Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the most common diseases of the liver globally. Non-alcoholic steatohepatitis (NASH) has a complicated pathophysiology which includes lipid buildup, oxidative stress, endoplasmic reticulum stress, and lipotoxicity. Recently, there has been tremendous improvement in understanding of NASH pathogenesis due to advancements in the scientific field. It is being investigated how non-invasive circulating and imaging biomarkers can help in NAFLD and NASH diagnosis and monitoring the progress. Multiple medications are now undergoing clinical trials for the treatment of NASH, and lifestyle changes have been acknowledged as one of the main treatment methods. The purpose of this review article is to discuss the incidence of NAFLD globally, management issues with NASH, and its relation to the metabolic syndrome. It explains pathophysiology as well as therapeutic strategies using natural items, dietary changes, and pharmaceutical treatments. While emphasizing the necessity for surrogate endpoints to facilitate medication development for NASH, the study also considers the potential of non-invasive imaging biomarkers including magnetic resonance imaging (MRI) and magnetic resonance elastography (MRE).
Keywords: therapeutic, diagnostic, innovations, non-alcoholic steatohepatitis, non-alcoholic fatty liver disease
Introduction and background
An abnormality in the metabolism of hepatic fatty acids (FA) results in the development of non-alcoholic fatty liver disease (NAFLD). Non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver (NAFL) are the two main kinds. While NASH is characterized by steatosis combined with inflammation, hepatocellular damage, lobular inflammation, and fibrosis, NAFL refers to the presence of fat in the liver without considerable inflammation [1]. Insulin resistance (IR)-related regulation of lipolysis at the level of adipose tissue plays a major role in developing NAFLD [2].
The accumulation of FA in the liver is facilitated by the over-expression of CD36 fatty acid translocase and adipocyte fatty acid-binding proteins (FABP), particularly FABP-4 and FABP-5 [3]. De novo lipogenesis (DNL), a detoxification activity that formulates new FA from excess glucose, is also a significant contributor to hepatic lipid accumulation in NAFLD. The activation of two transcription factors, sterol regulatory element-binding protein-1c (SREBP-1c) and carbohydrate-responsive element-binding protein (ChREBP), plays a vital part in the upregulation of hepatic DNL [4]. Additionally, a small amount of the FA pool in NAFLD is derived from dietary triglycerides associated with chylomicrons [5].
A recent increase in metabolic syndrome and its associated conditions like visceral obesity, diabetes mellitus type 2, and dyslipidemia has caused an increase in the incidence of NAFLD. This syndrome raises death rates as well as the risk of developing cardiovascular disorders [1,4]. Furthermore, NAFLD has been linked to liver cancer. Therefore, early detection and timely treatment of NAFLD is crucial [5]. This paper provides a comprehensive overview of the advancements in therapeutic techniques and diagnostic approaches for NAFLD, highlighting their evolution over time.
Review
Pathogenesis of NAFLD
The pathogenesis of NAFLD involves a "two-hit" hypothesis. The "first hit" is insulin resistance, which leads to excessive FA flow into the liver. The "second hit" is inflammation attributed to gut-derived endotoxin, oxidative stress, and mitochondrial dysfunction [3]. Oxidation occurs due to many factors, such as cytokine injury, hyperinsulinemia, changes in the function of the immune system, and energy homeostasis. According to the studies conducted by Anstee et al. in 2013 and Vetrano et al. in 2023, several factors contribute to the development of NASH, which is characterized by excessive accumulation of cholesterol, inflammation, liver cell injury, and hepatocyte cell death. These factors can lead to liver disease and the development of hepatocellular carcinoma (HCC), a type of liver cancer [2, 6].
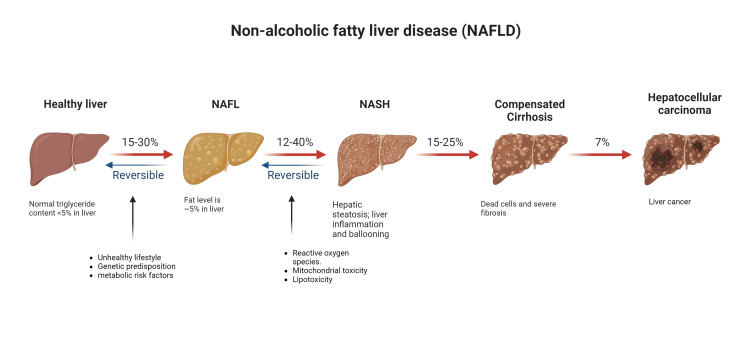
Recent research, as highlighted by Cholankeril et al. [7], has demonstrated that HCC can also arise in non-cirrhotic patients with NASH. The degree of fibrosis may play a crucial role in determining the future risk of HCC in the absence of cirrhosis, as evidenced by studies conducted by [1, 8]. Specifically, patients with NASH and advanced fibrosis have been found to face a heightened HCC risk. Figure 1 explains the development of NAF below.
