Abstract
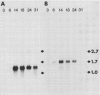
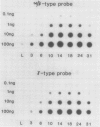
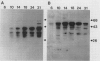
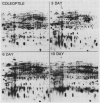
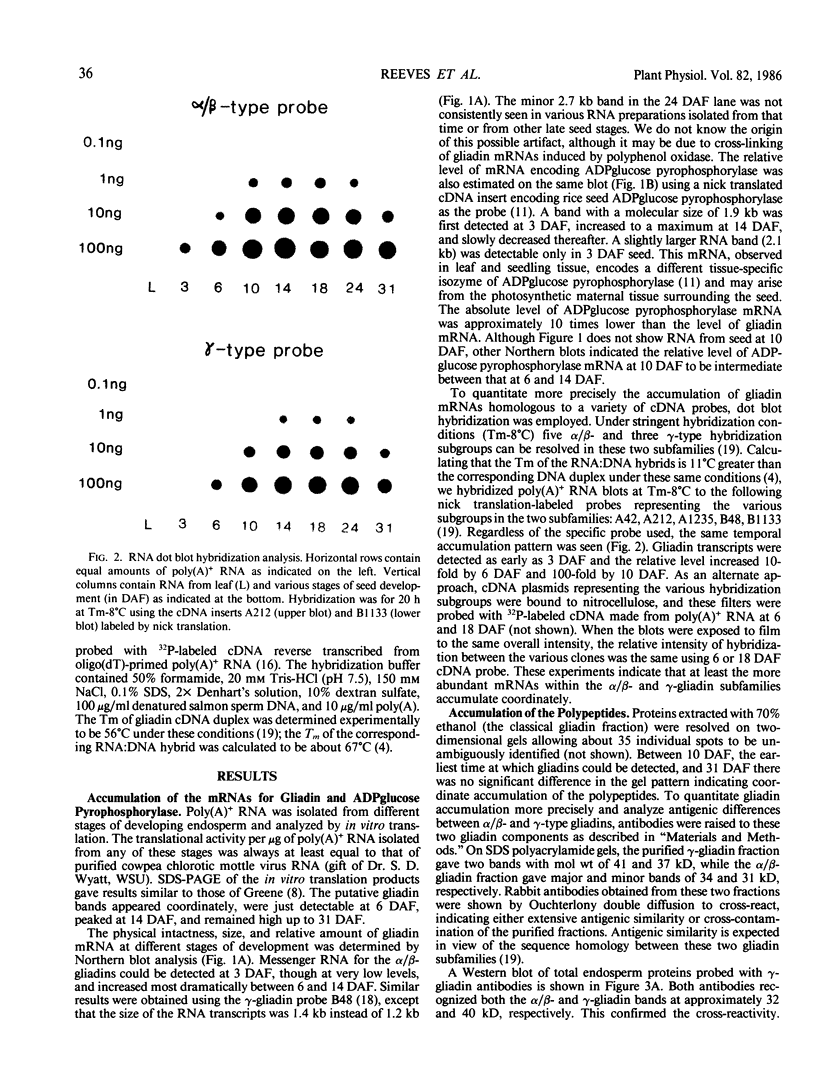
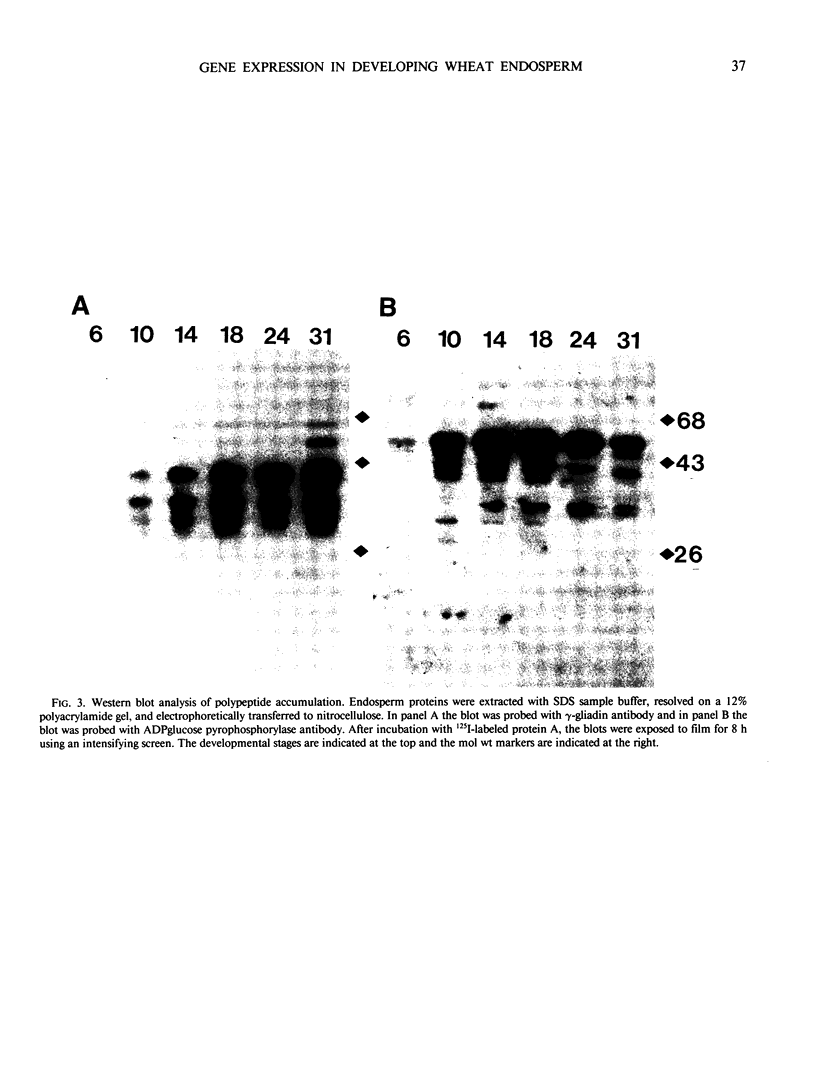
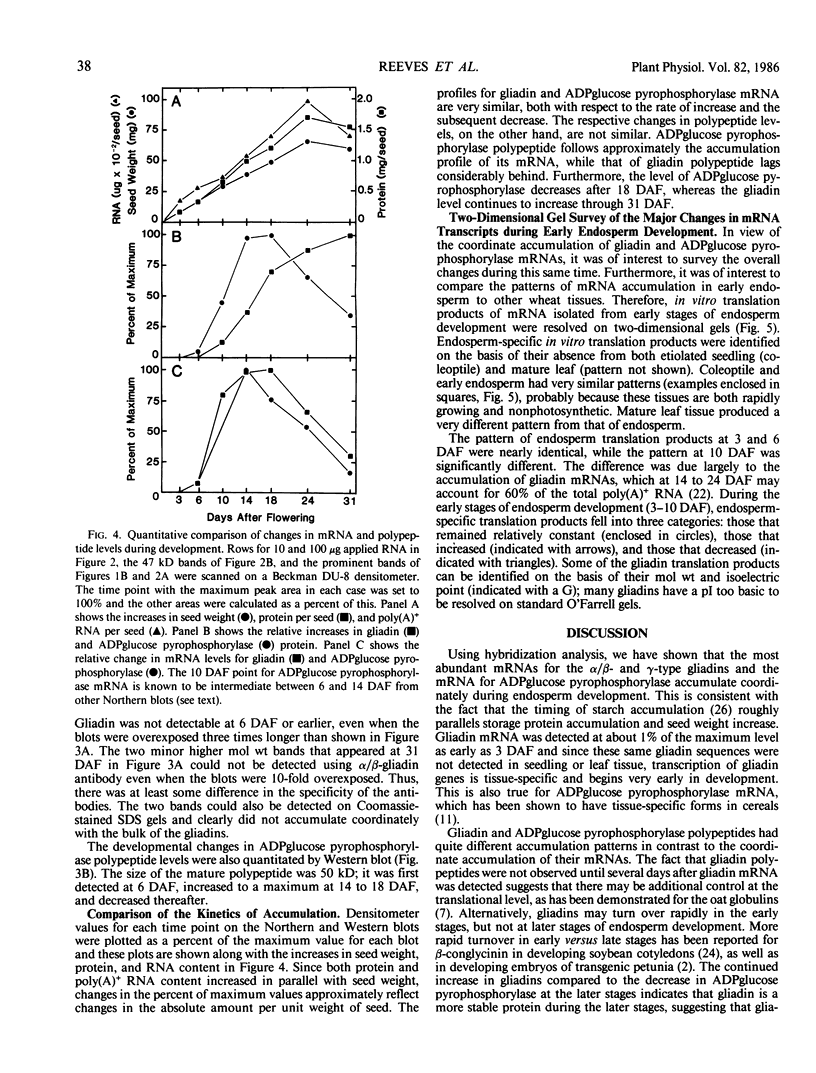
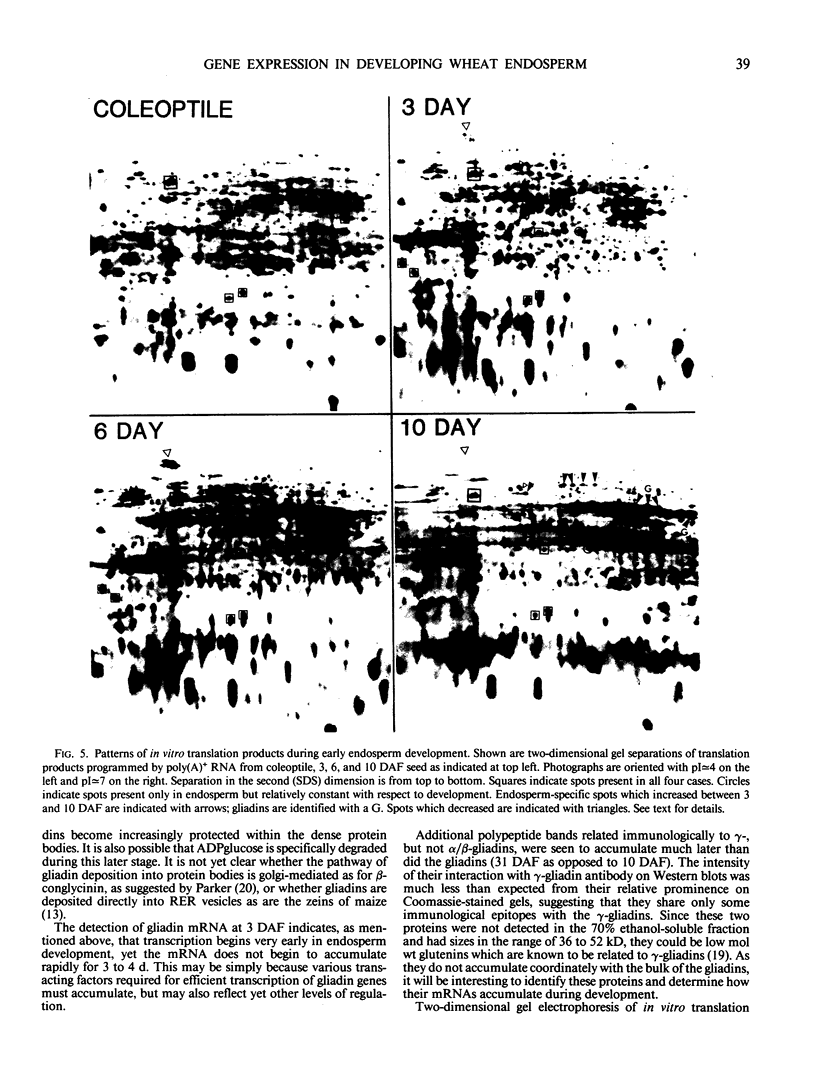
The developmental accumulation pattern of messenger RNA transcripts and polypeptides for wheat gliadins and ADPglucose pyrophosphorylase was determined using cDNA and antibody probes. Gliadin mRNA was detected on Northern and RNA dot blots at 3 days after flowering, it increased 100-fold by 10 days and decreased subsequent to 14 days. The abundant mRNAs encoding α/β- and γ-type gliadins and mRNA for ADPglucose pyrophosphorylase, a key regulatory enzyme of starch biosynthesis, accumulated coordinately. Despite the coordinate accumulation of their mRNA transcripts, the accumulation of gliadin and ADPglucose pyrophosphorylase polypeptides, as determined by Western blot, differed significantly. The time at which gliadin and ADPglucose pyrophosphorylase mRNAs began accumulating was also the