SUMMARY
Despite its central importance in cellular metabolism, many details remain to be determined regarding subcellular lactate metabolism and its regulation in physiology and disease, as there is sensitive spatiotemporal resolution of lactate distribution, and dynamics remains a technical challenge. Here, we develop and characterize an ultrasensitive, highly responsive, ratiometric lactate sensor, named FiLa, enabling the monitoring of subtle lactate fluctuations in living cells and animals. Utilizing FiLa, we demonstrate that lactate is highly enriched in mammalian mitochondria and compile an atlas of subcellular lactate metabolism that reveals lactate as a key hub sensing various metabolic activities. In addition, FiLa sensors also enable direct imaging of elevated lactate levels in diabetic mice and facilitate the establishment of a simple, rapid, and sensitive lactate assay for point-of-care clinical screening. Thus, FiLa sensors provide powerful, broadly applicable tools for defining the spatiotemporal landscape of lactate metabolism in health and disease.
Graphical Abstract
In brief
Li et al develop a series of high-performance lactate sensors for tracking transient changes in lactate metabolism both in vitro and in vivo. Measurements with the lactate sensor FiLa reveal an enrichment for lactate in mitochondria. The ultrasensitive lactate sensors also provide fast and convenient lactate assays for point-of-care clinical screening.
INTRODUCTION
Lactate was initially considered a waste product derived from glycolysis; however, it has now emerged as a major metabolic fuel (Faubert et al., 2017; Hui et al., 2017; Rabinowitz and Enerbäck, 2020), an important anabolic building block (Brooks, 2018), and a significant signaling molecule (Daw et al., 2020; Zhang et al., 2019b). Owing to its fundamental importance, lactate metabolism is woven into a large number of physiologic or pathological processes, including histone modification (Zhang et al., 2019a), hypoxia response (Lee et al., 2015), autophagy (Velentzas et al., 2018), neural activity (Magistretti and Allaman, 2018; Suzuki et al., 2011), spermatogenesis (Boussouar and Benahmed, 2004), embryonic development (Oginuma et al., 2017), aging (Iatsenko et al., 2018), immunity (Marin et al., 2019; Xu et al., 2021), and cancer metabolism (Doherty and Cleveland, 2013; Faubert et al., 2017; Watson et al., 2021). In vivo, lactate shows dynamic spatial distributions in different subcellular compartments, cell species, tissues, and body fluids. Lactate has long been used as a metabolic indicator of disease states. Lactate therapy may be efficacious for the treatment of diseases such as poorly controlled diabetes, traumatic brain injury, heart failure, inflammation, and immunosuppression (Brooks, 2018). Thus, dissecting the biological significance and increasingly recognized complexity of lactate metabolism require the development of molecular tools that provide sensitive spatiotemporal resolution for real-time monitoring and imaging.
Despite their urgent need, tools for in vivo and in situ tracking of lactate are limited. Currently, researchers depend largely on measuring lactate in extracellular medium or cell lysate by enzymatic cycling assays, chromatography, or mass spectrometry or measuring extracellular acidification rate (ECAR) by a Seahorse analyzer. However, these methods are not only time intensive or costly but also are incompatible with the study of spatiotemporal dynamics in single, live cells or in vivo. Recent advances have shown that challenges of live-cell metabolism monitoring may be resolved by the use of genetically encoded fluorescent sensors, which bind and respond to specific metabolites such as ATP (Berg et al., 2009; Lobas et al., 2019), NAD(H) (Bilan et al., 2014; Cambronne et al., 2016; Hung et al., 2011; Zhao et al., 2015; Zhao et al., 2011, 2016; Zou et al., 2020), or oxidized and reduced nicotinamide adenine dinucleotide phosphate (NADP(H)) (Cameron et al., 2016; Tao et al., 2017; Zou et al., 2018). These sensors form intrinsic fluorophores in vivo, are generally capable of specific cell or subcellular organelle targeting, and allow accurate spatiotemporal monitoring of metabolites. Genetically encoded fluorescent sensors have been reported for lactate monitoring (Bekdash et al., 2021; Harada et al., 2020; Koveal et al., 2022; Nasu et al., 2021; San Martín et al., 2013); however, these sensors have rather small dynamic ranges in vitro and/ or in live cells, undesirable response to calcium, or inappropriate (physiologically) affinity. Such limitations make them difficult to quantify subtle lactate fluctuations in vitro and in vivo.
