Abstract
Perceptual filters formed early in development provide an initial means of parsing the incoming auditory stream. However, these filters may not remain fixed, and may be updated by subsequent auditory input, such that, even in an adult organism, the auditory system undergoes plastic changes to achieve a more efficient representation of the recent auditory environment. Songbirds are an excellent model system for experimental studies of auditory phenomena due to many parallels between song learning in birds and language acquisition in humans. In the present study, we explored the effects of passive immersion in a novel heterospecific auditory environment on neural responses in NCM, a songbird auditory area similar to secondary auditory cortex in mammals. In zebra finches, a well-studied species of songbirds, NCM responds selectively to conspecific songs and contains a neuronal memory for tutor and other familiar conspecific songs. Adult male zebra finches were randomly assigned to either a conspecific or heterospecific auditory environment. After 2, 4 or 9 days of exposure, subjects were presented with heterospecific and conspecific songs during awake electrophysiological recording. The neural response strength and rate of adaptation to the testing stimuli were recorded bilaterally. Controls exposed to conspecific environment sounds exhibited the normal pattern of hemispheric lateralization with higher absolute response strength and faster adaptation in the right hemisphere. The pattern of lateralization was fully reversed in birds exposed to heterospecific environment for 4 or 9 days and partially reversed in birds exposed to heterospecific environment for 2 days. Our results show that brief passive exposure to a novel category of sounds was sufficient to induce a gradual reorganization of the left and right secondary auditory cortices. These changes may reflect modification of perceptual filters to form a more efficient representation of auditory space.
INTRODUCTION
Over the course of a lifetime, an individual is exposed to a wide and varying range of acoustic stimuli. Although the peripheral auditory system initially parses sounds into their constituent frequencies and phase, evidence is increasing that the auditory cortex, and perhaps earlier stations, can represent more complex sound features (Woolley et al., 2009; Woolley and Casseday 2004; Schreiner 1995; O’Connor et al., 2010). These representations are established during development (Miller and Knudsen, 2001; Bao et al., 2013; Amin et al, 2013), but they are not fixed (Edeline et al., 1993; David et al., 2012; Fritz et al., 2007; Gentner et al., 2004; Meliza 2011; Cheung et al., 2005; Gentner and Margoliash, 2003). As new sounds are experienced, the cortex may adapt dynamically in order to represent the statistics of the current acoustic environment in an efficient manner (Nelken 2008; Scheich et al., 2007; Sharpee et al., 2011). In many vertebrates, early exposure to a set of species-specific sounds shapes perceptual filters to respond preferentially to these highly relevant communication signals (Suga et al., 1997; Maier and Scheich,1987; Portfors et al., 2009; Ehret 1987; Belin et al., 2000; Amin et al., 2013). In some species that are capable of learning a complex set of vocal communication signals, both auditory perception and vocal production of these salient sounds have been shown to be lateralized, e.g. for native language in humans and song in songbirds (Dronkers et al., 2007; Wernicke,1911; Springer et al., 1999; Nottebohm et al., 1976; Floody and Arnold, 1997; Wild et al., 2000; Voss et al., 2007; Poirier et al., 2009; Phan and Vicario, 2010; Moorman et al., 2012; Ocklenburg et al, 2013). This raises the question of how adaptive changes in acoustic representations induced by experience with new sounds in adulthood interact with pre-existing patterns of lateralized processing.
In humans, the acoustic categories of native vs. non-native language are established by the first year of age and, in adulthood, robust lateralization is seen only for the native language (Doupe and Kuhl, 1999; Kovelman et al., 2011; Hickok and Poeppel, 2007). Similarly, in songbirds, lateralized processing is dependent on normal auditory exposure during an early period, and is not seen for simple sounds (Phan et al., 2010). Although this groundwork for recognition and categorization of sounds is laid down during the juvenile period, the auditory cortex retains a level of plasticity into adulthood (Recanzone et al., 1993, Bao et al., 2004); this could contribute to the ability to master a new language or to refine pitch perception in musicians. When the acoustic environment changes, e.g., when an English-speaking adult is immersed in a foreign language environment containing new contrasts and categories not encountered in the native language, the auditory cortex may change to represent the new auditory information. The challenge of changing acoustic statistics could drive a process of neural reorganization that affects the existing pattern of lateralization if the modification of perceptual filters to represent new sound categories occurs separately in left and right hemispheres. In the present study, we tested this scenario by immersing adult songbirds in a novel acoustic environment (the vocalizations of a different species) and then assessing changes in auditory responses in the two brain hemispheres.
The many parallels between songbird vocal learning and human speech acquisition (Doupe and Kuhl 1999), including, most importantly, hemispheric lateralization for both motor and auditory processing (Nottebohm et al., 1976; Williams et al. 1992; Cynx et al., 1992; Floody and Arnold, 1997; Wild et al., 2000; Voss et al., 2007; Poirier et al., 2009; Phan and Vicario, 2010), make the songbird the best model system for addressing this question with invasive physiological techniques. In addition, recent anatomical evidence suggests close analogies, if not homologies, between the thalamo-cortical auditory system of birds and mammals (Wang et al., 2010; Bolhuis et al., 2010; Haesler et al., 2004; Dugas-Ford et al, 2012; Karten, 2013; Jarvis et. al., 2013), though the homology hypothesis is not undisputed (Montiel and Molnár, 2013). This study focuses on neural responses in the caudo-medial neostriatum (NCM) of the zebra finch, a forebrain area that is analogous to superficial layers of mammalian A1 or perhaps a secondary auditory cortex (Wang et al., 2010; Theunissen et al., 2000). Figure 1 illustrates the circuitry of the birdsong auditory system used in sound perception (Bolhuis et al., 2012). NCM neurons respond selectively to vocalizations with acoustic features typical of their own species and undergo stimulus-specific adaptation to the unique songs of individual conspecifics, which represents a form of long-lasting neuronal memory (Chew et al, 1995; Stripling et al., 2001; Phan et al., 2006). Lateralization of auditory processing in NCM has been demonstrated in both electrophysiological and IEG studies (Phan and Vicario, 2010; Bolhuis et al., 2012). Neural responses to song are stronger and adapt faster in the right NCM hemisphere than in the left; this lateralization depends on early auditory experience (Phan and Vicario, 2010). These properties make songbird NCM an excellent place to study the interaction of novel auditory experience with lateralization for complex vocal sounds.
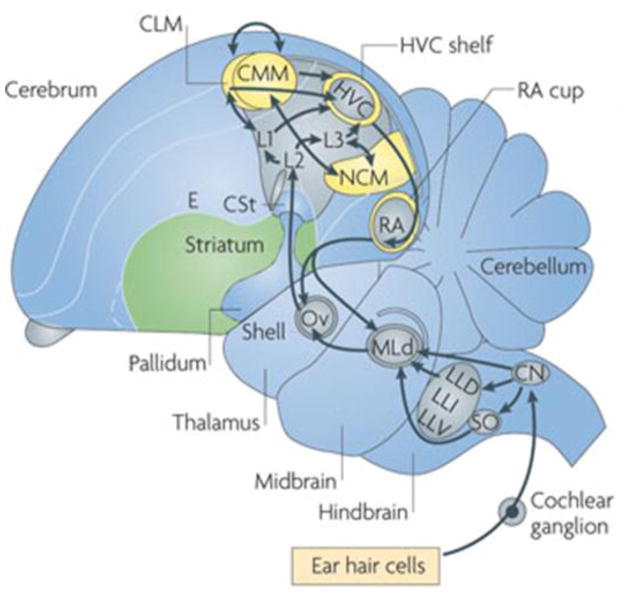
Figure 1.
Avian auditory system. Ascending auditory inputs travel from hair cells to nucleus mesencephalicus lateralis pars dorsalis (MLD; avian homolog of mammalian central nucleus of the inferior colliculus), through auditory thalamus, nucleus Ovoidalis (Ov; avian homolog of mammalian medial geniculate nucleus). Ov then projects to forebrain region Field L2, thought to be homologous to thalamo-recipient layers III–IV of mammalian cortex (Wang et al., 2010; Dugas-Ford et al., 2012). Field L2 sends projections to adjacent areas, Field L1 and L3, which in turn project to the caudo-medial nidopallium (NCM) and caudal mesopallium (CLM and CMM). Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Neuroscience, Bolhuis et al., 2010.
