Abstract
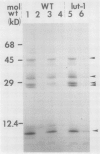
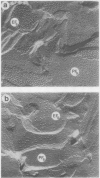
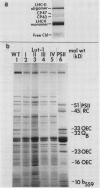
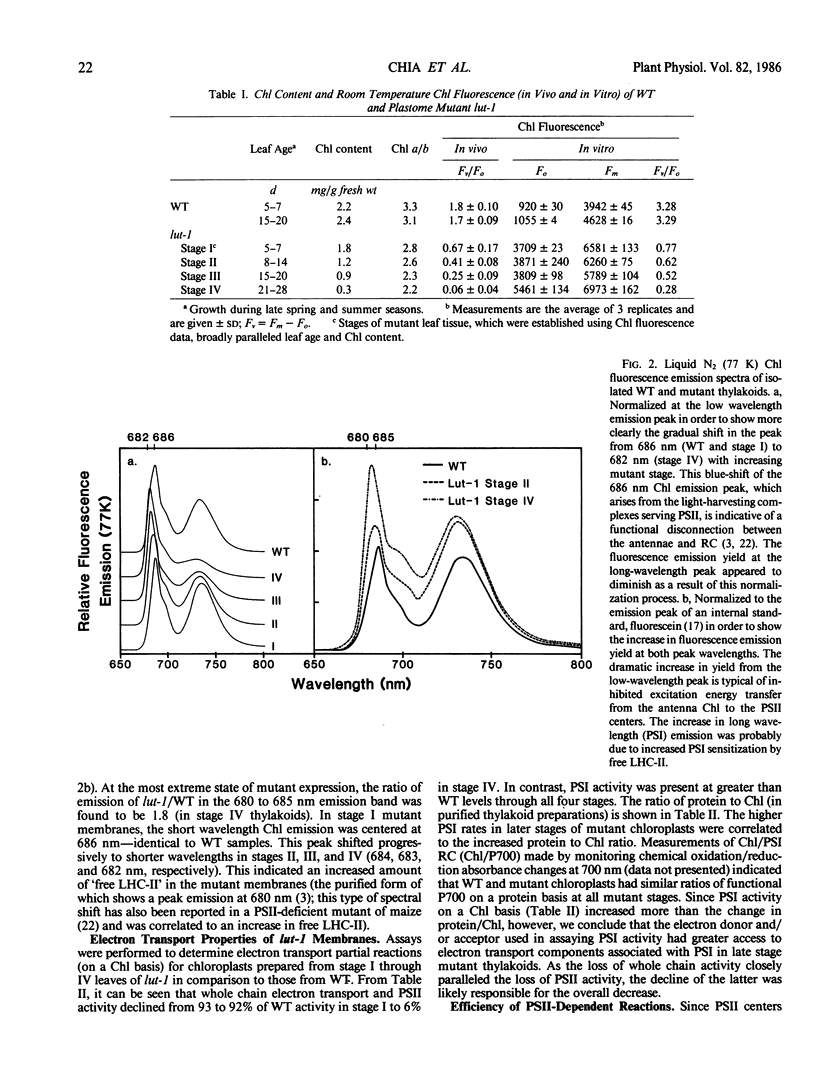
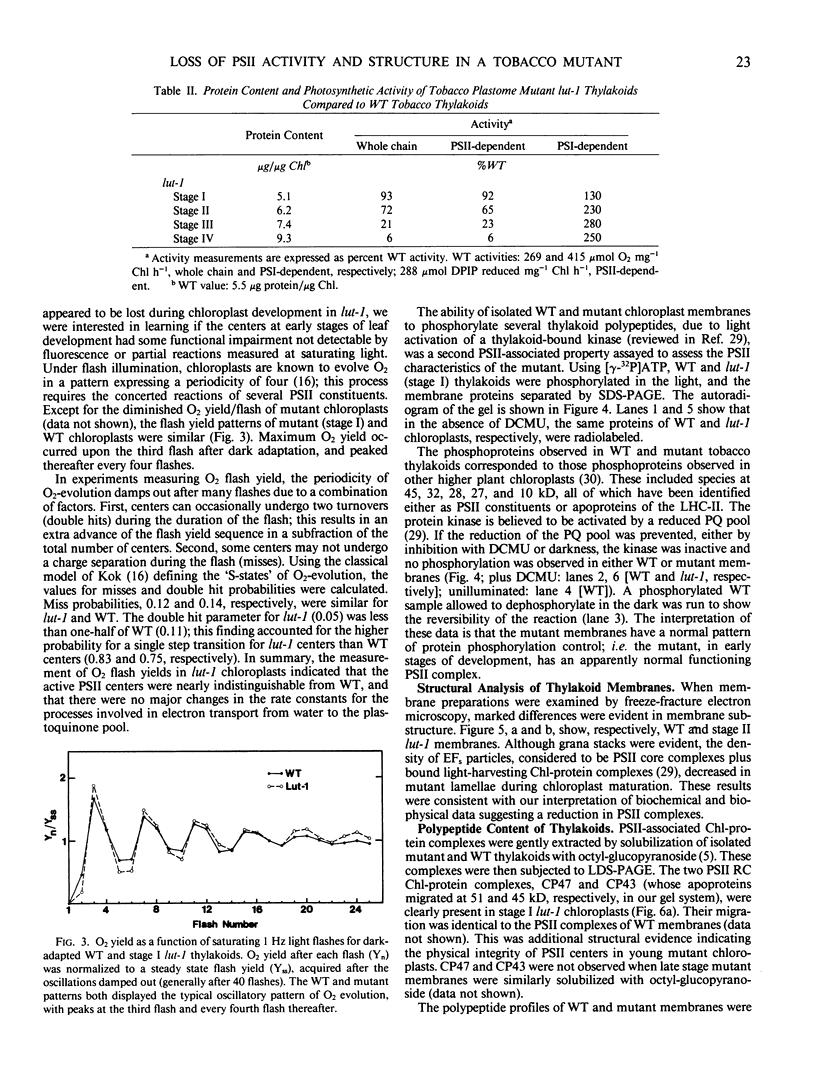
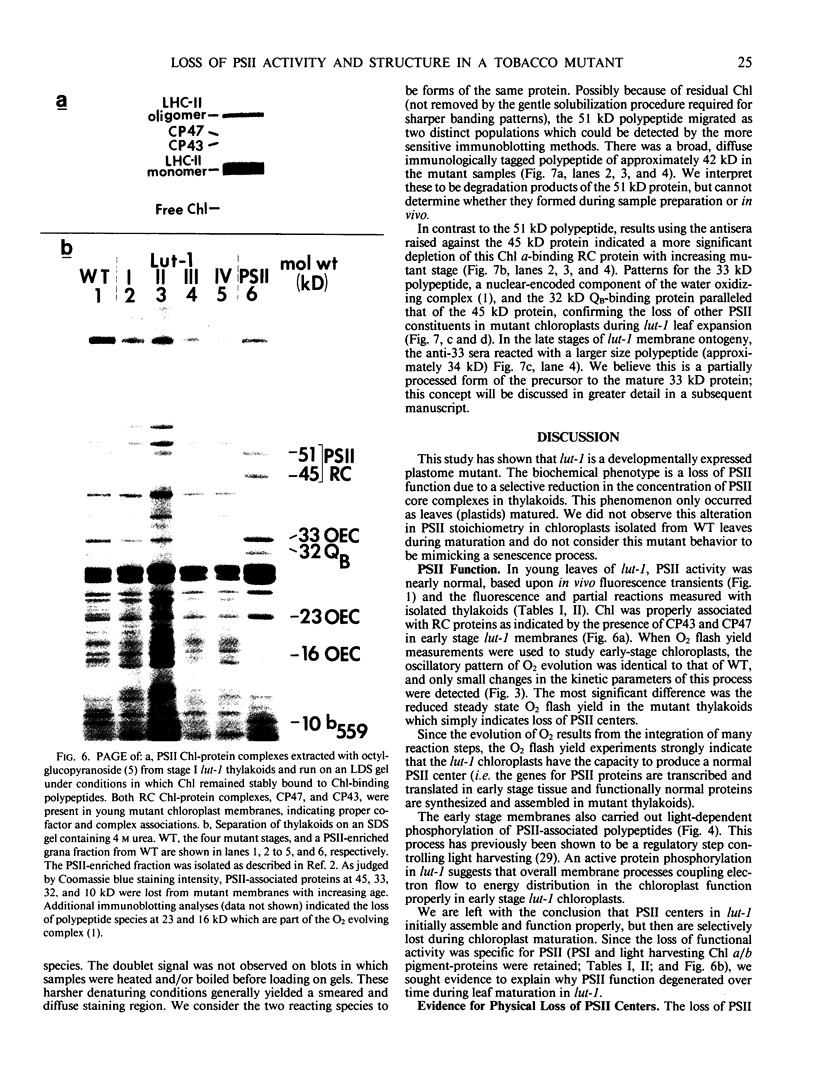
Lutescens-1, a tobacco mutant with a maternally inherited dysfunction, displayed an unusual developmental phenotype. In vivo measurement of chlorophyll fluorescence revealed deterioration in photosystem II (PSII) function as leaves expanded. Analysis of thylakoid membrane proteins by polyacrylamide gel electrophoresis indicated the physical loss of nuclear- and chloroplast-encoded polypeptides comprising the PSII core complex concomitant with loss of activity. Freeze fracture electron micrographs of mutant thylakoids showed a reduced density, compared to wild type, of the EFs particles which have been shown previously to be the structural entity containing PSII core complexes and associated pigment-proteins. The selective loss of PSII cores from thylakoids resulted in a higher ratio of antenna chlorophyll to reaction centers and an altered 77 K chlorophyll fluorescence emission spectra; these data are interpreted to indicate functional isolation