To fill the current technical gap, we herein have developed a series of ultrasensitive, highly responsive, ratiometric, genetically encoded lactate indicators, denoted FiLa (fluorescent indicators of lactate). We demonstrate the utility of FiLa sensors in subcellular organelles, single cells, live mice, high-throughput screening, and human serum and urine by monitoring lactate dynamics. FiLa sensor shall pave the way for precision diagnostics of lactate metabolism in fundamental, translational, and clinical research.
DESIGN
For single fluorescent protein sensors, a circularly permuted fluorescent protein (cpFP) is usually utilized and inserted into a loop region of a ligand-sensing protein or between tandem units of proteins for developing important metabolite sensors (Zhao and Yang, 2015; Zhao et al., 2018). Previous studies have shown that lactate binding induces significant conformational changes in Escherichia coli LldR (Aguilera et al., 2008), which is, therefore, a promising candidate for sensor design (Figure 1A). To engineer ultrasensitive and highly responsive lactate sensors, we first designed 90 chimeric proteins by inserting circularly permuted yellow fluorescent protein (cpYFP) between two complete or truncated subunits of LldR or between amino acid residues located on the random coiled loops of LldR with or without the DNA-binding domain of LldR (Figures 1B and S1A). Among them, the chimera with cpYFP inserted between Y186 and P189 of LldR, deleting the DNA-binding domain in the process, showed a ~200% increase in the ratio of fluorescence when excited at 485 and 420 nm upon lactate addition (Figure S1B). We then created a series of truncated variants of this chimera in the 182–189 residue regions of LldR and found that the M185/P189 variant exhibited a ~420% increase in the fluorescence ratio (R485/420) upon lactate addition (Figure S1C). To further expand the dynamic range and increase the selectivity toward lactate, we created a library of random mutants (~700 variants) targeting residues P189 and P190 based on the M185/P189 chimera (Figures 1B and S1D). Among all of these encoded variants, P189C/P190D (CD), P189M/P190D (MD), P189H/P190D (HD), and P189F/P190D (FD) displayed a dynamic range (∆F/F) of ~470%, ~740%, ~1,400%, and ~2,600% (named FiLa-H), respectively, and their affinities for lactate ranged from 20 to 50 μM (Figures S1D and S1E). Physiological lactate levels have been estimated as 0.8–2 mM in blood (Brooks, 2018) or 0.6–1 mM within mammalian cells (Brooks, 2018; Zhang et al., 2019b), possibly exceeding the affinities of these sensors for lactate. Thus, to tune the affinity of these lactate indicators (Figure 1B), we next created a library of site-saturation mutants targeting residue M185, based on the CD, MD, HD, and FD chimera (Figure S1F). These lactate indicators had various affinities, ranging from ~19 μM to ~1 mM, as shown by the ratio of fluorescence when excited at 485 and 420 nm. Considering the maximum fluorescence ratio change, affinity, and expression level, we chose the variant (M185L/P189H/P190D), denoted FiLa, for further characterization (Figure S1F; Table S1).
Figure 1. Engineering of ultrasensitive and highly responsive lactate sensors.