Figure 1. Different stages of NAFLD.
The figure depicts the stages of NAFLD, from healthy liver to NAFL that progresses to NASH. The disease progression leads to cirrhosis and hepatocellular carcinoma (HCC).
NAFL: Non-alcoholic fatty liver, NASH: Non-alcoholic steatohepatitis
Figure created by the authors with BioRender.com.
The pathogenesis of NAFLD involves several interconnected mechanisms that contribute to its development and progression.
Lipid Accumulation
One of the primary mechanisms is lipid accumulation. When the intake of energy exceeds energy expenditure, excess energy is stored as lipids, leading to the buildup of triglycerides in hepatocytes. This lipid accumulation arises from multiple sources, including white adipose tissue, de novo lipogenesis, and a high-fat and/or high-sugar diet [9]. Such excess triglyceride synthesis contributes to the manifestation of hepatic steatosis, characterized by the accumulation of fat in the liver [3].
Oxidative Stress
Another critical mechanism is oxidative stress. In NAFLD, an overabundance of fatty acids compromises mitochondrial function and beta-oxidation, resulting in mitochondrial dysfunction. This dysfunctional state gives rise to the production of reactive oxygen species (ROS), which are highly reactive molecules. ROS, in turn, induces oxidative stress, triggering inflammation and causing damage to hepatocytes. The interplay between lipid accumulation and oxidative stress creates a vicious cycle, exacerbating liver injury [10].
Endoplasmic Reticulum (ER) Stress
Endoplasmic reticulum (ER) stress is also closely associated with NAFLD. The ER is responsible for protein synthesis, folding, and quality control. Disrupted ER homeostasis leads to the accumulation of unfolded or misfolded proteins, triggering ER stress. Initially, the unfolded protein response (UPR) is activated to restore protein homeostasis. However, if the UPR fails to promote cell survival, proapoptotic ER stress pathways are activated, resulting in cell death. The presence of ER stress further contributes to the progression of NAFLD [11].
Lipotoxicity
Additionally, lipotoxicity plays a significant role in the pathogenesis of NAFLD. Lipotoxicity refers to the toxic effects caused by sustained high concentrations of lipids and metabolites in non-adipose tissues. In NAFLD, lipotoxic substances accumulate in hepatocytes, leading to liver damage. Insulin resistance, increased plasma free fatty acids (FFAs), mitochondrial dysfunction, oxidative stress, ER stress, and inflammatory responses collectively contribute to the lipotoxicity observed in NAFLD [9].
Management approaches for NAFLD
Natural products and lifestyle modifications are being explored as potential therapeutic options for NAFLD, as there are currently no FDA-approved drugs specifically for its treatment. These natural products can target various aspects of NAFLD pathogenesis, including lipid metabolism, oxidative stress, ER stress, and inflammation. The therapeutic techniques to treat NAFLD are mainly focused on inflammation, fibrosis, and hepatic steatosis because the pathogenesis of NAFLD is complex [12-14].
In terms of lipid metabolism, certain natural products have shown promise by modulating the adenosine monophosphate-activated protein kinase (AMPK) pathway. Examples include antrodan from Antrodia cinnamomea, emodin from Radix Polygoni Multiflori, and flavonoids from Lomatogonium rotatum [15].
Oxidative stress is a crucial factor in NAFLD, and natural products with antioxidant properties have been investigated. Hesperetin from citrus fruits, as well as Gastrodin, yellow loosestrife, geniposide, xyloketal B, chicory seed extract, Crataegus azarolus var. aronia, apigenin, scutellarin, and alpinetin have demonstrated antioxidant effects and the ability to regulate lipid metabolism through pathways such as nuclear factor erythroid-derived 2-like 2 (Nrf2) or peroxisome proliferator-activated receptor (PPAR) activation [10].
ER stress is closely associated with lipid accumulation and liver injury in NAFLD, and certain natural products have shown potential in alleviating ER stress. Coffee, Amomum villosum var. xanthioides, Eucommia ulmoides Oliver leaves, aucubin, geniposide, Ixeris dentata, and tanshinone IIA have demonstrated the ability to mitigate ER stress and related liver injury.
Inflammation plays a critical role in NAFLD progression, and natural products with anti-inflammatory properties have been studied. Resveratrol from grapes and red wine, Cynanchum atratum, Lycopus lucidus, Atractylodes macrocephala, and Salvianolic acid A have shown anti-inflammatory effects through mechanisms like AMPK activation [3-15].
