Abstract
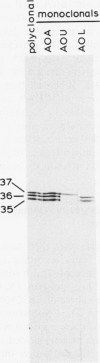
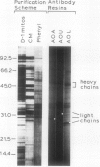
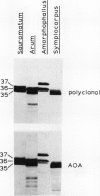
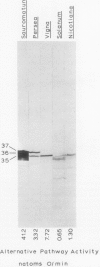
The higher plant mitochondrial electron transport chain contains, in addition to the cytochrome chain which terminates with cytochrome oxidase, an alternative pathway that terminates with an alternative oxidase. The alternative oxidase of Sauromatum guttatum Schott has recently been identified as a cluster of proteins with apparent Mr of 37, 36, and 35 kilodaltons (kD). Monoclonal antibodies have now been prepared to these proteins and designated as AOA (binding all three proteins of the alternative oxidase cluster), AOU (binding the upper or 37 kD protein), and AOL (binding the lower or 36 and 35 kD proteins). All three antibodies bind to their respective alternative oxidase proteins whether the proteins are in their native or denatured states (as on protein blots). AOA and AOU inhibit alternative oxidase activity around 49%, whereas AOL inhibits activity only 14%. When coupled individually to Sepharose 4B, all three monoclonal resins were capable of retaining the entire cluster of alternative oxidase proteins, suggesting that these proteins are physically associated in some manner. The monoclonals were capable of binding similar mitochondrial proteins in a number of thermogenic and nonthermogenic species, indicating that they will be useful in characterizing and purifying the alternative oxidase of different systems. The ability of the monoclonal-Sepharose 4B resins to retain the cluster of previously identified alternative oxidase proteins, along with the inhibition of alternative oxidase activity by these monoclonals, supports the role of these proteins in constituting the alternative oxidase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elthon T. E., McIntosh L. Characterization and Solubilization of the Alternative Oxidase of Sauromatum guttatum Mitochondria. Plant Physiol. 1986 Sep;82(1):1–6. doi: 10.1104/pp.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Identification of the alternative terminal oxidase of higher plant mitochondria. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8399–8403. doi: 10.1073/pnas.84.23.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Stewart C. R., McCoy C. A., Bonner W. D. Alternative Respiratory Path Capacity in Plant Mitochondria: Effect of Growth Temperature, the Electrochemical Gradient, and Assay pH. Plant Physiol. 1986 Feb;80(2):378–383. doi: 10.1104/pp.80.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Liang H. G., Lü C. S. A Comparative Study of CN-Resistant Respiration in Different Cultures of Tobacco Callus. Plant Physiol. 1984 Jul;75(3):876–878. doi: 10.1104/pp.75.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Márton L. Streptomycin-resistant plants from callus culture of haploid tobacco. Nat New Biol. 1973 Jul 4;244(131):29–30. doi: 10.1038/newbio244029a0. [DOI] [PubMed] [Google Scholar]
- Raskin I., Ehmann A., Melander W. R., Meeuse B. J. Salicylic Acid: a natural inducer of heat production in arum lilies. Science. 1987 Sep 25;237(4822):1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Schwitzguebel J. P., Siegenthaler P. A. Purification of peroxisomes and mitochondria from spinach leaf by percoll gradient centrifugation. Plant Physiol. 1984 Jul;75(3):670–674. doi: 10.1104/pp.75.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanker L. H., Vanderlaan M., Juarez-Salinas H. One-step purification of mouse monoclonal antibodies from ascites fluid by hydroxylapatite chromatography. J Immunol Methods. 1985 Jan 21;76(1):157–169. doi: 10.1016/0022-1759(85)90488-0. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]