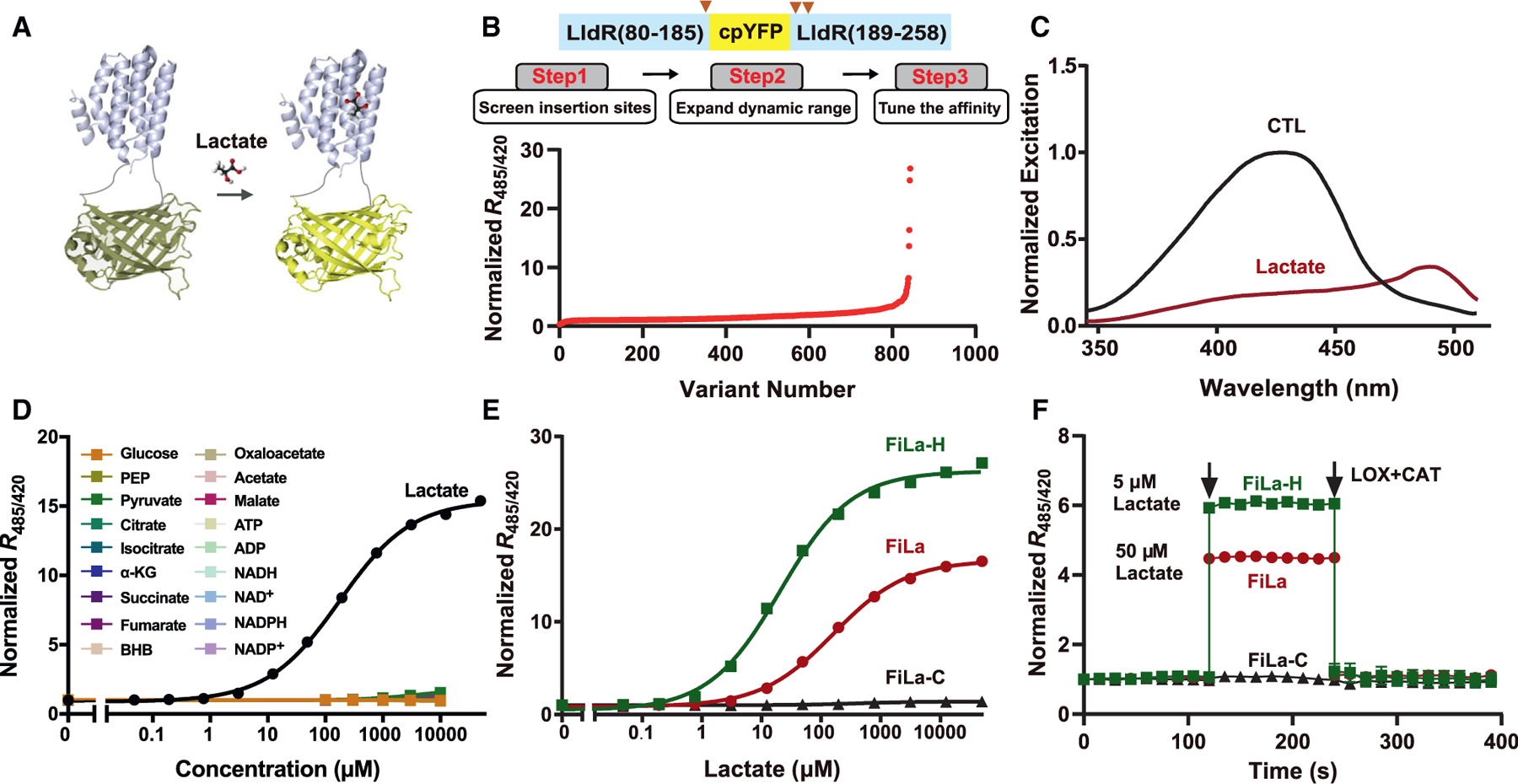
(A) Schematic representation of the lactate sensor FiLa. The fluorescent protein cpYFP was inserted into a monomer of the lactate-binding bacterial protein LldR. Binding of lactate induces changes in protein conformation and fluorescence.
(B) Engineering of ultrasensitive and highly responsive lactate sensors. A total of 843 variants was screened, including cpYFP insertion sites, truncated variants, random mutants, and site-saturation mutants.
(C) Excitation spectra of purified FiLa in the control condition (black) and saturated with lactate (dark red). The excitation spectrum recorded at an emission wavelength of 530 nm has maxima around 425 and 490 nm. Data are normalized to the peak intensity in the control condition.
(D) Fluorescence response of FiLa at the indicated concentration of lactate or other metabolites. Data are normalized to the initial value (n = 3). PEP, phosphoenolpyruvate; α-KG, α-ketoglutarate; BHB, β-hydroxybutyrate.
(E) Lactate titration curves of FiLa, FiLa-H, and FiLa-C sensors. Data are normalized to the initial value (n = 3).
(F) Kinetics of fluorescence response of purified FiLa and FiLa-H protein to 50 μM lactate and 5 μM lactate, respectively. Lactate was subsequently decreased by the addition of 100 μM lactate oxidase (LOX) and 500 U/mL catalase (CAT). Data are normalized to the initial value (n = 3). Data are the mean ± SEM (D–F). See also Figure S1 and Tables S1 and S2.