of light-harvesting chlorophyll a/b complexes in the absence of PSII centers. Examination of PSII reaction centers (which were present at lower levels in mutant membranes) by monitoring the light-dependent phosphorylation of PSII polypeptides and flash-induced O2 evolution patterns demonstrated that the PSII cores which were assembled in mutant thylakoids were functionally identical to those of wild type. We conclude that the lutescens-1 mutation affected the correct stoichiometry of PSII centers, in relation to other membrane constituents, by disrupting the proper assembly and maintenance of PSII complexes in lutescens-1 thylakoid membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camm E. L., Green B. R. Fractionation of Thylakoid Membranes with the Nonionic Detergent Octyl-beta-d-glucopyranoside: RESOLUTION OF CHLOROPHYLL-PROTEIN COMPLEX II INTO TWO CHLOROPHYLL-PROTEIN COMPLEXES. Plant Physiol. 1980 Sep;66(3):428–432. doi: 10.1104/pp.66.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Darr S., Souza Machado V., Arntzen C. J. Uniparental inheritance of a chloroplast photosystem II polypeptide controlling herbicide binding. Biochim Biophys Acta. 1981 Jan 14;634(1):219–228. doi: 10.1016/0005-2728(81)90140-7. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Edelman M., Hallick R. B. Chloroplast-coded atrazine resistance in Solanum nigrum: psbA loci from susceptible and resistant biotypes are isogenic except for a single codon change. Nucleic Acids Res. 1984 Dec 21;12(24):9489–9496. doi: 10.1093/nar/12.24.9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Melis A., Thielen A. P. The relative absorption cross-sections of photosystem I and photosystem II in chloroplasts from three types of Nicotiana tabacum. Biochim Biophys Acta. 1980 Feb 8;589(2):275–286. doi: 10.1016/0005-2728(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Miller K. R., Cushman R. A. A chloroplast membrane lacking photosystem II. Thylakoid stacking in the absence of the photosystem II particle. Biochim Biophys Acta. 1979 Jun 5;546(3):481–497. doi: 10.1016/0005-2728(79)90083-5. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., Bose S., Kyle D. J. Phosphorylation of the light-harvesting chlorophyll-protein regulates excitation energy distribution between photosystem II and photosystem I. Arch Biochem Biophys. 1982 Jun;216(1):356–361. doi: 10.1016/0003-9861(82)90221-1. [DOI] [PubMed] [Google Scholar]