METHODS
Animals
Adult male zebra finches (>120 days of age) were obtained from the zebra finch breeding aviary at Rutgers University, New Brunswick, NJ or purchased from a local supplier. At the start of the experiment, subjects were removed from the general aviary and individually housed in soundproof isolation boxes for 9, 4, or 2 consecutive days. A separate group of birds housed in the general aviary served as aviary controls (AV), with exposure to the general acoustics of the aviary. All subjects were maintained on a 12/12 light cycle with ad libitum access to food and water.
Environment
Subjects were randomly assigned to groups and exposed to either a control conspecific environment (9d and 4d CONENV) or an experimental heterospecific environment (2d, 4d, and 9d HETENV). During lights-on, individually housed birds received continuous 12h playback of either zebra finch (CONENV) or canary (HETENV) aviary sounds. Playback environments were recorded from zebra finch and canary aviaries at Rockefeller University, Millbrook, NY to ensure that all acoustic environments were novel at the start of the experiment. Exposure to canary songs and calls in the HETENV group, simulated a ‘foreign’ acoustic environment, to replicate acoustic experience of zebra finches in the “cross housed” condition described in Terleph et al, 2008. Exposure of the CONENV group to zebra finch songs and calls represented the ‘native’ acoustic environment. HETENV stimuli contain relatively more energy at higher frequencies and longer durations than CONENV stimuli (Figure 2A). The sounds presented in HETENV contained acoustic features typical of canary vocalizations such as rapid trills and high pitched whistles with long durations and many repetitions. In contrast, sounds presented in CONENV contained acoustic features commonly found in zebra finch vocalizations such as broadband harmonic stacks, fast frequency modulations, and shorter syllables (Figure 2B).
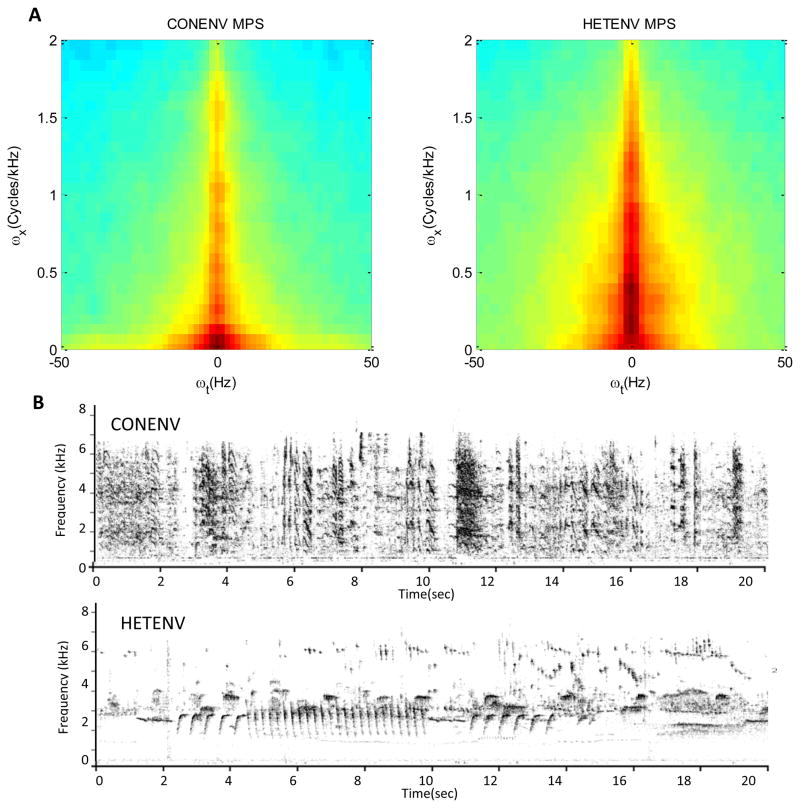
Figure 2.
A) Modulation Power Spectra (MPS) generated from zebra finch (CONENV) and canary (HETENV) playback tapes show the temporal and spectral modulations of vocalizations recorded in the two species environments. The X axis represents the temporal modulation as oscillations of power over time. The Y axis represents the spectral modulation as oscillations of power over a short-interval frequency spectrum. HETENV stimuli contain relatively more energy at higher frequencies and longer durations than CONENV stimuli. B) Spectrograms of CONENV and HETENV from a representative 20s sample of each environment.
Vocalizations in the two auditory environments occupied distinct, but partially overlapping, sectors of acoustic space, such that CONENV and HETENV present two separate combinations of sounds, both of which lie within the perceptual range of the zebra finch (Okanoya and Dooling, 1987). Sound intensities for the two playback environments were intentionally matched to each other and to sound intensity in the general zebra finch aviary and were well below the threshold for causing peripheral damage (Rubel and Ryals, 1982). Playback amplitude at the end of the cage closest to the speaker averaged 67dB SPL (A-scale) with occasional brief peaks not exceeding 74dB SPL (A-scale, fast) in both the CONENV and HETENV. All procedures conformed to a protocol approved by the Animal Care and Use Committee at Rutgers University.
Electrophysiology
Two days prior to testing, subjects were deeply anesthetized with 1.5–2.0% isoflurane in oxygen and placed in a stereotaxic apparatus. A metal pin was attached to the skull with dental cement and a craniotomy was performed on the skull above NCM in preparation for awake, head-fixed electrophysiological recording. Post-surgery, subjects were observed for recovery from the short acting anesthetic (Robertson et al, 2000). After full recovery (approximately 30min), subjects were returned to their respective acoustic environments for continued exposure to sound playback. 48hpost-surgery, subjects were removed from their isolation boxes and placed in a soundproof booth (IAC Inc., Bronx, NY) for electrophysiological recording. Subjects were restrained in a custom made plastic tube and head-fixed to the stereotaxic apparatus using the previously implanted head pin. A multi-electrode microdrive (Thomas Recording, Giessen, Germany) was used to place16 tungsten micro-electrodes (Type ESI2ec, impedance: 2–4 M ohm, Thomas Recording) in NCM bilaterally (8 in each hemisphere). Coordinates used for placement of micro electrodes within the boundaries of NCM were 0.5–1.5mm rostral and 0–1 mm lateral to the bifurcation of the mid-sagittal sinus (the zero point for songbird stereotaxis). Each electrode was lowered into the brain while white noise stimuli with the amplitude envelope of canary song were played to identify the first responsive site along the track. Once responsive sites were located on all electrodes, playback of the testing stimuli commenced. Multi-unit recordings of neural spike activity were taken simultaneously from all electrodes using Spike 2 software (CED, Cambridge, England). Recorded activity was amplified (x19,000) and band-pass filtered from 0.5–5kHz.
Testing Stimuli
Auditory stimuli at each group of simultaneously recorded sites consisted of a set of 5 novel zebra finch (zfstim) and 5 novel canary songs (canstim) that had never been heard in the playback environments. In AV controls, auditory stimuli at each group of sites consisted of alternating sets of either 5 novel zebra finch or 5 novel canary songs. Each song was presented for 25 repetitions as part of a shuffled set (duration: 800–1500ms; sampling rate: 40 KHz; 8s ISI). All song stimuli were played back at 70dB through a speaker centered 30cm in front of the subject. Presentation of each set of song stimuli was followed by a set of pure tone stimuli to obtain tuning curves and best frequencies for each recording site. Tone stimuli ranged from 500Hz to 5000Hz at 250Hz increments (20 stimuli; duration: 260ms; sampling rate: 40 KHz; 3 repetitions in shuffled order; 6s ISI). After presentation of both song and tone stimuli, all electrodes were lowered 300μm along the dorsal-ventral axis and a new set of song stimuli was played, followed by the tone set. This was repeated until all 4 sets of novel songs had been played at their respective depths in NCM with the exception of the 9D experimental groups. In the 9D CONENV and 9D HETENV birds, testing order was randomly decoupled from dorsal-ventral depth in different birds (for example, the first site tested could be at the deepest point in the penetration) in order to control for a possible interaction between depth and time in the recording situation. There was no effect of testing order on ARMs (F(3, 338)=0.7228, P =.5389, n.s.), so time was dropped as a factor from our analyses. Upon completion of testing, 3 small electrolytic lesions (10μA for 10s) were made at the final recording depth, in each hemisphere, for histological verification that recording sites were within the boundaries of NCM.