RESULTS
Characterization of ultrasensitive and highly responsive lactate sensors
FiLa has two excitation peaks around 425 and 490 nm and one emission peak near 514 nm (Figures 1C, S1G, and S1H). Upon lactate binding, FiLa showed a 3.0-fold increase and 5.3-fold decrease in fluorescence when excited at 485 and 420 nm, respectively (Figure S1I), leading to a ~1,500% ratiometric fluorescence change that was essentially unaffected by temperature fluctuations between 20°C and 40°C (Figure S1J). Fluorescence titration studies showed that FiLa had an apparent dissociation constant (Kd) ~130 μM at pH 7.4 (Figures 1D and 1E). FiLa has high selectivity toward lactate, showing no apparent fluorescence changes toward or in the presence of nucleotides (i.e., ADP/ATP, NAD+/NADH, and NADP+/NADPH), the metabolites in glycolysis and the tricarboxylic acid (TCA) cycle, all 20 amino acids, and Ca2+/Mg2+ (Figures 1D and S1K–S1R), and its extinc coefficients, quantum yields, and brightness in the presence and absence of lactate were included in Table S2.
FiLa fluorescence excited at 420 nm was much less sensitive to pH, allowing the pH-resistant measurement of lactate levels (Figure S1S). By contrast, FiLa fluorescence excited at 485 nm was sensitive to pH, but its Kd is more pH insensitive (Figures S1T–S1V). To correct the potential pH effects, we also created a control FiLa sensor, FiLa-C, by introducing two mutations in LldR (P189R/P190G), which almost completely abolished ligand binding (Figures 1E, S1W, and S1X). FiLa-C and FiLa displayed similar sensitivities to pH; thus, the pH effects on FiLa fluorescence excited at 485 nm could be accounted for by normalizing to FiLa-C (Figures S1Y and S1Z) when modest pH fluctuations occurred. To confirm the reversibility of FiLa, we sequentially added lactate and lactate oxidase/catalase, which induced a stepped increase and subsequent decrease in lactate concentration (Figure 1F). The resulting data showed that FiLa responded rapidly and was suitable for use in real-time measurements. Collectively, FiLa sensors manifest excellent sensitivity, selectivity, and a wide dynamic range, rendering it a very useful reagent for monitoring lactate metabolism in vitro and in vivo.
Lactate is highly enriched in mammalian mitochondria
Recent studies have shown that lactate can feed the TCA cycle in the mitochondria (Hui et al., 2017) or lactylate histones in the nucleus (Zhang et al., 2019a); however, there is no direct evidence of local lactate availability in these compartments. To investigate subcellular lactate distribution in live mammalian cells, we, therefore, stably expressed the FiLa sensor in different subcellular compartments. Fluorescence imaging and a microplate assay showed slight differences between cytosolic and nuclear signals (Figures 2A and 2B). Surprisingly, however, FiLa analysis showed not only that there was a lactate pool inside mitochondria but also that the lactate level in mitochondria was significantly higher than that in the cytosol or nucleus (Figures 2A and 2B). Super-resolution microscopy revealed that the FiLa-Mit colocalized with the mitochondrial matrix probe mitochondria-tagRFP but not the mitochondrial intermembrane space probe MitoTracker Red (Figures S2A and S2B). The matrix localization of the FiLa-Mit sensor was further confirmed by sub-fractionation and immunoblotting of markers for the outer-membrane, the inner-membrane, the intermembrane space, and the matrix fractions (Feng et al., 2013; Marunouchi et al., 2013) (Figure S2C). Quantitative analysis in different cell lines showed that cytosolic/nuclear and mitochondrial lactate concentrations in glucose-fed cells were ~53–89 and ~268–1,025 mM, respectively (Figure 2B). Glucose deprivation significantly decreased lactate levels in these subcellular compartments, with mitochondria lactate remaining the highest (Figure 2B). These data suggested that lactate levels in various subcellular compartments are all sensitive to changes in glucose levels and metabolism.
Figure 2. Lactate is highly enriched in mammalian mitochondria.