Lifestyle Interventions and Mediterranean Diet in NAFLD
In addition to natural products, lifestyle interventions play a crucial role in improving the symptoms and signs of NAFLD. Weight loss and lifestyle changes, such as adopting a balanced diet, regular physical activity, and avoiding alcohol consumption, are the main approaches to improving outcomes of NAFLD [12]. The Mediterranean diet (MD) includes food rich in macronutrients that are helpful in modulating glycosidic and lipid metabolism and thus help with fatty liver disease. MD consists of 30-35% fat which comes from consuming extra virgin olive oil, nuts, and omega-3 containing foods (provides mono-unsaturated fatty acids (MUFAs) and poly-unsaturated fatty acids (PUFAs)), 25-30% of protein which comes from vegetable sources and 40-45% carbohydrates (50-70% of that carbohydrates should come from low glycemic index and high fibers [16]. The combination of all these effects visceral obesity, dyslipidemia, insulin resistance, and chronic inflammation which improves metabolic syndrome leading to improvement in NAFLD [16]. Multiple studies have shown that MD has anti-inflammatory and antioxidant properties which decrease the progression of NAFLD. These benefits are due to the nutraceutical effect of bioactive compounds and phytochemicals like fibers MUFAs, phytosterols, and omega-3 fatty acids [17]. MD diet also affects gut-microbiota production which also affects metabolic syndrome and NAFLD. Furthermore, between the 1950s and 1980s, Ancel Keys published multiple studies that showed improvement in cardiovascular and cancer mortality in people from Greece and Italy and their diet mostly was MD [17].
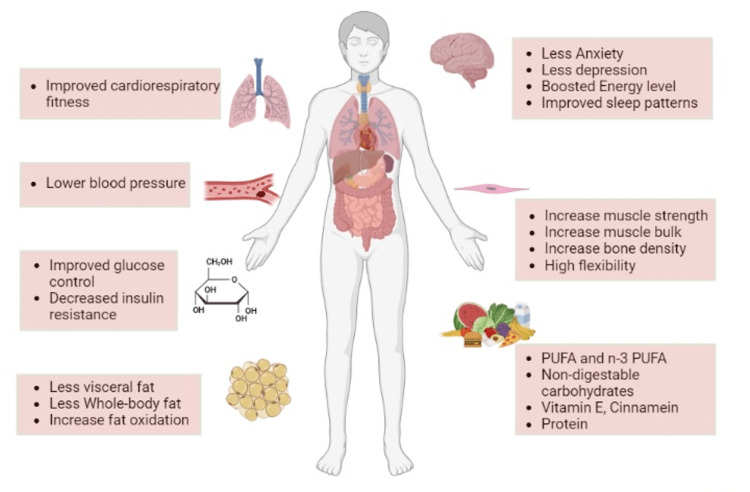
Figure 2 shows lifestyle interventions in NAFLD/NASH:
Figure 2. Lifestyle interventions in NAFLD/NASH.
The figure depicts lifestyle interventions in NAFLD/NASH, including improved brain functioning, muscle strength, volunteering exercise leading to respiratory fitness, less body fat, and improved diet.
NAFLD: Non-alcoholic fatty liver disease, NASH: Non-alcoholic steatohepatitis; PUFA: poly-unsaturated fatty acid
Figure created by the authors with BioRender.com.
Lifestyle modifications are particularly important in patients with diabetes, obesity, and metabolic syndrome, as these conditions frequently coexist with NAFLD and increase cardiovascular risks. Fatty liver disease encompasses a spectrum of hepatic pathology, ranging from simple steatosis to non-alcoholic steatohepatitis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease. The most recent guidelines suggest the management and treatment of patients with NAFLD considering both the liver disease and the associated metabolic co-morbidities. Diet and physical exercise are considered the first line of treatment for patients with NAFLD, but their results on therapeutic efficacy are often contrasting. Behavioral therapy is necessary most of the time to achieve a sufficient result [15, 18-20].
Pharmacological Therapy for NAFLD/NASH
Since the last decade, there has been an increase in pharmacological therapies in developing drugs treating NASH [21]. Statins (lipid-lowering agents), which are primarily used to reduce cardiovascular risk, have been found to be beneficial in treating patients with NAFLD, even in cases where the disease has progressed to non-alcoholic steatohepatitis (NASH) [4, 22]. Other non-statin hypolipidemic therapies, such as ezetimibe, bile acid sequestrants, PCSK9 inhibitors, and omega-3 fatty acids, may also confer liver benefits and reduce residual lipid risks in patients with NAFLD and NASH [23, 24].
