are expressed as mean ± standard error; (n = 10).
4. Discussion
HSL is considered a "functional" food that provides various organic components with biological activity such as polyphenols, flavonoids, anthocyanins, delphinidin, hibiscetin, quercetin and gossypetin, protocatechuic acid, alkaloids, l-ascorbic acid, carotenoids, anisaldehyde, galactose, mucopolysaccharides, pectins, polysacchrarides and stearic acid. At low concentrations, this food acts against OS and diabetes, it decreases hight blood pressure, obesity, hypertriglyceridemia, hypercholesterolemia, hyperglycemia, insulin resistance, hyperinsulinemia and LDL oxidation. HSL can also provide different minerals and essential amino acids that form part of the antioxidant system. It also fights cancer and modulates the immune response [[42], [43], [44]]. However, these "beneficial" characteristics could become detrimental if consumed in excess or for a prolonged lapse of time, increasing antioxidant enzymes and excessively depleting ROS [[42], [43], [44]]. This could lead to RS and to a redox imbalance [11,43,44]. Therefore, the objective of this study was to evaluate the alteration of vascular reactivity associated to excessive and chronic consumption of HSL infusions at different percentages.
Our results show a significant increase in the elastic fiber thickness of the tunica media and intima of the thoracic aorta when HSL is consumed. These changes suggest higher rigidity of the vessel and hypertrophy, which was reflected in the increase in SBP in the groups with HSL infusion at 3% and 6%. Structural alterations and hypertrophy in the conductive arteries is associated with stiffness, inflammation, and increased blood pressure [12,44]. In this sense, a study carried out in male "Carworth" rats was associated with an increase in collagen and elastic fibers in the renal artery and the presence of renovascular hypertension [45]. The same effect was observed in another study when analyzing the structure of the aorta in hypertensive "Wistar" rats, where the hypertrophy caused in the wall decreased the elasticity of fibers and collagen biosynthesis leading to increased SBP [12,44]. The hypertrophy of the aorta may stimulate the synthesis of angiotensin II and produce vasoconstriction favoring O2− production through the activation of NAD/NADH+. This leads to inactivation of eNOS in the endothelium, causing, in turn, over-expression of iNOS that contributes to an inflammatory process [17,44,46]. Furthermore, HSL contains tyrosine, and an excess of this amino acid could lead to an increased synthesis of NE by the sympathetic nerve endings, which could contribute to increase SBP in the groups with HSL infusions at 3% and 6% [44,47].
Our results show that vasoconstriction and vasodilation increased and decreased, respectively, in the rats that consumed the HSL infusion at 3% and 6%. This suggests that, the structural changes, associate to the excess consumption of HSL infusion may alter vascular function. This effect could be due to an increase in the antioxidants provided by the HSL infusion such as cyanidin-3-glucoside, quercetin and polyphenols that increase the activities of the SOD isoforms, CAT, GPx enzymes of the antioxidant system. However, decreased LPO, and the increase in these enzymes can, in turn, decrease the ROS [[42], [43], [44]] which could modulate vascular reactivity. In this sense, our results showed that the rats that consumed the 6% infusion of HSL, showed an increase in the ratio NO3−/NO2− are secondary metabolites of the oxidation of nitric oxide (NO). The presence of a high ratio of these secondary metabolites is associated with over-expression/activity of iNOS or by uncoupled eNOS. This could also influence the thickening of elastic fibers, in the middle muscle zone, and the loss of the elasticity in the thoracic aorta. The structural alterations and hypertrophy in conductive arteries such as the aorta may lead to chronic inflammation, increased SBP and hypertension [43]. In this sense, it is essential to maintain the redox balance in endothelial cells, and a decrease in ROS could contribute to the alteration of the vascular function and consequently lead to various vascular diseases such, endothelial dysfunction and hypertension [2]. The effects of ROS in the thoracic aorta are not uniform since they depend on which ROS molecule is acting and at what concentration [48]. In the thoracic aorta, the most important ROS are O2−, H2O2, NO and peroxynitrite (ONOO−), [49].