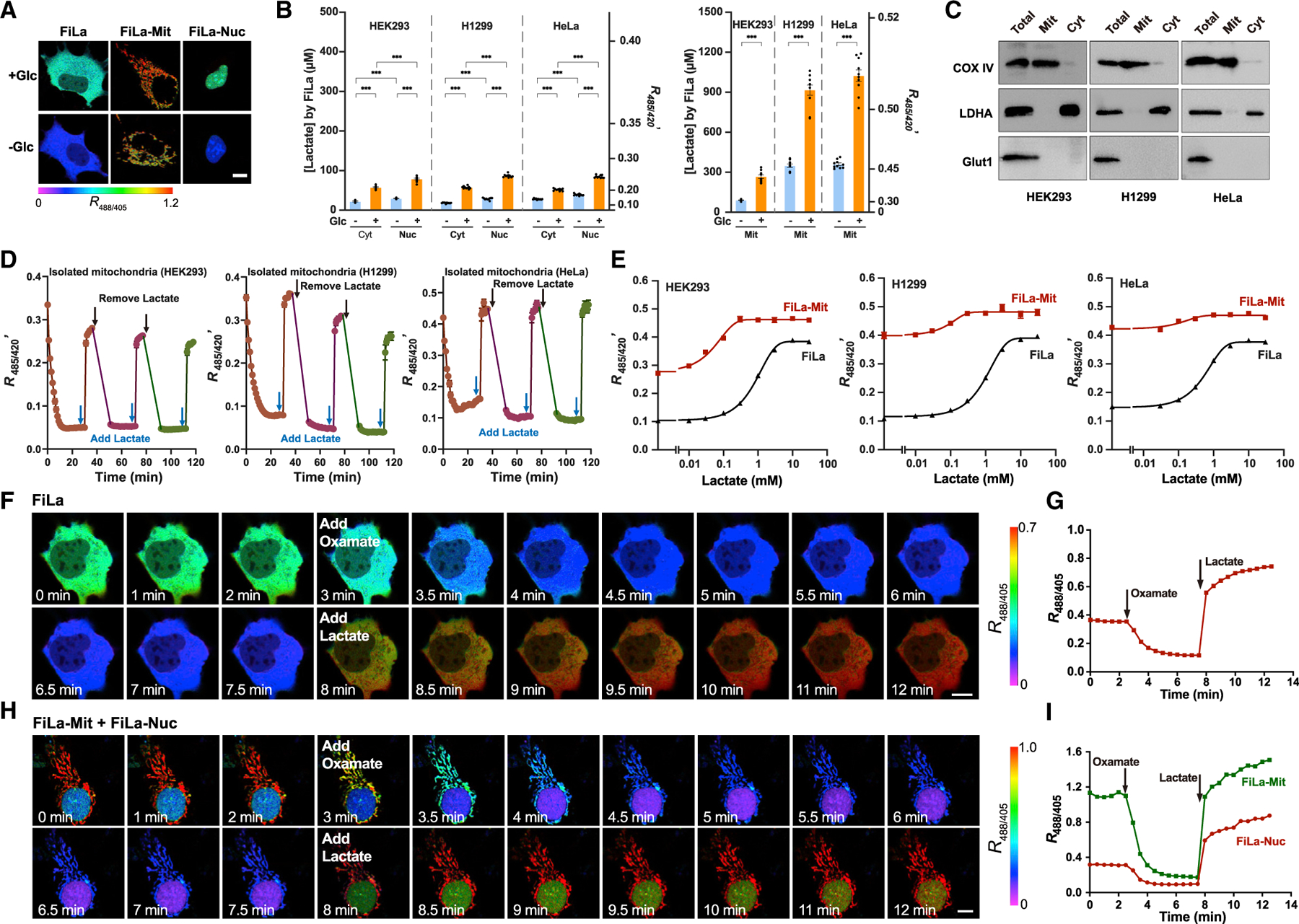
(A) Ratiometric fluorescence images of HEK293 cells expressing FiLa in cytosol, mitochondria, and nucleus. Images were pseudocolored by R488/405. Cells were cultured in the presence or absence of 25-mM glucose. Scale bars, 10 μm.
(B) Subcellular distribution (left: cytosol/nucleus, right: mitochondria) of lactate measured by FiLa in HEK293, H1299, and HeLa cells (n = 10).
(C) Western blot showing responses of total cellular, mitochondrial, and cytosolic fractions probed with antibodies to cytochrome c oxidase IV (COX IV, mitochondrial fraction marker), lactate dehydrogenase A (LDHA, cytosolic fraction marker), and glucose transporter type 1 (Glut1, plasma membrane marker).
(D) Kinetics of FiLa fluorescence in isolated mitochondria of three cell types in response to successive addition or removal of 1 mM lactate (n = 5). 0.95 × 108 mitochondria from HEK293, 1.09 × 108 from HeLa, and 2.25 × 108 from H1299 were used for each measurement, respectively.