Several antihyperglycemic drugs have shown promise in treating NAFLD/NASH, including pioglitazone, sitagliptin, GLP-1 receptor agonists, and SGLT2 inhibitors [21, 25]. Insulin sensitizers such as pioglitazone and high-dose vitamin E have been reported to improve the histology of patients with NASH. However, it's important to note that not all pharmacological interventions have been effective in improving liver histology in patients with NAFLD, such as metformin and ursodeoxycholic acid (UDCA). Liver biopsy is currently considered the gold standard for the diagnosis and staging of NAFLD because of the absence of noninvasive and specific biomarkers. Personalized medicine approaches and targeted therapies addressing the underlying mechanisms of NAFLD are also being explored [11, 22]. Table 1 shows the pharmacological interventions in NAFLD.
Table 1. Pharmacological interventions for NAFLD, their mechanisms and possible limitations.
Adapted from Negi et al. [22]
NAFLD: Non-alcoholic fatty liver disease
| Pharmacological Intervention | Mechanism of Action | Limitations |
| Pioglitazone | Improves insulin sensitivity, reduces liver inflammation | Weight gain, fluid retention, increased risk of heart failure |
| Vitamin E | Antioxidant properties, reduces oxidative stress in the liver | High doses may increase the risk of hemorrhagic stroke |
| Ursodeoxycholic acid (UDCA) | Modulates bile acid metabolism, reduces liver inflammation | Limited evidence of effectiveness, variable response among patients |
| Omega-3 fatty acids | Anti-inflammatory effects, improves lipid metabolism | High doses may increase the risk of bleeding, gastrointestinal side effects |
| Metformin | Improves insulin sensitivity, reduces glucose production in the liver | Gastrointestinal side effects, lactic acidosis (rare but serious complication) |
| Statins | Reduces cholesterol levels, may have anti-inflammatory effects | Muscle pain, liver toxicity, potential drug interactions |
| Fibrates | Lowers triglyceride levels, may improve liver steatosis | Gastrointestinal side effects, increased risk of gallstones |
| Pentoxifylline | Reduces inflammation and fibrosis in the liver | Gastrointestinal side effects, limited evidence of effectiveness |
| Vitamin D | Modulates immune response, may reduce liver inflammation | Limited evidence of effectiveness, potential for vitamin D toxicity |
| Antioxidant supplements | Neutralize oxidative stress, protect liver cells | Limited evidence of effectiveness, potential for adverse effects in high doses |
Advancement in the Therapeutic Strategies and Drugs
Managing patients with NAFLD involves addressing the disease stage and risk factors. Key strategies include lifestyle modification, targeting metabolic syndrome, managing cirrhosis complications, and pharmacotherapy for high-risk patients. Lifestyle modifications aim to reduce obesity, increase physical activity, and manage metabolic risk factors. Aggressive lifestyle modification is recommended for patients with severe steatohepatitis and fibrosis. Cirrhosis patients require HCC surveillance and treatments to reduce HCC risk. Therapeutic techniques target inflammation, fibrosis, and hepatic steatosis, focusing on weight loss, lipid metabolism improvement, cardiovascular risk reduction, and insulin sensitivity enhancement [11].
Monitoring the progression and identification advancement of disease in NAFLD/NASH
Circulating Biomarkers of NAFLD/NASH
To monitor the effectiveness of NAFLD treatment and assess disease progression, several biomarkers can be used. Serum hepatobiliary enzymes, hepatic steatosis, inflammation, and hepatocellular swelling can be measured to evaluate the impact of interventions. Additionally, biomarkers such as fibrosis markers (e.g., fibrosis-4 index) and non-invasive imaging techniques (e.g., transient elastography) can provide insights into the degree of fibrosis and liver stiffness [8].
Regarding the diagnosis of NAFLD, a combination of approaches is typically used. Blood tests, such as liver enzyme and liver function tests, chronic viral hepatitis tests, and lipid profiles, can help diagnose the condition and determine its severity. Imaging techniques also play a crucial role in the evaluation and management of NAFLD [26].
Identification of Advanced Fibrosis
Alanine aminotransferase (ALT) is an enzyme predominantly found in liver cells. Elevated levels of ALT in the blood indicate liver injury or inflammation, making it a valuable marker of liver damage in NAFLD. Aspartate aminotransferase (AST), another liver cell enzyme, is also used as a biomarker in NAFLD [27]. While elevated AST levels can indicate liver damage, they are less specific to liver disease compared to ALT. Gamma-glutamyl transferase (GGT) is an enzyme involved in liver and bile duct function. Elevated levels of GGT can indicate liver injury and are often used alongside other liver function tests to assess liver health in NAFLD. GGT is useful in identifying liver dysfunction and monitoring disease progression.