The excess of antioxidants and precursors of the antioxidant systems provided by HSL infusions such flavonoids and polyphenols could decrease O2− and H2O2, which, at physiological concentrations, act as second messengers [44,48]. Our results show that the vasoconstriction response in the experimental groups with infusions with different HSL percentages eliminated the significant difference found in the control group after being incubated with KO2. KO2 reacts violently with water to form KOH and, in this process, O2− is formed. This suggests the participation of O2− in the vascular response, and it also shows that an excess of antioxidants provided by HSL decreases the concentration of O2−. This result can be associated with the alteration of the vascular reactivity and with increased SBP.
In contrast, H2O2 favors vasodilation and produces NO through the activation of different signaling pathways such as PI3K/Akt, Erk1/2, and p38MAPK [49]. H2O2 not only stimulates eNOS but it can also increase its expression. Another protein that participates in the regulation of vasodilation is PKG1α. This protein kinase is sensitive to oxidation of H2O2 through the formation of a disulfide bond. The vasodilation that occurs is independent of the levels of cyclic guanosine monophosphate [48]. The results obtained in our study show that vasodilation increases after incubation with H2O2 in the group that consumed the HSL infusion at 6%, thus suggesting the participation of H2O2 in the vasodilatation response. Our results also suggest that the excess antioxidants provided by the HSL infusion at 6% decreases the H2O2 concentration, which is reflected in the alteration of the arterial function, probably due to a decrease of the eNOS pathway. In this sense, the tendency, and the increase in the NO3−/NO2− ratio in the groups that consumed the HSL infusion at 3% and 6% could indirectly reflect the over-expression/activity of iNOS [44,50]. Some reactive nitrosative species such as ONOO− may participate in the inflammatory processes due to an increase in the availability of NO provided by the iNOS pathway [50,51]. However, our results showed that NO3−/NO2− ratio had a tendency to increase, but that the 3-NT was decreased. These results could be paradoxical because an increase in protein nitrosylation was expected, through the iNOS metabolic pathway that would probably be increased in the aorta. However, the excess of antioxidants provided by the 6% HSL infusion could decrease the ONOO−, which participates in the nitrosylation process, thus explaining the decrease in 3-NT in the aortic homogenate. This decrease is also associated with the low concentration of thiol groups in the cysteines of proteins, by excessive consumption of HSL that decreases the concentration of thiol groups [50,51]. However, more studies are required to corroborate this hypothesis.
In our study we analyzed the activity of several enzymes including G6PD which is responsible for providing reducing equivalents such as NADPH and of controlling ROS production through of the antioxidant enzymes that are dependent on NADPH [52]. Overexpression of G6PD decreases the excess ROS in endothelial cells treated with H2O2 or TNFα. Moreover, the G6PD overexpression can increase the concentration of GSH through the GR pathway [52,53]. Furthermore, in vascular cells, some processes that generate inflammation, such as the hypertrophy of the vascular wall in MS and type 2 diabetes, could increase the expression or activity of G6PD and favor high levels of NADPH as a compensatory mechanism [53]. Our results showed an increase in the activity of G6PD in the aorta homogenate. This could be attributed to the alteration of the vascular function and structural changes associated with inflammation and hypertrophy of the aortic wall. The hypertrophy may cause activation of NADPH oxidase and the release of some interleukins that favor the pro-inflammatory state. These processes can stimulate the activation of G6PD and favor the production NADPH that is a cofactor for some antioxidant enzymes such as GPx, TrxR, and iNOS. HSL could also favor the G6PD overexpression or activity, leading to an increase in NADPH [54].
In addition to the fact that G6PD overexpression can increase GSH concentration, HSL can contribute to increase GSH by itself, since it contains cysteine, glycine, and glutamic acid, which are precursors of GSH. Therefore, this could increase the synthesis of GSH [19,44]. In this sense, the results showed a significant increase in the concentration of GSH in the groups that received the HSL infusion at 3% and 6%.