time when the overall pattern of gene expression, as seen by two-dimensional gel electrophoresis of in vitro translation products, changed most significantly. However, the accumulation of a number of other mRNAs or polypeptides having unknown function occurred at other times during endosperm development. The pattern of expression in the earliest stages of development was strikingly similar to that of coleoptile, another rapidly growing, nonphotosynthetic tissue. Thus, the pattern of gene expression reflects the program of development observed cytologically.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Straus J. W., Dudock B. S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Jacobs K. A., Eickbush T. H., Cherbas P. T., Kafatos F. C. Isolation of multigene families and determination of homologies by filter hybridization methods. Methods Enzymol. 1983;100:266–285. doi: 10.1016/0076-6879(83)00061-0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Greene F. C. Expression of Storage Protein Genes in Developing Wheat (Triticum aestivum L.) Seeds : Correlation of RNA Accumulation and Protein Synthesis. Plant Physiol. 1983 Jan;71(1):40–46. doi: 10.1104/pp.71.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H. B., Reeves C. D., Okita T. W. ADPglucose Pyrophosphorylase Is Encoded by Different mRNA Transcripts in Leaf and Endosperm of Cereals. Plant Physiol. 1986 Jun;81(2):642–645. doi: 10.1104/pp.81.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M. Methods for the preparation of messenger RNA. Methods Enzymol. 1983;96:24–38. doi: 10.1016/s0076-6879(83)96006-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Cheesbrough V., Reeves C. D. Evolution and heterogeneity of the alpha-/beta-type and gamma-type gliadin DNA sequences. J Biol Chem. 1985 Jul 5;260(13):8203–8213. [PubMed] [Google Scholar]
- Pernollet J. C., Vaillant V. Characterization and Complexity of Wheat Developing Endosperm mRNAs. Plant Physiol. 1984 Sep;76(1):187–190. doi: 10.1104/pp.76.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttuck-Eidens D. M., Beachy R. N. Degradation of beta-Conglycinin in Early Stages of Soybean Embryogenesis. Plant Physiol. 1985 Aug;78(4):895–898. doi: 10.1104/pp.78.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]