The fatty liver index (FLI) is a scoring system that combines several parameters, including body mass index (BMI), waist circumference, triglyceride levels, and GGT levels [27]. FLI is a noninvasive tool used to estimate the likelihood of having a fatty liver and assess the severity of hepatic steatosis. It provides a practical approach to identifying individuals at risk of NAFLD. The fibrosis-4 (FIB-4) index is a noninvasive marker used to assess the degree of liver fibrosis in NAFLD. It combines age, AST, ALT, and platelet count to estimate the fibrosis stage. The FIB-4 index is helpful in identifying patients with advanced fibrosis who may require further evaluation or intervention [26].
The enhanced liver fibrosis (ELF) test is a blood-based panel that measures specific markers associated with liver fibrosis. This panel includes hyaluronic acid, amino-terminal propeptide of type III collagen, and tissue inhibitor of metalloproteinase 1. The ELF test provides a quantitative assessment of fibrosis severity in NAFLD, aiding in the evaluation of disease progression and the efficacy of therapeutic interventions [23].
Genetic and Inflammatory Biomarkers in NAFLD/NASH
In addition to these biomarkers, inflammatory biomarkers such as C-reactive protein (CRP) and interleukin-6 (IL-6) are evaluated in NAFLD. CRP is an indicator of systemic inflammation, while IL-6 is a pro-inflammatory cytokine. These biomarkers reflect the inflammatory state associated with NAFLD and may help guide treatment strategies [27]. Adipokines and cytokines, such as adiponectin, tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and leptin, are also investigated as biomarkers in NAFLD. These molecules are involved in the regulation of inflammation, insulin sensitivity, and metabolic processes. Their measurement helps in understanding the complex interplay between adipose tissue, inflammation, and metabolic dysfunction in NAFLD [28].
Genetic and molecular markers, along with the gut microbiota, are pivotal in NAFLD treatment. Genetic variants like PNPLA3 rs738409 C>G, TM6SF2 E167K, and GCKR rs780094 influence hepatic lipid accumulation and disease progression. Omics-based markers provide insights into molecular profiles, while microRNAs serve as potential mechans [22]. The gut microbiota exhibits specific signatures linked to NAFLD severity. Understanding these markers enhances diagnosis, prognosis, and treatment strategies for NAFLD [1, 29].
Scoring Systems to Identify NAFLD/NASH
The non-invasive diagnosis of NAFLD includes FLI, NAFLD liver fat score (NLFS), lipid accumulation product (LAP), and novel NAFLD biomarkers. The FLI is a well-predictive algorithm that estimates hepatic steatosis. It is a preferred diagnosis technique due to the simplicity of the method. This method is based on BMI, waist circumference, serum TG, and gamma-glutamyl transferase (GCT). In this algorithm, an FLI score of <30 indicates no fatty liver, a score from 30 to 60 indicates undetermined conditions, and a score ≥60 predicts the development of hepatic steatosis.
The other diagnostic technique to predict NAFLD is the NLFS. In this technique, the liver fat content is measured with the help of proton magnetic resonance spectroscopy (H-MRS). Then it is compared to the standard hepatic steatosis fat content. This scoring system includes fasting serum insulin level, and the sensitivity level is much higher than the FLI. Statistically, the AST ratio can be noted with a sensitivity of 86% and a specificity of 71%. Another diagnostic technique widely used to identify fatty liver diseases in patients is LAP. Initially, it was developed for the US National Health and Nutrition Examination survey. It is now known as a biomarker of central obesity. This diagnostic technique separates the patients according to their fatty liver level with the help of ultrasound results. This makes it useful for checking the presence and stage of NAFLD. LAP is a powerful and easy tool to predict NAFLD in childhood. If LAP is ≥42.7, NAFLD should be suspected [28,29].
Ultrasonography
In the diagnosis and management of non-alcoholic fatty liver disease (NAFLD), various imaging techniques are employed. Conventional ultrasound (US) is commonly used as an initial imaging modality to detect fatty liver disease. It is a non-invasive technique that assesses hepatic steatosis by evaluating the liver's echogenicity [30]. Ultrasonography allows for reliable and accurate detection of moderate-severe fatty liver, compared to histology. Because of its low cost, safety, and accessibility, ultrasound is likely the imaging technique of choice for screening for fatty liver in clinical and population settings. However, it has limitations in accurately quantifying hepatic fat content and differentiating between simple steatosis and non-alcoholic steatohepatitis (NASH). Controlled attenuation parameter (CAP) is a technique that quantifies liver fat using ultrasonography, but it can be affected by obesity and has limitations in obese patients [28].