HSL in various concentrations also provides Se and the amount provided depends on the crop, type of soil, pH, type of plant species, and salinity [55,56]. Se is considered as an essential micronutrient and its excess or depletion in the body favors the appearance of some diseases such as hypothyroidism, cardiovascular disorders, cognitive impairment, and weakening of the immune system [56]. Furthermore, Se is indispensable for 25 seleno enzymes such as GPx and TrxR, which contribute to maintain the redox homeostasis [56]. Our results show that the Se concentration was increased in the groups of rats that received HSL infusion at 3% and 6%. This suggests that the excessive consumption the HSL could lead to selenosis, which is caused by excess intake of Se. However, more studies are required to corroborate this hypothesis.
Se may be oxidized in the presence nitrogen species and GSH [56] ,[57].Se compounds such as selenite and selenium dioxide can react with the thiol groups present in GSH to produce O2−. This has been observed in experiments with isolated mitochondria treated with selenium-containing components, including selenite and selenocysteine [57].This suggests that excess Se could lead to generation of O2− and ONOO−and that the excessive antioxidants provided by the HSL infusion can decrease it.
A high level of Se concentration favors LPO, which causes DNA damage and favors oxidation of polyunsaturated fatty acids and degradation of proteins [57],[58]. A high Se concentration could cause thiol oxidation. Our results showed that the concentration of total thiol was decreased but that LPO was increased in the rats that received the HSL infusion at 3% and 6%. The low levels of thiols may contribute to the alteration of the vascular function and to structural changes in the thoracic aorta in these rats and are linked to cardiovascular events [59]. Another study associated the decrease in thiols and the increase in LPO with the severity of artery coronary disease lesions [60].
To maintain thiol homeostasis in cysteines of proteins, mammalian cells depend on the TrxR system [55,61]. This system also participates in the regulation of the vascular tone, and when the TrxR is overexpressed, vascular reactivity is improved by increasing NO bioavailability [62]. A study in mice showed that the TrxR deletion generated a lower response to vasodilation and a greater ONOO− production [63,64]. Our results showed an increase in the activity of this enzyme in the group of rats that consumed the HSL infusion at 6%. These results suggest that an increase in the TrxR activity could be attributed to the excess Se and the increase of the activity of G6PD [[62], [63], [64]]. This could be a compensatory mechanism that is aimed to increase the levels of thiols [61] and could be associated with the excessive consumption of antioxidants such as polyphenols, quercetin, flavonoids, Se, and gallic, protocatechuic, tannic acids, which may be provided by the HSL infusion.
Another enzyme that increased its activity in this study was GPx. This enzyme contains a selenium-cysteine at its catalytic site and was overactive in the aorta homogenate from the rats of the group that consumed the HSL infusion at 6%. This increased activity could occur because of substrate surplus such as GSH and NADPH that were provided by the G6PD and to the Se elevation. Furthermore, moderate ROS are required for keeping the formation of disulfide (thiols) in the cell and the GPx overexpression can lead to a decrease of protein disulfides that is related to reduce signaling from growth factors and decreased mitochondrial function [65].
On the other hand, GST is an antioxidant enzyme that participates in the detoxification process caused by oxidative damage, xenobiotic agents, and oxidized lipids, and it may prevent the effects of LPO [66]. This enzyme conjugates GSH with electrophilic and xenobiotic agents such as drugs, toxins, carcinogens, and detoxifies the organism [67].Our results showed a decrease in the GST activity in the group of rats that consumed the HSL infusion at 3% and 6%. The tannic acid administration at doses of 80 mg/kg reduces the activity of the “mu” and “alpha” subunits of the GST in the liver [66]. It also decreases the “pi” subunit of the GST when protocatechuic acid is provided [66,67]. Another study demonstrated that high flavonoid concentrations could inhibit GST activity and favor an increase in LPO [67]. This suggests that the excess consumption of flavonoids, tannic and protocatechuic acids, which are provided in the HSL infusion at 3% and 6%, favored the decrease in the GST activity in the aorta and contributed to increase LPO [67].
