Abstract
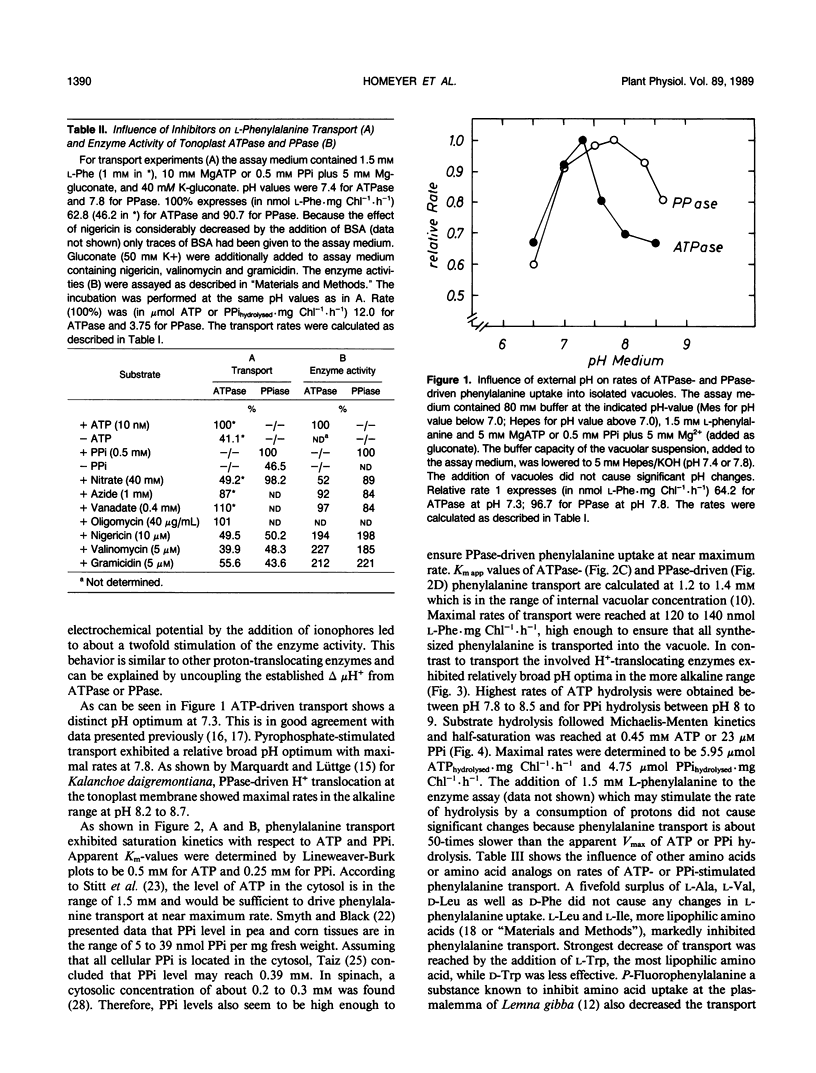
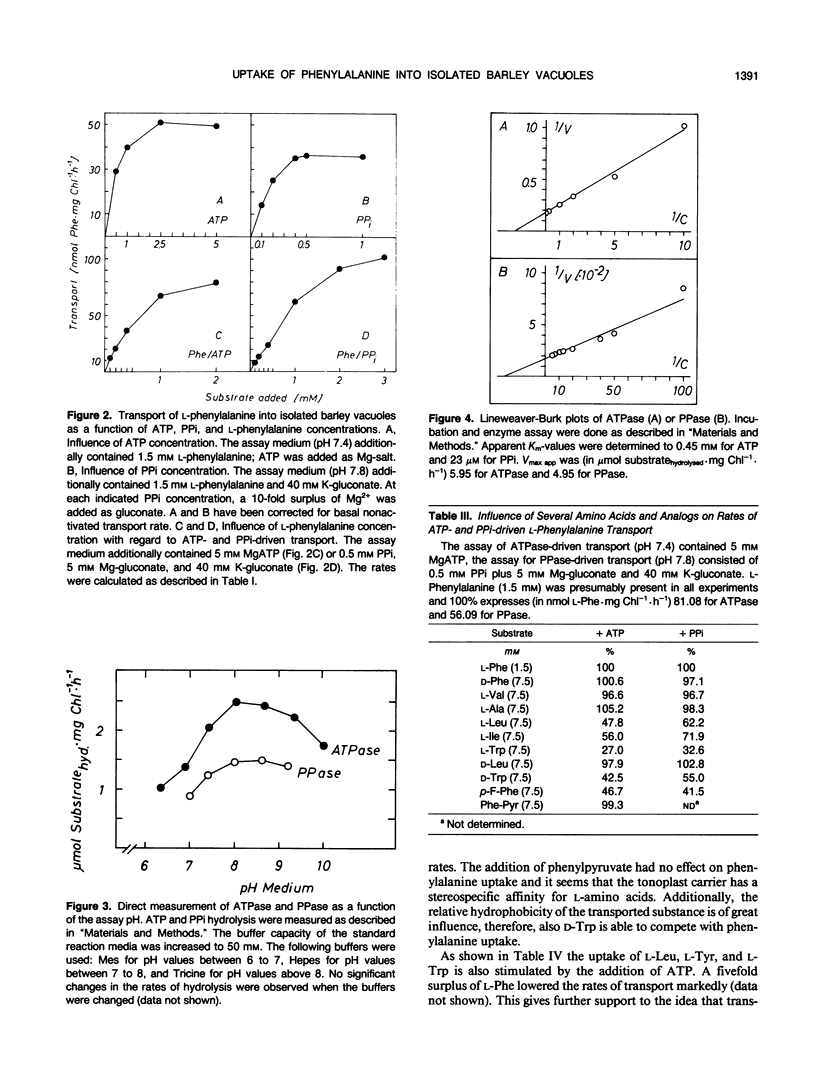
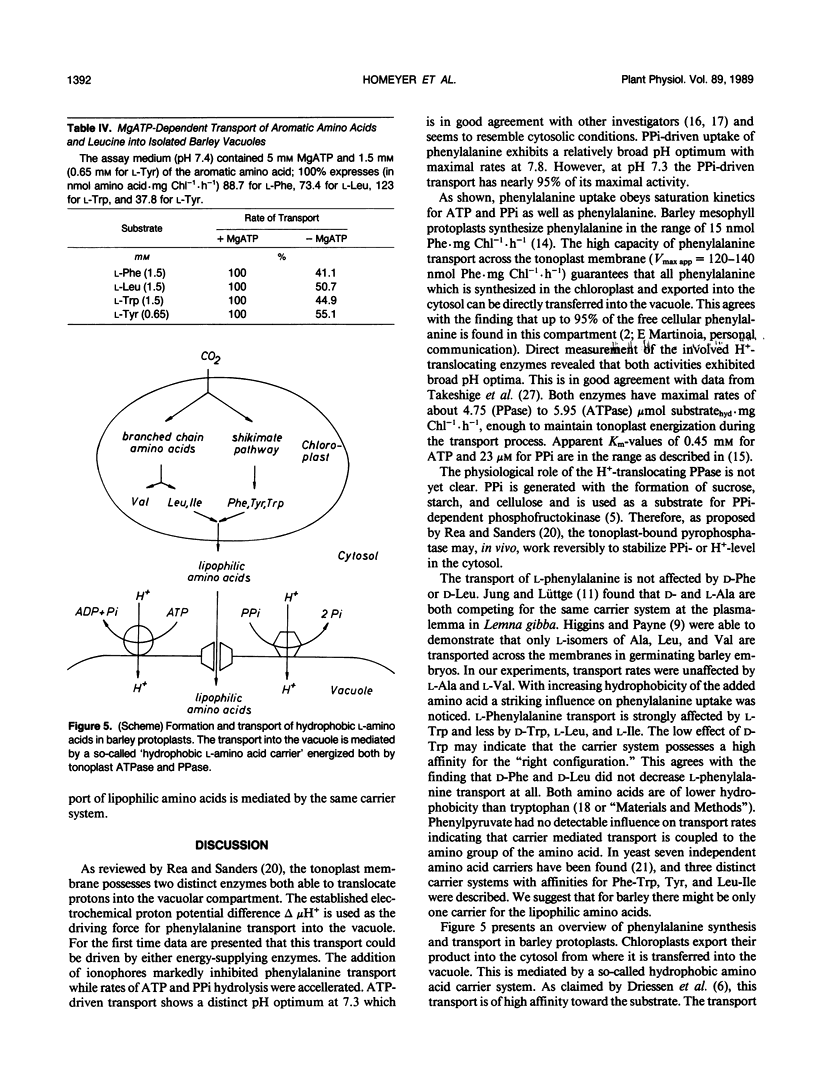
The uptake of phenylalanine was studied with vacuole isolated from barley mesophyll protoplasts. The phenylalanine transport exhibited saturation kinetics with apparent Km-values of 1.2 to 1.4 millimolar for ATP- or PPi-driven uptake; Vmax app was 120 to 140 nanomoles Phe per milligram of chlorophyll per hour (1 milligram of chlorophyll corresponds to 5 × 106 vacuoles). Half-maximal transport rates driven with ATP or PPi were reached at 0.5 millimolar ATP or 0.25 millimolar PPi. ATP-driven transport showed a distinct pH optimum at 7.3 while PPi-driven transport reached maximum rates at pH 7.8. Direct measurement of the H+-translocating enzyme activities revealed Km app values of 0.45 millimolar for ATPase (EC 3.6.1.3) and 23 micromolar for pyrophosphatase (PPase) (EC 3.6.1.1). In contrast to the coupled amino acid transport, ATPase and PPase activities had relative broad pH optima between 7 to 8 for ATPase and 8 to 9 for PPase. ATPase