Magnetic Resonance Imaging (MRI) and Magnetic Resonance Spectroscopy (MRS)
MRI and MRS provide more precise assessments of hepatic steatosis [31]. MRI techniques, such as proton density fat fraction (PDFF) measurement, offer an accurate evaluation and can differentiate between simple steatosis and NASH. MRS, a specialized MRI technique, allows for the quantification of hepatic triglyceride content and aids in the diagnosis and monitoring of NAFLD but less widely available. MRI determines the liver fat at 5.56% in a population compared to the healthy individual [32]. PET is valuable for NAFLD treatment, detecting liver metabolic activity to assess severity and guide therapy. However, PET's limitations include limited spatial resolution, high cost, and the need for radioactive tracers. Single-photon emission computed tomography (SPECT) provides 3D images for functional assessment, evaluating perfusion and liver function in NAFLD, but has lower spatial resolution and longer acquisition times [33].
Elastography and Computed Tomography (CT)
Elastography measures liver stiffness as a non-invasive marker of fibrosis in NAFLD, though operator-dependency and limited availability may be considerations [34]. Transient elastography, known as FibroScan, is a non-invasive method used to assess liver fibrosis by measuring liver stiffness. Liver stiffness correlates with the degree of fibrosis and helps in risk stratification for NAFLD patients. FibroScan is a valuable tool for identifying individuals with advanced fibrosis who may require closer monitoring or intervention [35].
Computed tomography (CT) can identify and quantify hepatic fat content in NAFLD. It also provides additional information about liver structure and the presence of complications such as hepatocellular carcinoma (HCC). However, CT involves radiation exposure and is not typically utilized as a first-line imaging technique for NAFLD evaluation. It has limited sensitivity for mild steatosis [2], [31].
These imaging techniques, particularly MRI-based techniques like PDFF and MRS, are increasingly being used for the non-invasive assessment of NAFLD due to their ability to accurately quantify hepatic steatosis and differentiate between different stages of the disease. They offer advantages over liver biopsy, which is the current gold standard but is invasive and subject to sampling variability [33]. Table 2 summarizes the current imaging being used in NAFLD.
Table 2. Different imaging techniques used in NAFLD, their advantages and limitations.
Adapted from Takahashi et al. [36]
NAFLD: non-alcoholic fatty liver disease
| Imaging Technique | Description | Advantages | Limitations |
| Ultrasonography | Uses high-frequency sound waves to produce images of internal organs | Non-invasive, widely available, real-time imaging, cost-effective | Limited tissue penetration, operator-dependent, image quality may be affected by body habitus or bowel gas |
| Computed tomography (CT) | Utilizes X-rays to create detailed cross-sectional images of the body | High-resolution, multiplanar imaging, rapid acquisition | Ionizing radiation exposure, contrast agent use may cause allergic reactions or kidney damage, limited soft tissue characterization |
| Magnetic resonance imaging (MRI) | Uses strong magnetic fields and radio waves to generate detailed images of the body | Excellent soft tissue contrast, multiplanar imaging, no ionizing radiation | Expensive, longer scan times, patient claustrophobia, contraindicated for patients with certain metallic implants or devices |
| Positron emission tomography (PET) | Combines functional and anatomical imaging by detecting radioactive tracers | Can detect metabolic activity, useful for cancer staging, whole-body imaging | Expensive, limited spatial resolution, requires injection of radioactive tracers |
| Single-photon emission computed tomography (SPECT) | Uses gamma cameras to detect gamma rays emitted by radioactive tracers | 3D imaging, functional assessment, wide availability | Lower spatial resolution compared to PET, longer acquisition times, limited quantitative accuracy |
| Elastography | Measures tissue stiffness as a marker of fibrosis using ultrasound or MRI | Non-invasive, can assess liver fibrosis, real-time imaging | Operator-dependent, limited availability, may be affected by obesity or other factors |
| Magnetic resonance elastography (MRE) | Applies low-frequency vibrations and MRI to assess tissue stiffness | Whole liver assessment, excellent diagnostic accuracy for fibrosis | Requires MRI facility, expensive, time-consuming |
Challenges and future advancements in the treatment of NAFLD
Challenges in treating NAFLD include barriers to lifestyle modifications, patient compliance with pharmacological therapies, and limited treatment options [23]. Overcoming these challenges requires addressing factors such as motivation, long-term behavioral changes, and access to resources for lifestyle interventions, as well as improving medication adherence [37]. The future of NAFLD treatment and research holds promise through personalized medicine based on genetic profiling, nanomedicine for enhanced drug delivery, modulation of the gut microbiota, and identification of novel therapeutic targets [21]. Future advancement of NAFLD therapy should focus on the mechanistic studies on cell-based and animal models and human clinical trials of exercise, as well as the combination of lifestyle intervention and pharmaceutical therapy specifically targeting main signaling pathways related to lipid metabolism, oxidative stress, and inflammation. Additionally, combination therapies that integrate lifestyle modifications, pharmacological agents, and innovative interventions can provide more effective disease management [25]. These efforts aim to advance NAFLD treatment, improve patient outcomes, and alleviate the burden of the disease.