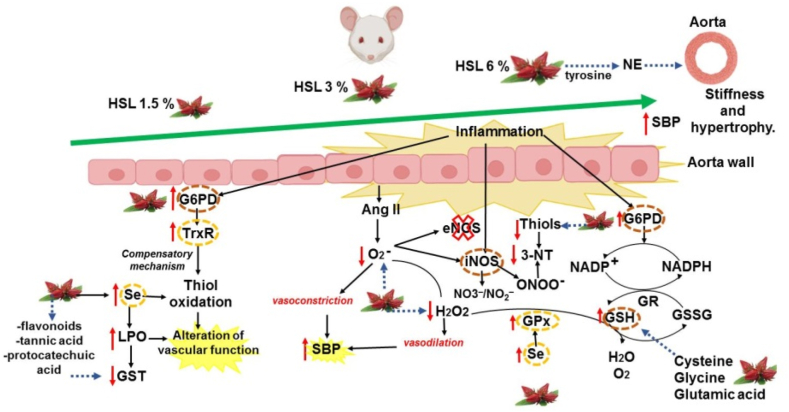
Fig. 8 summarizes the physiological alterations and anatomical changes in the thoracic aorta in response to different percentages HSL infusions.
Fig. 8.
Summary of the alteration in physiological and anatomical changes within the thoracic aorta in response to different percentages HSL infusions. These changes are accompanied by alterations of the enzymatic and non-antioxidant systems that contribute to the alteration of the redox homeostasis and generation of RS, which results in elevated blood pressure in rats. Abbreviations: eNOS = endotelial nitric oxide synthase, G6PD = Glucose-6-phosphate dehydrogenase, GR = glutathione reductase, GSH = glutathione, GSSG = oxidized glutathione, GST = glutathione-S-transferase, H2O2 = hydrogen peroxide, HSL= Hibiscus Sabdariffa Linnaeus, iNOS = inducible nitric oxide synthase, LPO = lipoperoxidation, NADPH = nicotinamide adenine dinucleotide phosphate, NE = norepinephrine, NO3−/NO2− = nitrate/nitrite ratio, 3-NT = 3-nitrityrosine, O2− = superoxide anion, SBP = systolic blood pressure, Se = selenium, TrxR = thioredoxin reductase.
5. Conclusions
The excessive and chronic consumption of antioxidants such as polyphenols, quercetin, flavonoids, Se and gallic, protocatechuic, tannic acids provided by the HSL infusion at 3% and 6% may deplete the ROS thus deteriorating vascular function in healthy rats. These effects were not observed at low concentrations such as 1.5%.
The deterioration of vascular reactivity is accompanied by an increase in the thickness of the thoracic aorta wall and with rupture of elastic fibers that contribute to increase the systolic blood pressure and favor hypertension.
These changes are accompanied with the over-activity of the antioxidant system that may eliminate ROS and alter the redox homeostasis, generating RS and contributing to hypertension.
Author contributions
Linaloe Manzano-Pech, PhD:. Conceived and designed the experiments; analyzed and interpreted the data; wrote the paper.
Verónica Guarner-Lans, PhD:. Contributed reagents, material, analysis tools or data; Wrote the paper.
María Elena Soto, PhD:. Conceived and designed the experiments; analyzed and interpreted the data.
Eulises Díaz-Díaz, PhD:. Conceived and designed the experiments; analyzed and interpreted the data.
Israel Pérez-Torres, PhD:. Conceived and designed the experiments; analyzed and interpreted the data; contributed reagents, material, analysis tools or data; Wrote the paper.
Funding
This research received no external funding. Instituto Nacional de Cardiología Ignacio Chavez paid the open access publication fee.
Institutional review board statement
The Laboratory Animal Care Committee of the National Institute of Cardiology “Ignacio Chávez” in México (SICUAE.DC.2019/3–4) approved the experiments in animals, and they were conducted in compliance with the Guide for the Care and use of laboratory animals of the National Institutes of Health (NIH).
Informed consent statement
Not applicable.
Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the payment of open access publishing was covered by the Institute National de Cardiology Ignacio Chávez. We thank Benito Chávez Rentería for histology technical support.
