Abstract
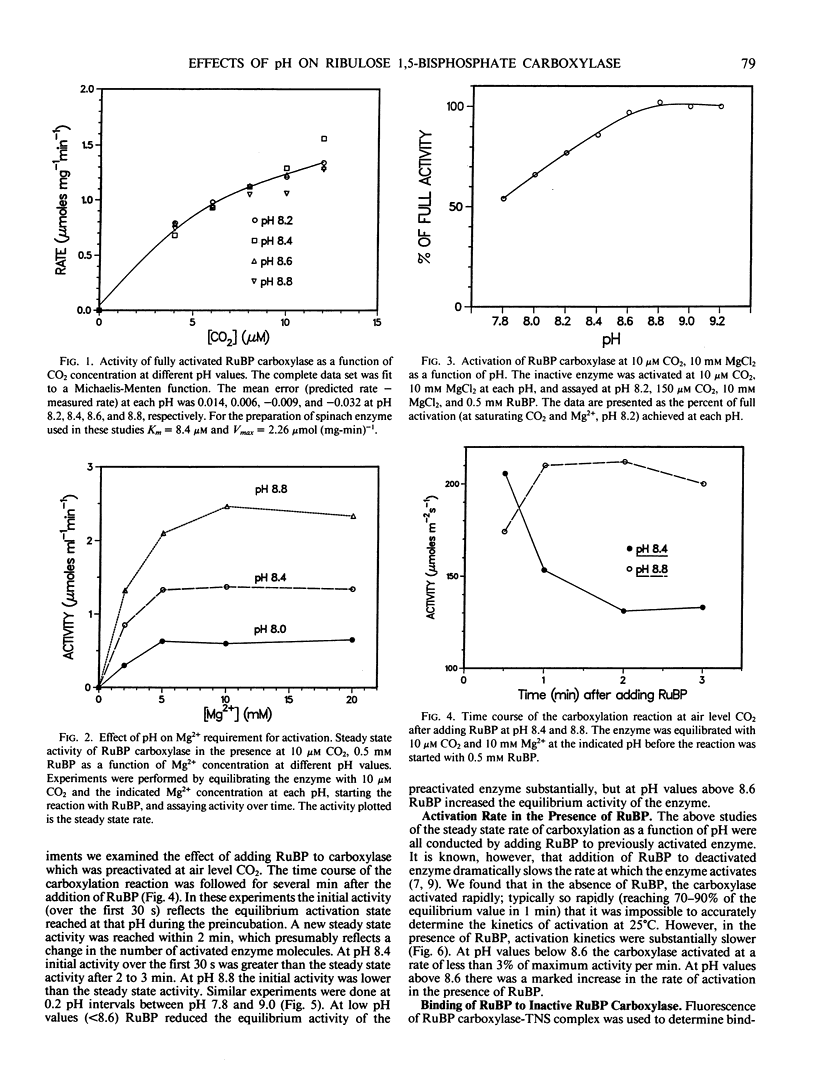
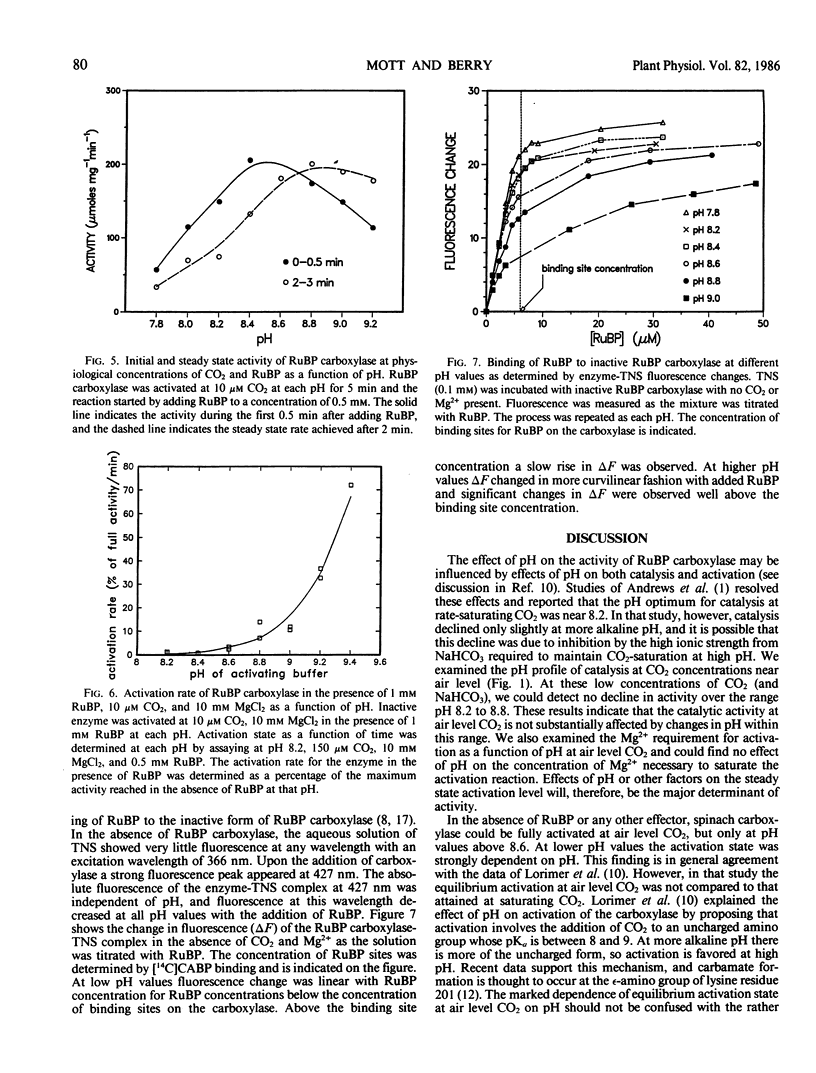
The effects of pH on catalysis and activation characteristics of spinach ribulose 1,5-bisphosphate (RuBP) carboxylase were examined at air level of CO2. Catalysis at limiting CO2 was independent of pH over the range of pH 8.2 to 8.8 However, the kinetics of activation and the apparent equilibrium between the activated and inactivated forms of the enzyme were strongly dependent upon the pH and the presence or absence of the substrate RuBP. When incubated at air level of CO2 at pH 8.2 in the absence of RuBP, the enzyme activation state was approximately 75% of that achieved with saturating CO2 at that pH. The extent of activation increased with pH reaching 100% at pH values of 8.6 or higher. Adding RuBP to the activation medium after equilibrium activation state had been established decreased the apparent equilibrium activation level at pH values below 8.6. This effect was reversed at pH values above 8.6. Activation of inactive enzyme by CO2 and Mg2+ was inhibited dramatically at pH values below 8.6 and less so at pH values above 8.6. Studies showed that binding of RuBP to the inactive form of the enzyme was pH dependent with tighter binding occurring at lower pH values. It is suggested that the tight binding of RuBP to the inactive enzyme tends to decrease the equilibrium concentration of the activated form at pH values less than 8.6. These studies indicate that stromal pH could have a strong effect on the activation state of this enzyme in vivo, and possible feedback interactions which might adjust the apparent Vmax to match the rate of RuBP regeneration are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Badger M. R., Lorimer G. H. Factors affecting interconversion between kinetic forms of ribulose diphosphate carboxylase-oxygenase from spinach. Arch Biochem Biophys. 1975 Nov;171(1):93–103. doi: 10.1016/0003-9861(75)90011-9. [DOI] [PubMed] [Google Scholar]
- Badger M. R., Lorimer G. H. Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1981 Apr 14;20(8):2219–2225. doi: 10.1021/bi00511a023. [DOI] [PubMed] [Google Scholar]