Conclusions
One of the most prevalent liver diseases, NAFLD still has a long way to go before it can be properly diagnosed and treated. Since there is no FDA-approved medication to treat it, the majority of care strategies rely on altering one's lifestyle and managing underlying conditions that are associated with metabolic syndrome (obesity, hypertension, diabetes, and hyperlipemia). To determine the severity and stages of NAFLD, several biomarkers are also being employed. However, more study is required to fully comprehend the pathogenesis of NAFLD, and efforts must be made to enhance therapies and diagnostics. The recent advancements in diagnosis and interventions were highlighted in this article.
The authors have declared that no competing interests exist.
References
- 1.Molecular characterization and cell type composition deconvolution of fibrosis in NAFLD. Pantano L, Agyapong G, Shen Y, et al. Sci Rep. 2021;11:18045. doi: 10.1038/s41598-021-96966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Non-alcoholic fatty liver disease (NAFLD), type 2 diabetes, and non-viral hepatocarcinoma: pathophysiological mechanisms and new therapeutic strategies. Vetrano E, Rinaldi L, Mormone A, et al. Biomedicines. 2023;11:468. doi: 10.3390/biomedicines11020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The nuclear envelope in lipid metabolism and pathogenesis of NAFLD. Östlund C, Hernandez-Ono A, Shin JY. Biology (Basel) 2020;9:338. doi: 10.3390/biology9100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NAFLD and cardiovascular diseases: a clinical review. Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. Clin Res Cardiol. 2021;110:921–937. doi: 10.1007/s00392-020-01709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Targher G, Corey KE, Byrne CD, Roden M. Nat Rev Gastroenterol Hepatol. 2021;18:599–612. doi: 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 6.Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Anstee QM, Targher G, Day CP. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 7.Current challenges and future direction in surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Cholankeril G, El-Serag HB. Semin Liver Dis. 2023;43:89–99. doi: 10.1055/a-1957-8540. [DOI] [PubMed] [Google Scholar]
- 8.SWOT analysis of noninvasive tests for diagnosing NAFLD with severe fibrosis: an expert review by the JANIT Forum. Kamada Y, Nakamura T, Isobe S, et al. http://10.1007/s00535-022-01932-1. J Gastroenterol. 2023;58:79–97. doi: 10.1007/s00535-022-01932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Standardisation of diet and exercise in clinical trials of NAFLD-NASH: Recommendations from the Liver Forum. Glass O, Filozof C, Noureddin M, et al. J Hepatol. 2020;73:680–693. doi: 10.1016/j.jhep.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Narasimhan S, Gokulakrishnan K, Sampathkumar R, Farooq S, Ravikumar R, Mohan V, Balasubramanyam M. Clin Biochem. 2010;43:815–821. doi: 10.1016/j.clinbiochem.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Pathophysiological mechanisms and clinical evidence of relationship between nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease. Galiero R, Caturano A, Vetrano E, et al. Rev Cardiovasc Med. 2021;22:755–768. doi: 10.31083/j.rcm2203082. [DOI] [PubMed] [Google Scholar]
- 12.Managing NAFLD in type 2 diabetes: the effect of lifestyle interventions, a narrative review. Parry SA, Hodson L. Adv Ther. 2020;37:1381–1406. doi: 10.1007/s12325-020-01281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effect of a six-month lifestyle intervention on the physical activity and fitness status of adults with NAFLD and metabolic syndrome. Mascaró CM, Bouzas C, Montemayor S, et al. Nutrients. 2022;14:1813. doi: 10.3390/nu14091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Physiopathology of lifestyle interventions in non-alcoholic fatty liver disease (NAFLD) Carneros D, López-Lluch G, Bustos M. Nutrients. 2020;12:3472. doi: 10.3390/nu12113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Non-alcoholic fatty liver disease (NAFLD) pathogenesis and natural products for prevention and treatment. Guo X, Yin X, Liu Z, Wang J. Int J Mol Sci. 2022;23:15489. doi: 10.3390/ijms232415489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effect of dietary and lifestyle interventions on the amelioration of NAFLD in patients with metabolic syndrome: the FLIPAN study. Montemayor S, Bouzas C, Mascaró CM, et al. Nutrients. 2022;14:2223. doi: 10.3390/nu14112223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mediterranean diet and nonalcoholic fatty liver disease. Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5960814/ World J Gastroenterol. 2018;24:2083–2094. doi: 10.3748/wjg.v24.i19.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NAFLD and diabetes mellitus. Tilg H, Moschen AR, Roden M. Nat Rev Gastroenterol Hepatol. 2017;14:32–42. doi: 10.1038/nrgastro.2016.147. [DOI] [PubMed] [Google Scholar]