References
- 1.Steinberg S. Oxidative stress and sarcomeric proteins. Cir. Res. 2013;112:393–405. doi: 10.1161/CIRCRESAHA.111.300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taverne Y., Bogers A., Duncker B., Merkus D. Reactive oxygen species and the cardiovascular system. Oxid. Med. Cell. Longev. 2013 doi: 10.1155/2013/862423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benhar M., Stamler J. A central role for S-nitrosylation in apoptosis. Nat. Cell Biol. 2005;7:645–646. doi: 10.1038/ncb0705-645. [DOI] [PubMed] [Google Scholar]
- 4.Rhee S., Kang S., Jeong W., Tong-Shin C., Hyun A.W. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Grune T. Oxidants and antioxidative defense. Hum. Exp. Toxicol. 2002;21:61–62. doi: 10.1191/0960327102ht210oa. [DOI] [PubMed] [Google Scholar]
- 6.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Inter. J. Biochem. Cell. Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Zitka O., Skalickova S., Gumulec J., Masarik M., Adam V., Hubalek J., Trnkova L., Kruseova J., EckschlagerKizek T.R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Let. 2012;4:1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branco V., Carvalho V. The thioredoxin system as a target for mercury compounds. Biochim. Biophys. Acta, Gen. Subj. 2019;1863 doi: 10.1016/j.bbagen.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Singh F., Charles A.L., Schlagowski A.I., Bouitbir J., Bonifacio A., Piquard F., Krähenbühl S., Geny B., Zoll J. Reductive stress impairs myoblasts mitochondrial function and triggers mitochondrial hormesis. Biochim. Biophys. Acta. 2015;1853:1574–1585. doi: 10.1016/j.bbamcr.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H., Limphong P., Pieper J. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB. J. 2012;26:1442–1451. doi: 10.1096/fj.11-199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Torres I., Guarner V., Rubio M. Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents. Int. J. Mol. Sci. 2017;18:2098. doi: 10.3390/ijms18102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berillis P. The role of collagen in the Aorta's structure. Open Circ. Vasc. J. 2013;6:1–8. doi: 10.2174/1877382601306010001. [DOI] [Google Scholar]
- 13.Silver F., Horvath I., Foran D. Visco elasticity of the vessel wall: the role of the collagen and elastic fibers. Crit. Rev. Biomed. Eng. 2001;29:279–301. doi: 10.1615/critrevbiomedeng.v29.i3.10. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D., Pannier B., Vlachopoulos C., Wilkinson I., Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 15.Janus A., Szahidewicz-Krupska E., Mazur G., Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediat. Inflamm. 2016;2016 doi: 10.1155/2016/3634948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandoo A., Veldhuijzen van Zanten J.J.C.S., Metsios G.S., Carroll D., Kitas G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010;4:302–312. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Torres I., Zúñiga-Muñoz A., Beltrán-Rodríguez U., Díaz-Díaz E., Martínez-Memije R., Guarner-Lans V. Modification of the liver fatty acids by Hibiscus sabdariffa Linnaeus (Malvaceae) infusion, its possible effect on vascular reactivity in a metabolic syndrome model. Clin. Exp. Hypertens. 2014;36:123–131. doi: 10.3109/10641963.2013.789046. [DOI] [PubMed] [Google Scholar]
- 18.Norhaizan M., Hern N., Shin F., Amin I., Lye Y.C. Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 2010;122:1055–1060. [Google Scholar]
- 19.Costa-Rocha I., Bonnlaender B., Sievers H., Pischel I., Heinrich M. Hibiscus sabdariffa L.-A phytochemical and pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Tseng T., Kao T.W., Chu C.Y., Chou F.P., Lin W.L., Wang C.J. Induction of apoptosis by hibiscus protocatechuic acid in human leukaemia cells via reduction of retinoblastoma (RB) phosphorylation and Bcl-2 expression. Biochem. Pharmacol. 2000;60:307–315. doi: 10.1016/s0006-2952(00)00322-1. [DOI] [PubMed] [Google Scholar]