Histology
Three days post-test, subjects were deeply anesthetized with Nembutal and transcardially perfused with saline and 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde. Sagittal sections (50μm) were taken through left and right NCM on a Vibratome, stained with cresyl violet and visualized under light microscopy to identify lesioned recording sites (Figure 3). Sites determined to be outside of the histological boundaries of NCM were excluded. In addition, only those sites in NCM that showed adaptation profiles (see Data Analysis, below) characteristic of NCM neurons were included in the analyses. The vast majority of neurons and sites in NCM are known to exhibit stimulus specific adaptation, while those in the adjacent primary auditory area, Field L, do not (Chew et al, 1995). Thus, we eliminated recording sites with that did not meet the adaptation criterion (rate < -0.05) established in earlier work (Phan et al, 2006). Across the different groups, 7–10% of sites were excluded based on this criterion.
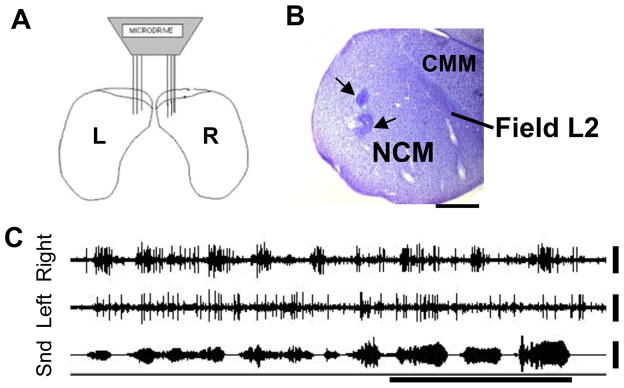
Figure 3.
NCM anatomy and electrophysiology. A) Schematic in coronal view of bilateral electrode placement in NCM. B) Sagittal section of NCM stained with cresyl violet. Black arrows indicate location of 2 electrolytic lesions made in NCM at the conclusion of recording. Scale bar: 1mm. Labels show locations of NCM, CMM and the primary auditory area, Field L2. C) Raw electrophysiological recording from electrodes placed in left and right NCM showing bilateral responses to conspecific song. X-axis time bar: 0.5s. Y-axis scale bar: 25μV
Data Analysis
Neural recordings were quantified using absolute response magnitude and adaptation rate. Both these measures have been shown to differ in a lateralized manner in NCM (Phan and Vicario 2010).
Absolute Response Magnitude (ARM) measures the strength of neural responses to auditory stimuli, and is calculated as follows: the root mean square (rms) of neural activity during the control period (500ms prior to stimulus onset) was subtracted from the rms of the stimulus-on period (stimulus duration plus 100ms), following established methods in the laboratory (Phan et al, 2006; Phan and Vicario, 2010). ARMs were averaged for trials 2–6 of each song stimulus and each stimulus category (zfstim or canstim) to yield a response strength for conspecific songs and a response strength for heterospecific songs for each recording site to be used in data analysis. The above analysis was repeated for ARMs of trials 6–25 to determine the response strengths to later stimulus presentations.
Adaptation Rate measures the normalized rate of decrease in response size to a repeated stimulus (Chew et al, 1995; Phan et al., 2006). Adaptation rate is calculated as the slope of the regression line over repeated presentations for trials 6–25 with a given stimulus divided by the average ARM of those trials. Dividing by the average ARM normalizes for differences in absolute response strength between recording sites. Thus, adaptation rate is the percentage drop in response amplitude per stimulus repetition at each recording site. Repeated novel stimuli elicit robust responses which rapidly adapt, while responses to familiar stimuli are already adapted and only undergo minimal additional adaptation. Thus, high adaptation rates signify novelty, while low rates indicate familiarity for a stimulus - a kind of long-lasting neuronal memory (Chew et al, 1996).
Effects of auditory environment on ARM and adaptation rate were quantified using repeated measures ANCOVA for main effects and interactions of auditory environment (CONENV, HETENV) and hemisphere (left, right) with stimulus category (zfstim, canstim) as the repeated measure. We noted that ARMs for both trials 2–6 and trials 6–25 both decreased with depth along the dorsal-ventral axis; therefore, our analyses included depth as a covariate. Bonferroni post-hocs were performed as appropriate to determine significant effects.
Tuning
To quantify the ARM of a given site to tone stimuli, the control rms (500ms prior to stimulus onset) was subtracted from the phasic response (the 10–60ms interval after stimulus onset) and averaged across all 3 repeats of each stimulus frequency per recording site. Best frequency and tuning width per recording site were calculated using an established algorithm (Terleph et al., 2008). Tuning width is defined as the contiguous frequency range over which phasic responses were at least two standard deviations above baseline. A separate ANOVA was performed on tuning ARM, width and best frequency for main effects and interactions between auditory environment, hemisphere and depth. Bonferroni post-hocs were once again performed to determine significant effects.
RESULTS
Changes in Hemispheric Lateralization of Absolute Response Magnitude
In zebra finches (n=12) with 9d auditory exposure to CONENV (conspecific zebra finch aviary sounds), mean ARMs during trials 2–6 were higher in the right hemisphere than the left to both zfstim and canstim (ANOVA for left vs. right hemisphere with stimulus type at each site as a repeated measure; Hemisphere F(1, 179)= 5.58, P < 0.019; Figure 4A, Top). These results are consistent with previous findings (Phan and Vicario, 2010). Also, there was a clear response bias for conspecific song over heterospecific song (ZF vs. CAN stimuli, F(1, 179)=29.422, P <0.00001), as previously shown (Chew et al., 1995). Furthermore, there was a strong trend towards an interaction between hemisphere and stimulus type (F(1, 179)=3.8615, P =0.051, n.s.), suggesting a greater degree of lateralization when tested with conspecific stimuli than with heterospecific stimuli. In the same group, ARMs for later trials 6–25 showed a similar pattern to the early trials 2–6. Again, the right hemisphere responded at a higher level than the left (F(1, 179)=5.8544, P<0.017) and the conspecific bias was maintained F(1, 179)=40.269, P<0.00001). However, the interaction between hemisphere and stimulus type is weaker (F(1,179)=3.2022, P=0.0753, n.s.). Both hemispheres respond more to zebra finch than to canary song.
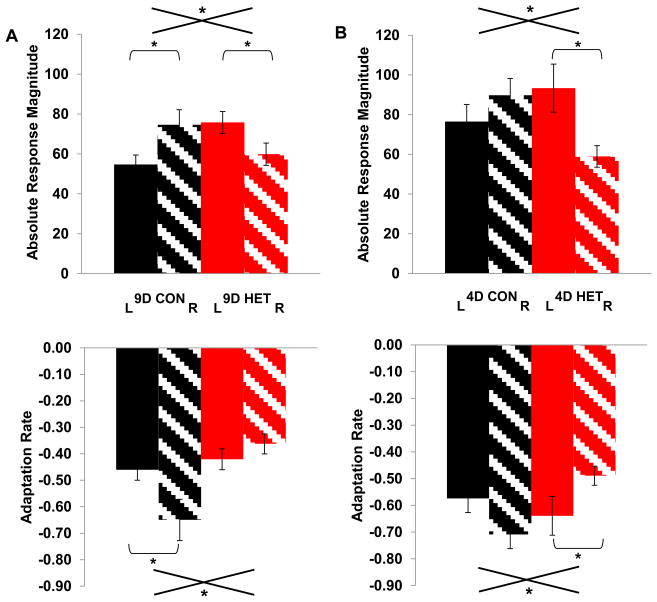
Figure 4.
A) Effects of 9d auditory exposure on neural responses. Top: Absolute Response Magnitudes (ARMs) for trials 2–6 in the left (solid bar) and right (striped bar) hemispheres for birds that spent 9d in either CONENV (black) or HETENV (red). CONENV birds had higher ARMs in the right hemisphere while HETENV birds showed higher ARMs in the left hemisphere, yielding a significant environment by hemisphere interaction (F(1,369)=8.5064, P<0.0038). Bottom: Adaptation Rates for trials 6–25. CONENV birds had faster adaptation in the right hemisphere while HETENV birds did not have a hemispheric difference in adaptation rates, once again showing a significant environment by hemisphere interaction (F(1,369)=5.5079, P<0.0195). B) Effects of 4d auditory exposure on neural responses. Top: ARMs trials 2–6 in the left and right hemispheres for birds that spent 4d in either CONENV or HETENV. CONENV birds had slightly higher ARMs in the right hemisphere while HETENV birds had higher ARMs in the left hemisphere, yielding a significant environment by hemisphere interaction (F(1,258)=8.2314, P<0.0045). Bottom: Adaptation Rates for trials 6–25. CONENV birds had faster adaptation rates in the right hemisphere while HETENV birds had faster adaptation rates in the left hemisphere, leading to a significant environment by hemisphere interaction (F(1, 258)=8.0441, P<0.0049). Error bars represent standard error of the mean (SEM). Asterisks denote significant main effects and interactions.