- 19.Effectiveness of lifestyle interventions in NAFLD (nonalcoholic fatty liver disease) - how are clinical trials affected? Michel M, Schattenberg JM. Expert Opin Investig Drugs. 2020;29:93–97. doi: 10.1080/13543784.2020.1716333. [DOI] [PubMed] [Google Scholar]
- 20.NAFLD in type 2 diabetes mellitus: Still many challenging questions. Cernea S, Raz I. Diabetes Metab Res Rev. 2021;37:0. doi: 10.1002/dmrr.3386. [DOI] [PubMed] [Google Scholar]
- 21.Applications of quantitative systems pharmacology (QSP) in drug development for NAFLD and NASH and its regulatory application. Siler SQ. Pharm Res. 2022;39:1789–1802. doi: 10.1007/s11095-022-03295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Metabolism. 2022;126:154925. doi: 10.1016/j.metabol.2021.154925. [DOI] [PubMed] [Google Scholar]
- 23.Therapeutic landscape for NAFLD in 2020. Neuschwander-Tetri BA. Gastroenterology. 2020;158:1984–1998. doi: 10.1053/j.gastro.2020.01.051. [DOI] [PubMed] [Google Scholar]
- 24.The effect of pharmacological treatment and lifestyle modification in patients with nonalcoholic fatty liver disease: An umbrella review of meta-analyses of randomized controlled trials. Cho K, Park S, Koyanagi A, et al. Obes Rev. 2022;23:0. doi: 10.1111/obr.13464. [DOI] [PubMed] [Google Scholar]
- 25.Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial. Della Pepa G, Russo M, Vitale M, et al. Diabetes Res Clin Pract. 2021;178:108984. doi: 10.1016/j.diabres.2021.108984. [DOI] [PubMed] [Google Scholar]
- 26.Differential DNA methylation and changing cell-type proportions as fibrotic stage progresses in NAFLD. Johnson ND, Wu X, Still CD, et al. Clin Epigenetics. 2021;13:152. doi: 10.1186/s13148-021-01129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Insights Into Extracellular Vesicles as Biomarker of NAFLD Pathogenesis. Garcia-Martinez I, Alen R, Rada P, Valverde AM. Front Med (Lausanne) 2020;7:395. doi: 10.3389/fmed.2020.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Interobserver reproducibility of the controlled attenuation parameter (CAP) for quantifying liver steatosis. Ferraioli G, Tinelli C, Lissandrin R, et al. Hepatol Int. 2014;8:576–581. doi: 10.1007/s12072-014-9573-1. [DOI] [PubMed] [Google Scholar]
- 29.Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Carlsson B, Lindén D, Brolén G, Liljeblad M, Bjursell M, Romeo S, Loomba R. Aliment Pharmacol Ther. 2020;51:1305–1320. doi: 10.1111/apt.15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Relationship between controlled attenuated parameter and magnetic resonance imaging-proton density fat fraction for evaluating hepatic steatosis in patients with NAFLD. An Z, Liu Q, Zeng W, et al. Hepatol Commun. 2022;6:1975–1986. doi: 10.1002/hep4.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. Dulai PS, Sirlin CB, Loomba R. J Hepatol. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical utility of magnetic resonance imaging biomarkers for identifying nonalcoholic steatohepatitis patients at high risk of progression: a multicenter pooled data and meta-analysis. Andersson A, Kelly M, Imajo K, et al. Clin Gastroenterol Hepatol. 2022;20:2451–2461. doi: 10.1016/j.cgh.2021.09.041. [DOI] [PubMed] [Google Scholar]
- 34.Non-invasive diagnosis and staging of non-alcoholic fatty liver disease. Kechagias S, Ekstedt M, Simonsson C, Nasr P. Hormones (Athens) 2022;21:349–368. doi: 10.1007/s42000-022-00377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Impact of a pilot structured mobile technology based lifestyle intervention for patients with nonalcoholic fatty liver disease. Tincopa MA, Lyden A, Wong J, Jackson EA, Richardson C, Lok AS. Dig Dis Sci. 2022;67:481–491. doi: 10.1007/s10620-021-06922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Takahashi Y, Sugimoto K, Inui H, Fukusato T. World J Gastroenterol. 2015;21:3777–3785. doi: 10.3748/wjg.v21.i13.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Non-alcoholic fatty liver diseases: current challenges and future directions. Roeb E. Ann Transl Med. 2021;9:726. doi: 10.21037/atm-20-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]