- Evans J. R., Seemann J. R. Differences between Wheat Genotypes in Specific Activity of Ribulose-1,5-bisphosphate Carboxylase and the Relationship to Photosynthesis. Plant Physiol. 1984 Apr;74(4):759–765. doi: 10.1104/pp.74.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Jordan D. B., Chollet R. Inhibition of ribulose bisphosphate carboxylase by substrate ribulose 1,5-bisphosphate. J Biol Chem. 1983 Nov 25;258(22):13752–13758. [PubMed] [Google Scholar]
- Kwok S. U., Wildman S. G. Studies on a conformational change of tobacco ribulose diphosphate carboxylase induced by D-ribulose-1,5-diphosphate. Arch Biochem Biophys. 1974 Apr 2;161(2):354–359. doi: 10.1016/0003-9861(74)90315-4. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J. 1976 Dec 1;159(3):563–570. doi: 10.1042/bj1590563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- McClure W. O., Edelman G. M. Fluorescent probes for conformational states of proteins. I. Mechanism of fluorescence of 2-p-toluidinylnaphthalene-6-sulfonate, a hydrophobic probe. Biochemistry. 1966 Jun;5(6):1908–1919. doi: 10.1021/bi00870a018. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Mott K. A., Jensen R. G., O'leary J. W., Berry J. A. Photosynthesis and Ribulose 1,5-Bisphosphate Concentrations in Intact Leaves of Xanthium strumarium L. Plant Physiol. 1984 Dec;76(4):968–971. doi: 10.1104/pp.76.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Jensen R. G. Photosynthesis and Activation of Ribulose Bisphosphate Carboxylase in Wheat Seedlings : Regulation by CO(2) and O(2). Plant Physiol. 1983 Apr;71(4):955–960. doi: 10.1104/pp.71.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater J., Salnikow J., Kleinkauf H. A fluorimetric study of substrate and effector binding of D-ribulose-1,5-biphosphate carboxylase/oxygenase from spinach. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1618–1625. doi: 10.1016/0006-291x(77)90628-3. [DOI] [PubMed] [Google Scholar]
- Yokota A., Kitaoka S. Correct pK values for dissociation constant of carbonic acid lower the reported Km values of ribulose bisphosphate carboxylase to half. Presentation of a nomograph and an equation for determining the pK values. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1075–1079. doi: 10.1016/0006-291x(85)90200-1. [DOI] [PubMed] [Google Scholar]