In zebra finches (n=13) with 9d auditory exposure to HETENV (heterospecific canary aviary sounds), mean ARMs during trials 2–6 were higher in the left hemisphere than the right (ANOVA F(1, 191)=4.5665, P <0.034; Figure 4A, Top), a striking reversal of the pattern of lateralization seen in zebra finches with conspecific auditory exposure. However, there was no change in the conspecific bias in these birds who heard heterospecific sounds for 9d: zebra finch songs still evoked a significantly higher response than canary songs (F(1, 191)=27.253, P<0.00001). Furthermore, there was no interaction between hemisphere and stimulus type (F(1, 191)= 2.4787, P=0.117, n.s.); and zebra finch songs evoke significantly higher responses than canary songs in both hemispheres. For trials 6–25, mean ARMs in the left hemisphere were also higher than in the right, but not significantly (F(1, 191)=1.9174, P=0.168, n.s.). The response bias for conspecific song remained (F(1, 191)=41.179, P<0.00001) and there was no significant interaction between hemisphere and stimulus type (F(1, 191)=0.2315, P=0.631, n.s.).
Data for birds exposed for 9d to CONENV and HETENV were combined in an overall model ANCOVA where ARMs for trials 2–6 were analyzed for main effects of environment and hemisphere, with stimulus type as the repeated measure and depth as the covariate. There were no main effects of environment or hemisphere, but there was a significant interaction of environment by hemisphere (ANCOVA: F(1, 369)=8.5064, P<0.0038), demonstrating a significant difference in the pattern of lateralization between the two exposure conditions. CONENV birds showed the expected pattern of lateralization, with the right hemisphere responding more robustly than the left (Phan and Vicario, 2010), but this was reversed in HETENV birds, with the left hemisphere responding more strongly than the right (Figure 4A, Top; Table 1). This change in lateralization was seen for both canary and zebra finch testing stimuli.
Table 1.
Summary table of lateralization effects. This table contains the direction of lateralization and the corresponding p-values for each experimental group and each dependent variable measured (ARMs and Adaptation Rates). The last row contains the Environment by Hemisphere interactions and their respective p-values.
| 9D | 4D | 2D | |||||
|---|---|---|---|---|---|---|---|
| CONENV | ARM | R>L | 0.019 | R>L | 0.062 | n/a | n/a |
| ADPT | R>L | 0.021 | R>L | 0.053 | n/a | n/a | |
| HETENV | ARM | L>R | 0.034 | L>R | 0.003 | R>L | 0.039 |
| ADPT | R=L | 0.279 | L>R | 0.031 | R=L | 0.874 | |
| ENV x HEMI | ARM | ------ | 0.0038 | ------ | 0.0023 | ------ | 0.800 |
| ADPT | ------ | 0.0005 | ------ | 0.0049 | ------ | 0.125 | |
Changes in Hemispheric Lateralization of Adaptation Rate
Adaptation rates during trials 6–25 are used to measure novelty or familiarity with a stimulus following established procedures (Phan et al., 2006). In the analysis of data from birds in the 9d CONENV group, adaptation rates were faster in the right hemisphere than the left (F(1, 179)=5.3922, P<0.021; Figure 4A, Bottom), in agreement with previous results (Phan and Vicario, 2010). Unexpectedly, adaptation to canary stimuli was slower than to zebra finch stimuli (F(1, 179)=6.3909, P<0.012). This could be due to weaker responses to canary stimuli that approach a minimum ARM.
In contrast, for birds hearing HETENV sounds for 9d, adaptation rates were not lateralized. Although mean adaptation rate was faster on the left than the right, contrary to the pattern seen in birds exposed to CONENV, the difference in adaptation rates in the two hemispheres was not significant (F(1, 191)=1.178, P=0.279, n.s.; Figure 4A, Bottom). Canary stimuli again showed slower adaptation than zebra finch stimuli (F(1, 191)=11.490, P<0.0009). There was no interaction of hemisphere with stimulus type because both types adapted slightly, but not significantly, faster in the left hemisphere.
When adaptation rates for birds exposed for 9d to CONENV and HETENV were combined in an ANCOVA and evaluated for main effects and interactions, rates in HETENV birds were slower than in CONENV birds (F(1, 369)=12.2325, P<0.0005) for both stimulus types. There was also a significant interaction of environment with hemisphere (F(1, 369)=5.5079, P<0.0195; Figure 4A, Bottom, Table 1). In CONENV birds most of the effect was due to the right hemisphere having steeper adaptation than all of the other groups, including left hemisphere in CONENV birds and both hemispheres in HETENV birds (P<0.05 for each Bonferroni post-hoc comparison).
Time Course of Changes in Hemispheric Lateralization
In order to assess how long the exposure to HETENV must be to affect the pattern of lateralization, additional groups of zebra finches were exposed to HETENV sounds for shorter periods of time, either 4d (n=6) or 2d (n=5). An additional group of birds was exposed to CONENV for 4d (n=5) to control for the non-specific effects of the procedure. ANOVAs and overall ANCOVAs were performed on the ARMs and adaptation rates from the neural data collected after 4d exposure, as previously described.
Analysis of ARMs on trials 2–6 for birds exposed for 4d to either CONENV or HETENV sounds trials revealed a significant interaction of environment with hemisphere (ANCOVA F(1,258)=9.4431, P<0.0023, Figure 4B, Top; Table 1), just as seen in 9d birds. The interaction is due to ARMs being slightly higher in the right hemisphere in CONENV birds and significantly higher in the left hemisphere in HETENV birds. Within CONENV birds, a repeated measures ANOVA revealed a trend towards higher ARMs in the right hemisphere (F(1,135)=3.5418, P=0.0620, n.s.). A repeated measures ANOVA in HETENV birds revealed the opposite pattern, with the left hemisphere showing significantly higher ARMs than the right (F(1,125)=8.9489, P<0.0034).
A significant interaction between environment and hemisphere was also seen in the ARMs for trials 6–25: (ANCOVA F(1,258)=8.5014, P<0.0039), similar to the pattern of results in ARMs for trials 2–6. Interestingly, there is an additional effect in the 6–25 ARMs data for the 4d birds that is not seen in the 9d birds. There is a significant environment by hemisphere by stimulus type interaction (F(1,258)=8.2314, P<0.0045); the left hemisphere in HETENV birds shows a response bias for zebra finch song, while the right does not. In contrast, in CONENV birds, the conspecific bias is only seen in the right hemisphere.
Analysis of adaptation rates for birds exposed for 4d to either CONENV or HETENV sounds showed a pattern of results similar to the birds with 9d of exposure. There is an interaction of environment with hemisphere (F(1,258)=8.0441, P<0.0049, Figure 4B, Bottom; Table 1). When CONENV birds were tested with a repeated measures ANOVA, the right hemisphere shows a trend towards faster adaptation (F(1,135)=3.6226, P<0.0591) while the reverse is true in HETENV birds where the left hemisphere adapts significantly faster than the right (F(1,125)=4.7560, P<0.0311).
Exposure to HETENV for only 2d did not induce the pattern of changes seen in 4d and 9d HETENV birds. When ARMs on trials 2–6 were compared between 2d HETENV vs. 9d CONENV controls, there were no significant interactions between environment and hemisphere (ANCOVA F(1,285)=0.0646, P=0.7996, n.s.; Table 1). There was a main effect of hemisphere consistent with the normal pattern of lateralization: ARMs in the right hemisphere in both groups were significantly higher than left (F(1, 285)=4.2847, P<0.0394). However, there was a significant main effect of environment, with higher mean ARMs in 2d HETENV birds (F(1, 285)=7.0087, P<0.0086), that was not seen at either 4d or 9d HETENV birds; this appears to be an early effect of HETENV exposure.