(E) Fluorescence responses of cells expressing FiLa-Mit or FiLa to different concentrations of lactate. Fluorescence was measured immediately after lactate addition and normalized to FiLa-C sensor (n = 4).
(F and G) Fluorescence images (F) and quantification (G) of FiLa in HEK293 cells upon 5 mM oxamate and 2 mM lactate in succession at the indicated time. Scale bars, 10 μm.
(H and I) Fluorescence images (H) and quantification (I) of FiLa expressed in the nucleus and mitochondria of HEK293 cells. Cells were treated with 5 mM oxamate and 2 mM lactate successively at the indicated time. Scale bars, 10 μm. Data are the mean ± SEM (B, D, and E). All p values were obtained using unpaired two-tailed Student’s t-test. ***p < 0.001.
See also Figure S2.
To further validate our surprising finding that lactate was highly abundant in mitochondria, we isolated intact mitochondria from cells expressing the FiLa-Mit sensor and investigated lactate dynamics. The isolated HEK293, H1299, or HeLa mitochondrial fractions had little contamination of cytosol or plasma membrane preparations, as shown by immunoblots of subcellular organelle markers (Figure 2C). The mitochondrial membrane integrities were assessed by NADH oxidation-based oxygen consumption rate (OCR) measurement, citrate synthase activity analysis, and the respiratory control ratio (RCR, state 3/state 4) detection, respectively. The addition of NADH did not change the OCR, and quantitative analysis of citrate synthase activity showed that mitochondrial preparations were ~90%–92% intact (Figures S2D and S2E). The RCR values of isolated mitochondria were >10 for HEK293 and H1299 cells and >7.0 for HeLa cells, suggesting the respiration activity of isolated mitochondria were well preserved (Figures S2F and S2G).
Intriguingly, the isolated mitochondrial fractions showed minimal lactate content after ~30-min incubation as monitored by FiLa fluorescence (Figure 2D), consistent with biochemical assay (Figure S2H). When the mitochondria preparations were incubated with buffer containing different levels of lactate, mitochondrial lactate increased significantly and dose dependently, as shown by FiLa’s fluorescence (Figure S2I). To assure the majority of mitochondria were tracking exogenous lactate, we also performed fluorescence imaging and flow cytometric analysis of isolated mitochondria. Both measurements showed that FiLa-Mit fluorescence ratios of most mitochondria significantly raised in the presence of exogenous lactate, whereas the readout of FiLa-C-Mit showed minimal changes (Figures S2J–S2M). After the lactate incubation, mitochondria lactate levels were significantly higher than lactate levels in the buffer, and there was a small yet significant decrease of lactate level in the buffer, as measured by the FiLa sensor (Figure S2I) and biochemical assay (Figure S2N). All these data suggested the lactate enrichment in mitochondria. Furthermore, we found that the uncoupler, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), significantly decreased mitochondrial membrane potential and inhibited lactate enrichment in isolated mitochondria upon lactate incubation (Figures S2I, S2N, and S2O), suggesting that lactate transport into mitochondria depended on the membrane potential. When the mitochondria were centrifuged and resuspended in buffer containing no lactate, FiLa fluorescence again decreased rapidly within 10 min (Figures 2D and S2P). These data showed that mitochondria lose their lactate content during isolation and incubation and that lactate can be transported into mitochondria.
Addition of exogenous lactate into the culture medium induced a rapid, dose-dependent, and saturable increase in the fluorescence ratio in both cytosol or mitochondria of glucose-deprived HEK293, H1299, and HeLa cells (Figures 2E, S2Q, and S2R), suggesting that lactate was readily transported across the plasma membrane and mitochondrial membrane. Furthermore, FiLa responded immediately to sequential inhibition of lactate dehydrogenase (LDH) with oxamate or addition of lactate to single living cells (Figures 2F and 2G), again demonstrating its excellent performance in real-time assays. Similar results were obtained when FiLa was expressed in mitochondria and the nucleus simultaneously (Figures 2H and 2I). By contrast, slight changes in fluorescence were observed in control FiLa-C-expressing cells with oxamate treatment or lactate (Figures S2S and S2T). Quantitative results of microplate assay showed that mitochondrial FiLa ratios were 0.045 and 0.48 (~1,067% change) upon oxamate or lactate addition, respectively (Figure S2U). To simultaneously image two key metabolic parameters in single living cells, we expressed FiLa sensor in the cytosol using a nuclear export signal peptide, SoNar (a NADH/NAD+ ratio biosensor) (Zhao et al., 2015) in the nucleus (Figures S2V and S2W). Alternatively, we expressed FiLa sensor in the nucleus and SoNar in the cytosol (Figures S2V and S2W). In cells treated with exogenous lactate, we observed a marked increase in cytosolic and nuclear lactate levels and NADH/NAD+ ratios (Figures S2V and S2W). In cells treated with exogenous pyruvate, we observed an increase in cytosolic and nuclear lactate levels and a decrease in the cytosolic and nuclear NADH/NAD+ ratios (Figures S2V and S2W). In cells treated with oxamate, we observed a marked decrease in cytosolic and nuclear lactate levels and a marked increase in the cytosolic and nuclear NADH/NAD+ ratios (Figures S2V and S2W). As the control, FiLa-C and a control iNap sensor (iN-apc)’s fluorescence did not significantly change upon lactate, pyruvate, or oxamate treatment (Figures S2X and S2Y). Taken together, these data indicate that the FiLa sensor displays a superior response and is very useful for the real-time tracking of subtle differences in subcellular lactate metabolism and the multiparameter imaging.