- 21.Prasongwatana V., Woottisin S., Sriboonlue P., Kukongviriyapan V. Uricosuric effect of roselle (Hibiscus sabdariffa) in normal and renal-stone former subject. J. Ethnopharmacol. 2008;117:491–495. doi: 10.1016/j.jep.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Torres I., Torres-Narváez J.C., Guarner-Lans V., Díaz-Díaz E., Diazconti Perezpeña- M., Romero P., Manzano-Pech L. Myocardial protection from ischemia-reperfusion damage by the antioxidant effect of hibiscus sabdariffa linnaeus on metabolic syndrome rats. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/1724194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sousa R., Marletta M. Inhibition of cytochrome P-450 activity in rat liver microsomes by the naturally occurring flavonoid, quercetin. Arch. Biochem. Biophys. 1985;240:34–357. doi: 10.1016/0003-9861(85)90040-2. [DOI] [PubMed] [Google Scholar]
- 24.Rees D., Palmer R., Hodson H., Moncada S. Specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br. J. Pharmacol. 1989;96:418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda U., Shimada K. Nitric oxide and cardiac failure. Clin. Cardiol. 1997;20:837–841. doi: 10.1002/clc.4960201009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wätien W., Michels G., Steffan B., Niering P., Chovolou Y., Kampkötter A., Tran-Thi Q.H., Proksch P., Kahl R. Low concentrations of flavonoids are protective in rat H4IIE cells whereas high concentrations cause DNA damage and apoptosis. J. Nutr. 2005;135:525–531. doi: 10.1093/jn/135.3.525. [DOI] [PubMed] [Google Scholar]
- 27.Sergediene E., Jönsson K., Szymusiak H., Tyrakowska B., Rietjens I.M., Cenas N. Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: description of quantitative structure-activity relationships. FEBS Lett. 1999;462:392–396. doi: 10.1016/s0014-5793(99)01561-6. [DOI] [PubMed] [Google Scholar]
- 28.Robaszkiewicz A., Balcerczyk A., Bartosz G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol. Int. 2007;31:1245–1250. doi: 10.1016/j.cellbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Hodnick W., Kung F., Roettger W., Bohmont C.W., Pardini R.S. Inhibition of mitochondrial respiration and production of toxic oxygen radicals by flavonoids. A structure-activity study. Biochem. Pharmacol. 1986;14:2345–2357. doi: 10.1016/0006-2952(86)90461-2. [DOI] [PubMed] [Google Scholar]
- 30.Decker E. Phenolics: prooxidants or antioxidants? Nutr. Rev. 1997;55:396–398. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 31.Passi S., Picardo M., Nazzaro-Porro M. Comparative cytotoxicity of phenols in vitro. Biochem. J. 1987;245:537–542. doi: 10.1042/bj2450537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez-Torres I., Roque P., El-Hafidi M., Diaz-Diaz E., Baños G. Association of renal damage and oxidative stress in a rat model of metabolic syndrome. Influence of gender. Free Radic. Res. 2009;43:761–771. doi: 10.1080/10715760903045296. [DOI] [PubMed] [Google Scholar]
- 33.Jia Z., Tang M., Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 34.Lee J., Durst R., Wrolstad R. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the Ph differential method: collaborative study. J. AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- 35.Soto M.E., Manzano-Pech L.G., Guarner-Lans V., Díaz-Galindo J.A., Vásquez X., Castrejón-Tellez V., Gamboa R., Huesca C., Fuentevilla-Alvárez G., Pérez-Torres I. Oxidant/antioxidant profile in the thoracic aneurysm of patients with the Loeys-Dietz syndrome. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/5392454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J., Zhang X., Broderick M., Fein H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors. 2003;3:276–284. doi: 10.3390/s30800276. [DOI] [Google Scholar]