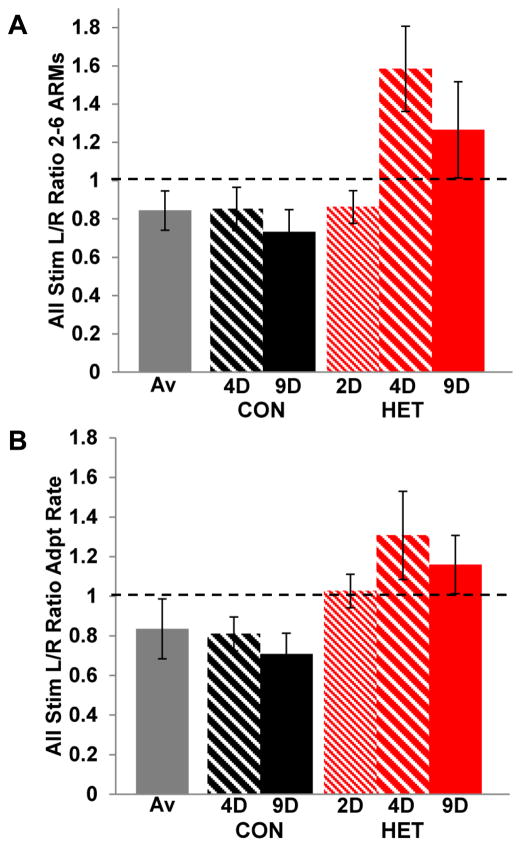
When ARMs on trials 2–6 were compared for birds exposed to HETENV for 2d and 9d, there was a significant interaction between environment and hemisphere(F(1, 297)=3.9972, P<0.046). The 9d HETENV birds show higher ARMs on the left, as previously described, while the 2d HETENV ARMs are higher on the right. For comparison between conditions and exposure durations, these data are plotted as left/right ratios in Figure 5A.
Figure 5.
Time course of exposure effects. A) ARMs are plotted as L/R ratios for each time point and housing condition: CON (black), HET (red), Av (gray). Ratios above 1 are indicative of higher ARMs in the left hemisphere, while ratios below 1 signify higher ARMs in the right hemisphere. B) Adaptation rates are plotted as L/R ratios for each time point and housing condition. Ratios above 1 represent faster adaptation rates in the left hemisphere and ratios below 1 represent faster adaptation rates in the right hemisphere. Error bars represent SEM.
Adaptation rates for trials 6–25 showed a weak trend towards an environment by hemisphere interaction for 2d HETENV vs. 9d CONENV controls (F(1, 297)=2.3671, P=0.125, n.s.; Table 1) but this effect was not significant. Closer analysis of the means revealed that adaptation rates were faster on the right than the left in 9d CONENV birds but were approximately equal in the left and right hemispheres in 2d HETENV birds, though this interaction does not reach significance.
When adaptation rates were compared between 2d and 9d HETENV, there was no significant interaction of environment by hemisphere (F(1,297)=0.1017, P=0.750, n.s.). Both groups had slightly faster adaptation in the left hemisphere than the right, contrary to the normal pattern of right side faster adaptation seen in 9d CONENV birds. Although the effect is small, it appears that the pattern of adaptation rates begins to change with exposure to the altered acoustic environment after only 2d. For comparison between conditions and exposure durations, these data are plotted as left/right ratios in Figure 5B.
Effects of Box vs. Aviary Housing
All data present so far was collected from box-housed birds to separate the acoustic variable from other factors such as visual stimulation and social interaction present in a general aviary. To test for potential effects of box housing vs. general aviary housing on patterns of lateralization, we compared 9d CONENV and 4d CONENV birds to a separate cohort of birds from the general aviary (n=16). When ARMs on trials 2–6 were compared using ANCOVA with environment (9d CONENV, 4d CONENV, AV), and hemisphere (left vs. right) as the factors and Depth as the covariate, there was a significant main effect of hemisphere (F(1, 900)=6.1897, P<0.013) with higher responses in the right hemisphere across all three groups. There was no interaction of environment with hemisphere (F(2, 900)=0.8602, P=0.4234, n.s.), indicating that the same pattern of right side higher ARMs was seen across all birds exposed to conspecific auditory environments, regardless of whether they were box-housed or aviary housed, consistent with the pattern of lateralization of ARMs previously reported in NCM (Phan and Vicario, 2010). Left/right lateralization ratios for ARMs were comparable in AV, 4d and 9d CONENV groups (Figure 5A).
Similarly, when adaptation rates for trials 6–25 were compared, there was a main effect of hemisphere with the right hemisphere exhibiting faster adaptation rates than the left (F(1, 900)=20.744, P<0.00001) across all three groups exposed to conspecific acoustics. There was no interaction of environment with hemisphere (F(2, 900)=1.2140, P=0.2975) indicating similar patterns of lateralization in box-housed and aviary housed birds, once again consistent with the lateralized adaptation rate reported in NCM (Phan and Vicario, 2010). Left/right lateralization ratios for adaptation rates were comparable in AV, 4d and 9d CONENV groups (Figure 5B). Whether birds were box-housed (and exposed to CONENV) or aviary-housed did not seem to have an effect on patterns of lateralization in auditory cortex, though other effects in brain regions not measured by our dependent variable cannot be ruled out.
Effects of Recent Auditory Experience on Responses to Tones
In order to assess whether the changes in hemispheric lateralization were specific to complex stimuli, or showed a general reversal of functional lateralization to all stimuli, we measured ARMs for pure tones in 9d HETENV and CONENV birds. ARMs for tones were defined as the average response across all trials at the best frequency of each site. There was no hemispheric difference in ARMs to pure tones in birds housed in either environment (ANOVA: CONENV: F(1,156)=0.002, P=0.969, HETENV: F(1,154)=0.799, P=0.373) and there was no interaction of environment with hemisphere (ANCOVA: F(1,321)=0.12695, P=0.722). Responses to simple stimuli like pure tones do not appear to be lateralized, as previously observed (Phan and Vicario, 2010), regardless of the environment to which the birds were exposed.
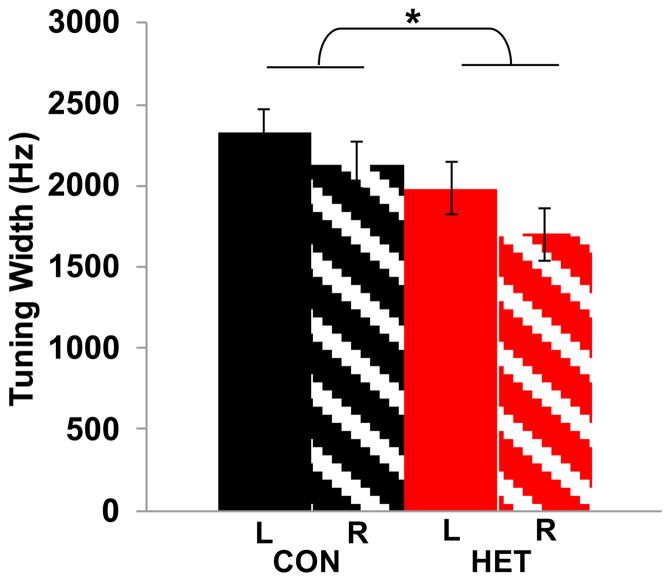
In a previous study in the laboratory that tested tone stimuli in birds exposed to novel sound environments for 9d, the width of tuning functions showed evidence of plasticity (Terleph et al., 2008). That study measured tuning width for pure tones as the contiguous frequency range over which absolute responses were at least two standard deviations above baseline. Using the same method, we found that tuning width was found to be significantly narrower in HETENV than CONENV birds (F(1, 304)=5.6451, P<0.018, Figure 6), consistent with the previous report, which found narrower tuning in birds housed in a heterospecific aviary. In that study, birds were individually moved from their home aviary to a heterospecific aviary, which could have had non-specific effects due to the presence of multi-sensory cues and social interactions. The present study presented CONENV or HETENV sounds to isolated birds, thus eliminating the non-acoustic factors, thus the present results confirm that narrowing of tuning width occurs primarily as a result of exposure to foreign acoustic stimuli, not other variables.
Figure 6.
Effects of exposure on tuning. Tuning widths in the left (solid bars) and right (striped bars) hemispheres for birds exposed to either CONENV(black) or HETENV (red) for 9 consecutive days are shown. Tuning width is significantly narrower in birds exposed to HETENV compared to CONENV (F(1, 304)=5.6451, P<0.018) but there was no effect of hemisphere. Error bars represent SEM. Asterisks denote significant main effects.