Subcellular lactate landscape reveals regulation of metabolism across various pathways
To understand how cellular metabolic pathways accommodate the Warburg effect, we developed high-throughput, live-cell chemical genetic assays of lactate metabolism in a 384-well plate format. With the two cancer cell lines (H1299 and HeLa cells), we profiled hundreds of drug responses of lactate metabolism, including 4 subcellular compartments (extracellular space, cytosol, nucleus, and mitochondria), 24 metabolic modulators targeting 10 standard metabolic pathways, and 2 nutritional states (glucose adequacy versus glucose deficiency) (Figures 3A and S3A). As expected, blocking glycolysis, either with the hexokinase II inhibitor 3-bromopyruvate (3-BrPA) or LDH inhibitor oxamate, led to a significant reduction in extracellular, cytosolic, nuclear, and mitochondrial lactate levels. Conversely, the suppression of oxidative phosphorylation, either with the complex I inhibitor rotenone or the complex V inhibitor oligomycin A, increased extracellular and subcellular lactate levels due to the compensatory upregulation of glycolysis (Figures 3B and S3B). Among them, mitochondrial FiLa exhibited the slightest increase, compared with that of cytosol and nucleus (Figure S3B). FiLa was also able to report sensitively the decrease in extracellular lactate level and the increase in subcellular lactate level upon inhibition of monocarboxylate transporters (MCT1, 2 inhibitors, AZD-3965 and AR-C155858) (Figures 3B and S3A; Table S3). Furthermore, extracellular, cytosolic, and nuclear lactate levels increased after ATP citrate lyase inhibition (ACLY inhibitors, SB-204990 and BMS-303141), mammalian target of rapamycin (mTOR) pathway inhibition (inhibitor, rapamycin), or ionomycin (Ca2+ ionophore), octyl-(R)-2-hydroxyglutarate, or octyl-(S)-2-hydroxyglutarate (two 2-HG isomer precursors) treatment. However, mitochondrial lactate levels decreased with ACLY inhibition or ionomycin treatment and increased with mTOR inhibition or R-2-hydroxyglutarate (R-2HG) precursor treatment (Figures 3B and S3A). Compared with glucose and fatty acids, transiently blocking glutamine catabolism (glutaminase inhibitors compound 968 and CB-839), often an alternative nutrient source for tumor cells, led to a much less significant change in lactate levels in all compartments (Figures 3B and S3A; Table S3).
Figure 3. Subcellular lactate landscape reveals regulation of metabolism across various pathways.