- 37.Rahman I., Kode A., Biswas S. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 38.Erel O., Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014;49:326–332. doi: 10.1016/j.clinbiochem.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 39.Gutteridge J. The use of standards for malonyldialdehyde. Anal. Biochem. 1975;69:518–526. doi: 10.1016/0003-2697(75)90155-4. [DOI] [PubMed] [Google Scholar]
- 40.Lee C. Glucose-6-phosphate dehydrogenase from mouse. Methods Enzymol. 1983;89:252–257. doi: 10.1016/s0076-6879(82)89045-9. [DOI] [PubMed] [Google Scholar]
- 41.Bellezza I., Giambanco I., Minelli A., Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Xiao W., Loscalzo J. Metabolic responses to reductive stress. Antioxidants Redox Signal. 2020;32:1330–1347. doi: 10.1089/ars.2019.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manzano-Pech L., Guarner-Lans V., Soto M.E., Díaz-Díaz E., Caballero-Chacón S., Díaz-Torres R., Rodríguez-Fierros F.L., Pérez-Torres I. Excessive consumption hibiscus sabdariffa L. Increases inflammation and blood pressure in male wistar rats via high antioxidant capacity: the preliminary findings. Cells. 2022;11:2774. doi: 10.3390/cells11182774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pérez-Torres I., Ruiz-Ramírez A., Baños G., El-Hafidi M. Hibiscus sabdariffa Linnaeus (Malvaceae), curcumin and resveratrol as alternative medicinal agents against metabolic syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2013;11:25–37. doi: 10.2174/1871525711311010006. [DOI] [PubMed] [Google Scholar]
- 45.Wolinsly H. Long-term effects of hypertension on the rat aortic wall and their relation to concurrent aging changes. Morphological and chemical studies. Circ. Res. 1972;30:301–309. doi: 10.1161/01.res.30.3.301. [DOI] [PubMed] [Google Scholar]
- 46.Tsutsum Y., Matsubara H., Masaki H., Kurihara H., Murasawa S., Takai S., Miyazaki M.Y., Nozawa Y., Ozono R., Nakagawa K., Miwa T., Kawada N., Mori Y., Shibasaki Y., Tanaka Y., Fujiyama S., Koyama Y., Fujiyama A., Takahashi H., Iwasaka T. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semsri S., Seatew C., Rattanabunyong S., Ruekit S., Horata N., Panya A., Yenchitsomanus P.T., Sawatdichaikul O., Choowongkomon K.T. In-vitro studies of anti-EGFR tyrosine kinase activity of Thai nutraceutical plants. Iran. J. Pharm. Res. 2020;19:199–206. doi: 10.22037/ijpr.2017.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bretón R., Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–534. doi: 10.1016/j.redox.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Touyz R., Rios F.J., Alves-Lopes R., Neves K.B., Camargo L.L., Montezano A.C. Oxidative stress: a unifying paradigm in hypertension. Can. J. Cardiol. 2020;6:659–670. doi: 10.1016/j.cjca.2020.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Torres I., Manzano-Pech, Rubio-Ruíz L., Soto M., Guarner-Lans M.E. V. Nitrosative stress and its association with cardiometabolic disorders. Molecules. 2020;25:2555. doi: 10.3390/molecules25112555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Handy D., Joseph J., Loscalzo J. Selenium, a micronutrient that modulates cardiovascular health via redox enzymology. Nutrients. 2021;13:1–22. doi: 10.3390/nu13093238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leopold J., Zhang Y., Scribner A., Stanton R.C., Loscalzo J. Glucose-6-Phosphate Dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler. Thromb. Vasc. Biol. 2003;23:411–417. doi: 10.1161/01.ATV.0000056744.26901.BA. [DOI] [PubMed] [Google Scholar]
- 53.Peiró C., Romacho T., Azcutia V., Villalobos L., Fernández E., Bolaños J.P., Moncada S., Sánchez-Ferrer C.F. Inflammation, glucose, and vascular cell damage: the role of the pentose phosphate pathway. Cardiovasc. Diabetol. 2016;15:82. doi: 10.1186/s12933-016-0397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ajiboye T., Raji H.O., Adeleye A.O., Adigun N.S., Giwa O.B., Ojewuyi O.B., Oladiji A.T. Hibiscus sabdariffa calyx palliates insulin resistance, hyperglycemia, dyslipidemia and oxidative rout in fructose-induced metabolic syndrome rats. J. Sci. Food Agric. 2016;96:1522–1531. doi: 10.1002/jsfa.7254. [DOI] [PubMed] [Google Scholar]