Effects on tuning width are also seen in birds exposed to HETENV for 4d. Tuning width in these birds was significantly narrower than the 4d controls (F(1, 267)=11.212, P<0.0009). Thus, 4dexposure to a foreign acoustic environment changes tuning in as little as 4d. Tuning width was no different in 2d HETENV birds when compared to 9d CONENV controls (F(1, 263)=0.0980, P=0.7545, n.s.), suggesting that narrowing of tuning requires a minimum duration of exposure to novel sounds.
Neural Responses as a Function of Recording Depth
In birds housed in CONENV and HETENV for 9d, effects of recording depth along the dorsal-ventral axis in NCM were analyzed in an ANOVA with environment, depth(from dorsal to ventral) and hemisphere as factors and stimulus type as a repeated measure. In 2–6 ARMs, there was a significant main effect of depth (F(3, 358)=6.039, P<0.0005). ARMs were lower at recording sites in ventral NCM across all birds consistent with previous findings (unpublished data). There was no interaction of environment by depth (F(3, 358)=0.6291, P<0.5966, n.s.); ARMs decreased ventrally for both CONENV and HETENV birds. However, there was a significant interaction of depth with stimulus type (F(3, 358)=3.4464, P<0.0169) where ARMs to zebra finch testing stimuli decreased more steeply along the dorsal-ventral axis than ARMs to canary stimuli due to zebra finch stimuli eliciting higher responses than canary stimuli at the dorsal sites.
Adaptation rates for trials 6–25 in 9d CONENV and HETENV birds were also analyzed for depth effects. Adaptation rates were faster at the most dorsal depth and became slower as recording sites moved more ventral (F(3, 358)=3.7005, P<0.0120). There was no environment by depth interaction (F(3, 358)=0.8882, P<0.4474), indicating that adaptation rates slowed at the same rate ventrally in both CONENV and HETENV birds. There was a significant depth by stimulus type interaction (F(3, 358)=4.4124, P<0.0046) driven by adaptation rates to both zebra finch and canary stimuli being about equal at dorsal-most sites, then zebra finch stimuli adapting faster than canary stimuli at ventral sites.
Tuning widths in the 9d CONENV and HETENV birds showed a trend towards narrowing at more ventral depths (F(3, 304)=2.5678, P<0.0546). However, there was no depth by environment interaction (F(3, 304)=0.7106, P=0.5463) and tuning was slightly narrower in HETENV than in CONENV at all recording depths.
DISCUSSION
These results demonstrate that hemispheric lateralization of auditory responses in adult birds is labile and subject to short term influences from the external environment. ARMs in birds exposed to HETENV for 9d were higher in the left hemisphere than in the right, a pattern that is clearly reversed from the one seen in CONENV birds, which is the same as that seen in AV birds, and previously documented in zebra finches (Phan and Vicario, 2010). In HETENV birds, left and right hemispheres adapted equally fast, a pattern which is partially reversed from that seen in CONENV and AV birds (where the right hemisphere adapted faster than the left). The same changes were seen after only 4d exposure to HETENV. However, after only 2d of HETENV exposure, there were subtle changes. Subjects showed no lateral difference in adaptation rates, in contrast to higher rates on the right in CONENV birds. In addition ARMs in both hemispheres in HETENV birds were elevated from CONENV though they remained higher on the right side, as seen in CONENV birds. These early trends in both adaptation rates and ARMs at the 2d timepoint may represent the beginning of reorganization in auditory cortex (c.f. Newman-Norland, 2006) which later results in a full reversal of lateralization at the 4d and 9d timepoints (Table 1). The duration and persistence of the reversals remain to be tested and could provide valuable insights into the time course of plasticity in auditory cortex. Given the malleability of hemispheric lateralization, birds that are returned to a conspecific auditory environment after 9d in a heterospecific auditory environment may revert back to the normal pattern of lateralization. If birds continue to be exposed to the foreign environment well beyond the 9d time point, lateralization patterns may also revert to normal, as the heterospecific sounds become encoded in now familiar categories.
The time course over which responses are modified in NCM could provide important clues about mechanisms underlying adult cortical plasticity. In our data, shifts in the pattern of lateralization are seen after as little as 2d of exposure and are fully present after 4d in the foreign environment while narrowing of tuning width requires at least 4d exposure. Changes in ARMs and adaptation rate occur after a similar time period, suggesting either a dose-dependent effect, where 4d of continuous exposure has provided the bird with enough exemplars to update its neural filters for the novel sounds, or that the 4d time point corresponds to an underlying biological process for cortical plasticity. A similar period is required for changes in the tonotopic map of rats operantly-trained to attend either to frequency or intensity components of a sound (Polley el al., 2006). Possible neural mechanisms operating either in NCM or in the ascending auditory system to change lateralization include formation of new synapses or modification of existing synapses. Incorporation of new neurons into existing circuitry could also contribute; NCM receives a continuous flux of new neurons in adulthood (Lipkind et al., 2002; Pytte et al., 2010).
Novel sounds presented in the HETENV condition effectively change the statistics of the bird’s acoustic environment by expanding the universe of probable sounds. The dramatic changes in lateralization that we observe appear to be due to reorganization of auditory processing in response to this challenge. We speculate that, in order to efficiently represent the new stimulus statistics, the auditory system modifies existing neural connectivity to represent the new sounds. The data show that the majority of the overall environment by hemisphere interaction in adaptation rates is contributed by the loss of faster right side adaptation in 9d, 4d and 2d HETENV birds. In contrast, the reversal of lateralization measured in ARMs is a result of both increased responding in the left and decreased responding in the right hemisphere in 9d and 4d HETENV birds when compared to CONENV birds. The right hemisphere responds to HETENV exposure with a slowing of adaptation rates, while the left side shows increased ARMs to song stimuli (Table 1). Both hemispheres are affected by changes in the auditory environment but each responds differently when exposed to novel categories of sounds.
In our data, exposure to novel sounds heightened responses to both conspecific and heterospecific song stimuli in the left hemisphere, suggesting a reorganization of categorical space that affects representations of native as well as non-native sounds. Studies of bilingual humans corroborate the idea that left hemisphere is plastic and responsive to changes in categorization of languages into adulthood. As proficiency with an artificial grammar increases, activation patterns to the trained artificial grammar shift from bilateral to left-dominant (Newman-Norlund et al., 2006), but effects of such training on lateralization for the native grammar are unknown. Similarly, a meta-analysis found that bilinguals who acquired a second language before age 6 show bilateral activation for both languages, whereas bilinguals who acquired their second language after age 6 had left hemisphere activation for the second language as well as the first (Hull and Vaid 2007). Our observation that left hemisphere responses to both conspecific and heterospecific songs were enhanced in our experimental group further supports the idea that experience with novel stimuli could induce plastic changes that are hemisphere-specific.
Hemispheric differences in reorganization to novel sounds are also seen in human studies. Training or recent exposure to novel sounds results in a greater change in the right hemisphere (Tremblay and Kraus, 2002; Brattico et al., 2005). A study that challenged human subjects with the task of discriminating new sounds and forming perceptual boundaries where none existed before found that peaks in the ERP response increase in the right hemisphere after training (Tremblay and Kraus, 2002). A similar process could be taking place in the birds (although in the absence of specific training)as they listen to a large set of canary vocalizations that contain different spectral and temporal features than those found in native conspecific vocalizations. Categorization of sound by songbirds could be based on the shared spectro-temporal features that characterize each species’ vocalizations, akin to human ability to segregate languages based on rhythm and prosody. The same mechanisms that underlie category formation for conspecific sounds during development could also be invoked when forming new categories of foreign sounds.
Our results reinforce the idea that auditory lateralization is not necessarily a fixed property of the brain but emerges in response to complex sounds. Both the original pattern of lateralization and the reversed pattern of lateralization were only seen in response to testing with natural songs which contain complex temporal structure and frequency modulations, not in response to simple tone stimuli (cf. Phan and Vicario, 2010; Terleph et al., 2008). These findings parallel results of human studies in which responses are lateralized to speech but not to non-speech stimuli or white noise (Belin et al., 2000). The dependence of lateralization on early experience in both songbirds and humans further suggests a critical role for prior auditory exposure (Phan and Vicario, 2010; Marcotte and Morere 1990). These results highlight the importance of the stimuli employed in auditory experiments. Caution should be exercised when comparing studies that employ different types of stimuli. Responses to naturalistic stimuli differ from responses to artificial stimuli or pure tones (Ramirez et al., 2011; Portfors et al., 2009), and this could affect the interpretation of results.