as well as ATP-driven transport was markedly inhibited by nitrate while PPase and PPi-coupled transport was not affected. The addition of ionophores inhibited phenylalanine transport suggesting the destruction of the electrochemical proton potential difference Δ μH+ while the rate of ATP and PPi hydrolysis was stimulated. The uptake of other lipophilic amino acids like l-Trp, l-Leu, and l-Tyr was also stimulated by ATP. They seem to compete for the same carrier system. l-Ala, l-Val, d-Phe, and d-Leu did not influence phenylalanine transport suggesting a stereospecificity of the carrier system for l-amino acids having a relatively high hydrophobicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami S., Hara-Nishimura I., Nishimura M., Akazawa T. Translocation of photosynthates into vacuoles in spinach leaf protoplasts. Plant Physiol. 1985 Apr;77(4):963–968. doi: 10.1104/pp.77.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Hellingwerf K. J., Konings W. N. Mechanism of energy coupling to entry and exit of neutral and branched chain amino acids in membrane vesicles of Streptococcus cremoris. J Biol Chem. 1987 Sep 15;262(26):12438–12443. [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Chromatographic resolution of h-translocating pyrophosphatase from h-translocating ATPase of higher plant tonoplast. Plant Physiol. 1986 May;81(1):126–129. doi: 10.1104/pp.81.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984 Sep 25;259(18):11505–11508. [PubMed] [Google Scholar]
- Smyth D. A., Black C. C. Measurement of the pyrophosphate content of plant tissues. Plant Physiol. 1984 Jul;75(3):862–864. doi: 10.1104/pp.75.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Lilley R. M., Heldt H. W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982 Oct;70(4):971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K., Tazawa M., Hager A. Characterization of the H Translocating Adenosine Triphosphatase and Pyrophosphatase of Vacuolar Membranes Isolated by Means of a Perfusion Technique from Chara corallina. Plant Physiol. 1988 Apr;86(4):1168–1173. doi: 10.1104/pp.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]