- 55.Hariharan S., Dharmaraj S. Selenium and selenoproteins: it's role in regulation of inflammation. Inflammopharmacology. 2020;28:1–29. doi: 10.1007/s10787-020-00690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White P. Selenium accumulation by plants. Ann. Bot. 2016;117:217–235. doi: 10.1093/aob/mcv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K., Jeong D. Biomodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: the selenium paradox. Mol. Med. Rep. 2012;5:299–304. doi: 10.3892/mmr.2011.651. submitted for publication. [DOI] [PubMed] [Google Scholar]
- 58.Terada A., Yoshida M., Seko Y., Kobayashi T., Yoshida K., Nakada M., Nakada K., Echizen H., Ogata H., Rikihisa T. Active oxygen species generation and cellular damage by additives of parenteral preparations: selenium and sulfhydril compounds. Nutrition. 1999;15:651–655. doi: 10.1016/s0899-9007(99)00119-7. [DOI] [PubMed] [Google Scholar]
- 59.Bourgonje M.F., Bourgonje A.R., Abdulle A.E., Kieneker L.M., Gemert S., Gansevoort R.T., Bakker S.J.L., Mulder D.J., Pasch A., Saleh J., Gordijn S.J., van Goor H. Systemic oxidative stress, aging and the risk of cardiovascular events in the general female population. Front. Cardiovasc. Med. 2021;9 doi: 10.3389/fcvm.2021.630543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed S.H., Kharroubi W., Kaoubaa N., Zarrouk A., Batbout F., Gamra H., Najjar M.F., Lizard G., Hininger-Favier I., Mohamed H.M. Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis. 2018;17:52. doi: 10.1186/s12944-018-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu J., Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H., Luo Y., Zhang E., He Y., Dai S., Zhang R., Huang Y., Bernatchez P., Giordano F.J., Shadel G., Sessa W.C., Min W. Endothelial- specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am. J. Pathol. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirsch J., chneider S.H., Pagel J.I., Rehberg M., Singer M., Hellfritsch J., Chillo O., Schubert K.M., Qiu J., Pogoda K., Kameritsch P., Uhl B., Pircher J., Deindl E., Müller S., Kirchner T., Pohl U., Conrad M., Beck H. Endothelial dysfunction, and a prothrombotic, proinflammatory phenotype is caused by loss of mitochondrial thioredoxin reductase in endothelium. Arterioscler. Thromb. Vasc. Biol. 2016;36:1891–1899. doi: 10.1161/ATVBAHA.116.307843. [DOI] [PubMed] [Google Scholar]
- 64.Kameritsch P., Singer M., Nuernbergk C., Rios N., Reyes A.M., Schmidt K., Kirsch J., Schneider H., Müller S., Pogoda K., Cui R., Kirchner T., de Wit C., Lange-Sperandio B., Pohl U., Conrad M., Radi R., Beck H. The mitochondrial thioredoxin reductase system (TrxR2) in vascular endothelium controls peroxynitrite levels and tissue integrity. Proc. Natl. Acad. Sci. U.S.A. 2021;118 doi: 10.1073/pnas.1921828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Handy D.E., Lubos E., Yang Y., Galbraith J.D., Kelly N., Zhang Y.Y., Leopold J.A., Loscalzo J. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J. Biol. Chem. 2009;284:11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krajka-Kuzniak V., Szaefer H., Baer-Dubowska W. Hepatic and extrahepatic expression of glutathione S-transferase isozymes in mice and its modulation by naturally occurring phenolic acids. Environ. Toxicol. Pharmacol. 2008;25:27–32. doi: 10.1016/j.etap.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Hayeshi R., Mutingwende I., Mavengere W., Masiyanise V., Mukanganyama S. The inhibition of human glutathione S-transferases activity by plant polyphenolic compounds ellagic acid and curcumin. Food Chem. Toxicol. 2007;45:286–295. doi: 10.1016/j.fct.2006.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.