Our data show that the challenge of novel auditory experience induces plasticity in different response parameters in the left and right hemispheres, suggesting an underlying difference in function. We speculate that, in the normal conspecific environment, the right hemisphere performs the fine discriminations necessary for recognition of particular conspecific songs as shown by rapid adaptation to each stimulus. In contrast, the left processes sounds according to their acoustic category (combinations of features that characterize conspecific vs. heterospecific song) and responds to the foreign environment with heightened responses to category violations, ultimately forming new categories as the need arises. This division of labor may be established early in life through an interaction between latent hemispheric predispositions and exposure to native sounds, i.e. conspecific songs or contrasts in the native language (Phan et al., 2010; Dehaene-Lambertz et al., 2002; Frasnelli et al., 2012; Rogers 2008). In humans, the left hemisphere responds preferentially to the contrasts that underlie the phonemes of a particular language, while the right hemisphere integrates inputs over a longer temporal window, allowing it to process stimuli with slower temporal and spectral modulation i.e. the prosody of speech (Telkemeyer et al., 2011). In the bat auditory cortex, the hemispheres differ in their selectivity for different classes of vocalizations; the left hemisphere seems tuned to social vocalizations, while the right processes echolocation calls (Kanwal 2012; Washington and Kanwal, 2012). Division of labor between the two hemispheres may lead to more efficient processing of sensory stimuli (Ocklenburg and Güntürkün, 2012; Hirnstein et al., 2008; Güntürkün et al., 2000). In songbirds, hemispheric lateralization may enable the rapid discrimination of specific sounds while concurrently allowing for plasticity in the formation of new categorical boundaries. Given the many parallels between humans and songbirds, the songbird model could serve as a window into the mechanisms underlying cortical plasticity for speech processing in humans.
Highlights.
Songbirds communicate using complex vocalizations learned by imitation, as humans do
Songs but not simple sounds elicit different responses in the two brain hemispheres
This lateralization is reversed by hearing a novel acoustic environment for 2–4 days
Auditory responses change in both hemispheres to represent new sound statistics
The brain adapts dynamically to novel patterns, e.g. when exposed to a new language
Acknowledgments
We thank Manda Pierce for technical assistance and Mimi Phan, Kasia Bieszczad, and Efe Soyman for helpful comments on the manuscript. This work was supported by National Institute on Deafness and Other Communication Disorders (DC008854).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lillian M. Yang, Email: lilyana0604@gmail.com.
David S. Vicario, Email: vicario@rci.rutgers.edu.
References
- Amin N, Gastpar M, Theunissen FE. Selective and efficient neural coding of communication signals depends on early acoustic and social environment. PLoS One. 2013 Apr 22;8(4):e61417. doi: 10.1371/journal.pone.0061417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chang EF, Teng CL, Heiser MA, Merzenich MM. Emergent categorical representation of natural, complex sounds resulting from the early post-natal sound environment. Neuroscience. 2013 Jun 6;248C:30–42. doi: 10.1016/j.neuroscience.2013.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004 Sep;7(9):974–81. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;(403):309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gobes SM, Terpstra NJ, den Boer-Visser AM, Zandbergen MA. Learning-related neuronal activation in the zebra finch song system nucleus HVC in response to the bird’s own song. PLoS One. 2012;7(7):e41556. doi: 10.1371/journal.pone.0041556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nat Rev Neurosci. 2010 Nov;11(11):747–59. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- Brattico E, Kujala T, Tervaniemi M, Alku A, Ambrosi L, Monitillo V. Long-term exposure to occupational noise alters the cortical organization of sound processing. Clinical Neurophysiology. 2005;(116):190–203. doi: 10.1016/j.clinph.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Nagarajan SS, Schreiner CE, Bedenbaugh PH, Wong A. Plasticity in primary auditory cortex of monkeys with altered vocal production. J Neurosci. 2005 Mar 9;25(10):2490–503. doi: 10.1523/JNEUROSCI.5289-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci U S A. 1995;92(8):3406–10. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc Natl Acad Sci USA. 1996;93:1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynx J, Williams H, Nottebohm F. Hemispheric differences in avian song discrimination. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1372–5. doi: 10.1073/pnas.89.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SV, Fritz JB, Shamma SA. Task reward structure shapes rapid receptive field plasticity in auditory cortex. Proc Natl Acad Sci U S A. 2012 Feb 7;109(6):2144–9. doi: 10.1073/pnas.1117717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002 Dec 6;298(5600):2013–5. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007 May;130(Pt 5):1432–41. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- Dugas-Ford J1, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16974–9. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993 Aug;107(4):539–51. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Ehret G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature. 1987;325(6101):249–51. doi: 10.1038/325249a0. [DOI] [PubMed] [Google Scholar]
- Floody OR, Arnold AP. Song lateralization in the zebra finch. Horm Behav. 1997 Feb;31(1):25–34. doi: 10.1006/hbeh.1997.1368. [DOI] [PubMed] [Google Scholar]
- Frasnelli E1, Vallortigara G, Rogers LJ. Left-right asymmetries of behavior and nervous system in invertebrates. Neurosci Biobehav Rev. 2012 Apr;36(4):1273–91. doi: 10.1016/j.neubiorev.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Adaptive changes in cortical receptive fields induced by attention to complex sounds. J Neurophysiol. 2007 Oct;98(4):2337–46. doi: 10.1152/jn.00552.2007. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Ball GF. Functional differences in forebrain auditory regions during learned vocal recognition in songbirds. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004 Dec;190(12):1001–10. doi: 10.1007/s00359-004-0556-x. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature. 2003;424(6949):669–74. doi: 10.1038/nature01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntürkün O, Diekamp B, Manns M, Nottelmann F, Prior H, Schwarz A, Skiba M. Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr Biol. 2000 Sep 7;10(17):1079–81. doi: 10.1016/s0960-9822(00)00671-0. [DOI] [PubMed] [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C. FoxP2 expression in avian vocal learners and non-learners. J Neurosci. 2004 Mar 31;24(13):3164–75. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hirnstein M, Hausmann M, Güntürkün O. The evolutionary origins of functional cerebral asymmetries in humans: does lateralization enhance parallel processing? Behav Brain Res. 2008 Mar 5;187(2):297–303. doi: 10.1016/j.bbr.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Hull R, Vaid J. Bilingual language lateralization: a meta-analytic tale of two hemispheres. Neuropsychologia. 2007;45(9):1987–2008. doi: 10.1016/j.neuropsychologia.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Yu J, Rivas MV, Horita H, Feenders G, Whitney O, Jarvis SC, Jarvis ER, Kubikova L, Puck AE, Siang-Bakshi C, Martin S, McElroy M, Hara E, Howard J, Pfenning A, Mouritsen H, Chen CC, Wada K. Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns. J Comp Neurol. 2013 Nov;521(16):3614–65. doi: 10.1002/cne.23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal JS. Right-left asymmetry in the cortical processing of sounds for social communication vs. navigation in mustached bats. Eur J Neurosci. 2012 Jan;35(2):257–70. doi: 10.1111/j.1460-9568.2011.07951.x. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Neocortical evolution: neuronal circuits arise independently of lamination. Curr Biol. 2013 Jan 7;23(1):R12–5. doi: 10.1016/j.cub.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Kovelman I, Yip JC, Beck EL. Cortical systems that process language, as revealed by non-native speech sound perception. Neuroreport. 2011 Dec 21;22(18):947–50. doi: 10.1097/WNR.0b013e32834cdc26. [DOI] [PubMed] [Google Scholar]
- Lipkind D, Nottebohm F, Rado R, Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav Brain Res. 2002 Jun 15;133(1):31–43. doi: 10.1016/s0166-4328(01)00416-8. [DOI] [PubMed] [Google Scholar]
- Maier V, Scheich H. Acoustic imprinting in guinea fowl chicks: age dependence of 2- deoxyglucose uptake in relevant forebrain areas. Brain Res. 1987 Jan;428(1):15–27. doi: 10.1016/0165-3806(87)90079-4. [DOI] [PubMed] [Google Scholar]
- Marcotte AC, Morere DA. Speech lateralization in deaf populations: evidence for a developmental critical period. Brain Lang. 1990 Jul;39(1):134–52. doi: 10.1016/0093-934x(90)90008-5. [DOI] [PubMed] [Google Scholar]
- Meliza CD. Effects of auditory recognition learning on the perception of vocal features in European starlings (Sturnus vulgaris) J Acoust Soc Am. 2011 Nov;130(5):3115–23. doi: 10.1121/1.3641420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GL, Knudsen EI. Early auditory experience induces frequency-specific, adaptive plasticity in the forebrain gaze fields of the barn owl. J Neurophysiol. 2001 May;85(5):2184–94. doi: 10.1152/jn.2001.85.5.2184. [DOI] [PubMed] [Google Scholar]
- Montiel JF, Molnár Z. The impact of gene expression analysis on evolving views of avian brain organization. J Comp Neurol. 2013 Nov;521(16):3604–13. doi: 10.1002/cne.23403. [DOI] [PubMed] [Google Scholar]
- Moorman S, Gobes SM, Kuijpers M, Kerkhofs A, Zandbergen MA, Bolhuis JJ. Human-like brain hemispheric dominance in birdsong learning. Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):12782–7. doi: 10.1073/pnas.1207207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken I. Processing of complex sounds in the auditory system. Curr Opin Neurobiol. 2008 Aug;18(4):413–7. doi: 10.1016/j.conb.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Newman-Norlund RD, Frey SH, Petitto LA, Grafton ST. Anatomical substrates of visual and auditory miniature second-language learning. J Cogn Neurosci. 2006 Dec;18(12):1984–97. doi: 10.1162/jocn.2006.18.12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinuscanarius. J Comp Neurol. 1976;165(4):457–86. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- O’Connor KN, Yin P, Petkov CI, Sutter ML. Complex spectral interactions encoded by auditory cortical neurons: relationship between bandwidth and pattern. Front Syst Neurosci. 2010 Nov 5;4:145. doi: 10.3389/fnsys.2010.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklenburg S, Güntürkün O. Hemispheric asymmetries: the comparative view. Front Psychol. 2012 Jan 26;3:5. doi: 10.3389/fpsyg.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklenburg S, Ströckens F, Güntürkün O. Lateralisation of conspecific vocalisation in non-human vertebrates. Laterality. 2013;18(1):1–31. doi: 10.1080/1357650X.2011.626561. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ. Hearing in passerine and psittacine birds: a comparative study of absolute and masked auditory thresholds. J Comp Psychol. 1987;101:7–15. [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A. 2006;103(4):1088–93. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Vicario DS. Hemispheric differences in processing of vocalizations depend on early experience. Proc Natl Acad Sci U S A. 2010;107(5):2301–6. doi: 10.1073/pnas.0900091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006 May 3;26(18):4970–82. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier C, Boumans T, Verhoye M, Balthazart J, Van der Linden A. Own-song recognition in the songbird auditory pathway: Selectivity and lateralization. J Neurosci. 2009;29:2252–2258. doi: 10.1523/JNEUROSCI.4650-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD, Jonson K. Over-representation of species-specific vocalizations in the awake mouse inferior colliculus. Neuroscience. 2009 Aug 18;162(2):486–500. doi: 10.1016/j.neuroscience.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Pytte CL, Parent C, Wildstein S, Varghese C, Oberlander S. Deafening decreases neuronal incorporation in the zebra finch caudomedialnidopallium (NCM) Behav Brain Res. 2010 Aug 25;211(2):141–7. doi: 10.1016/j.bbr.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AD, Ahmadian Y, Schumacher J, Schneider D, Woolley SM, Paninski L. Incorporating naturalistic correlation structure improves spectrogram reconstruction from neuronal activity in the songbird auditory midbrain. J Neurosci. 2011 Mar 9;31(10):3828–42. doi: 10.1523/JNEUROSCI.3256-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993 Jan;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Messana EP, Nedder AP, Cotanche DA. Endotracheal isoflurane anesthesia for chick auditory surgery. Hear Res. 2000 Mar;141(1–2):165–8. doi: 10.1016/s0378-5955(99)00219-1. [DOI] [PubMed] [Google Scholar]
- Rogers LJ. Development and function of lateralization in the avian brain. Brain Res Bull. 2008 Jun 15;76(3):235–44. doi: 10.1016/j.brainresbull.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Ryals BM. Patterns of hair cell loss in chick basilar papilla after intense auditory stimulation. Exposure duration and survival time. Acta Otolaryngol. 1982 Jan–Feb;93(1–2):31–41. doi: 10.3109/00016488209130849. [DOI] [PubMed] [Google Scholar]
- Scheich H, Brechmann A, Brosch M, Budinger E, Ohl FW. The cognitive auditory cortex: task-specificity of stimulus representations. Hear Res. 2007 Jul;229(1–2):213–24. doi: 10.1016/j.heares.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Schreiner CE. Order and disorder in auditory cortical maps. Curr Opin Neurobiol. 1995;5:489–496. doi: 10.1016/0959-4388(95)80010-7. [DOI] [PubMed] [Google Scholar]
- Sharpee TO, Atencio CA, Schreiner CE. Hierarchical representations in the auditory cortex. Curr Opin Neurobiol. 2011 Oct;21(5):761–7. doi: 10.1016/j.conb.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch Caudomedialneostriatum: role of genomic and electrophysiological activities. J Neurobiol. 2001;48:163–18. doi: 10.1002/neu.1049. [DOI] [PubMed] [Google Scholar]
- Suga N, Zhang Y, Yan J. Sharpening of frequency tuning by inhibition in the thalamic auditory nucleus of the mustached bat. J Neurophysiol. 1997;77(4):2098–2114. doi: 10.1152/jn.1997.77.4.2098. [DOI] [PubMed] [Google Scholar]
- Telkemeyer S, Rossi S, Nierhaus T, Steinbrink J, Obrig H, Wartenburger I. Acoustic processing of temporally modulated sounds in infants: evidence from a combined near-infrared spectroscopy and EEG study. Front Psychol. 2011;1:62. doi: 10.3389/fpsyg.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleph TA, Lu K, Vicario DS. Response properties of the auditory telencephalon in songbirds change with recent experience and season. PLoS One. 2008;3(8):e2854. doi: 10.1371/journal.pone.0002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen FE, Sen K, Doupe AJ. Spectral-temporal receptive fields of nonlinear auditory neurons obtained using natural sounds. J Neurosci. 2000;20(6):2315–31. doi: 10.1523/JNEUROSCI.20-06-02315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramo MJ. Biology and music. Music of the hemispheres. Science. 2001 Jan 5;291(5501):54–6. doi: 10.1126/science.10.1126/science.1056899. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Kraus N. Auditory training induces asymmetrical changes in cortical neural activity. J Speech Lang Hear Res. 2002 Jun;45(3):564–72. doi: 10.1044/1092-4388(2002/045). [DOI] [PubMed] [Google Scholar]
- Voss HU, Tabelow K, Polzehl J, Tchernichovski O, Maul KK, Salgado-Commissariat D, Ballon D, Helekar SA. Functional MRI of the zebra finch brain during song stimulation suggests a lateralized response topography. Proc Natl Acad Sci U S A. 2007 Jun 19;104(25):10667–72. doi: 10.1073/pnas.0611515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci U S A. 2010;107(28):12676–81. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kadia SC. Differential representation of species-specific primate vocalizations in the auditory cortices of marmoset and cat. J Neurophysiol. 2001 Nov;86(5):2616–20. doi: 10.1152/jn.2001.86.5.2616. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Beitel R, Schreiner CE. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J Neurophysiol. 1995 Dec;74(6):2685–706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- Washington SD, Kanwal JS. Sex-dependent hemispheric asymmetries for processing frequency-modulated sounds in the primary auditory cortex of the mustached bat. J Neurophysiol. 2012 Sep;108(6):1548–66. doi: 10.1152/jn.00952.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Suthers RA. Neural pathways for bilateral vocal control in songbirds. J Comp Neurol. 2000 Jul 31;423(3):413–26. doi: 10.1002/1096-9861(20000731)423:3<413::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Williams H, Crane LA, Hale TK, Esposito MA, Nottebohm F. Right-side dominance for song control in the zebra finch. J Neurobiol. 1992 Oct;23(8):1006–20. doi: 10.1002/neu.480230807. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Casseday JH. Response properties of single neurons in the zebra finch auditory midbrain: response patterns, frequency coding, intensity coding, and spike latencies. J Neurophysiol. 2004 Jan;91(1):136–51. doi: 10.1152/jn.00633.2003. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Gill PR, Fremouw T, Theunissen FE. Functional groups in the avian auditory system. J Neurosci. 2009;29(9):2780–93. doi: 10.1523/JNEUROSCI.2042-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]