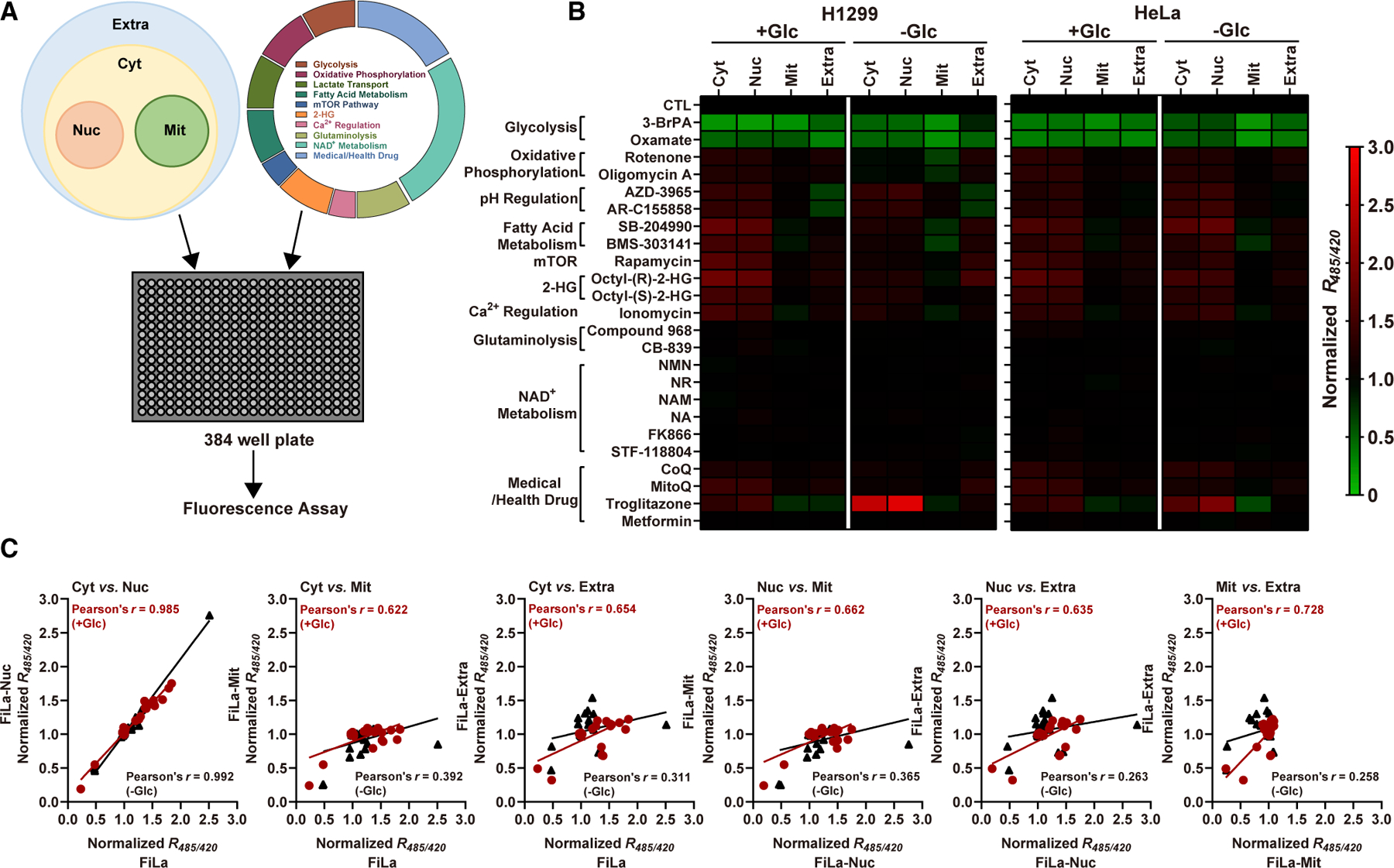
(A) Schematic of FiLa-based chemical assay. Mammalian cells stably expressing FiLa in different subcellular compartments were incubated with compounds in 384-well plates for 30 min and fluorescence measured.
(B) Effects of 24 metabolic modulators targeting 10 typical metabolic pathways on subcellular lactate levels in two cancer cell lines. Experiments were conducted in 25-mM glucose (glucose-fed) or 0-mM glucose (glucose-deprived) condition. FiLa fluorescence response was corrected by FiLa-C. Data are normalized to the control group and results are shown as a heatmap, from Table S3.
(C) Pearson correlation analysis of lactate levels between every two subcellular compartments in glucose-fed (dark red) or glucose-deprived (black) H1299 cells. See also Figure S3 and Tables S3 and S4.
It is also notable that supplementation of NAD+ precursors nicotinamide mononucleotide (NMN), nicotinamide riboside (NR), nicotinamide (NAM), or nicotinic acid (NA) or suppression of NAD+ synthesis (NAMPT inhibitors FK866, STF-118804) did not show sound effect on extracellular or subcellular lactate levels (Figures 3B and S3A; Table S3), which fails to support the hypothesis that cytosolic NAD+ pool can modulate glycolytic activity (Cantó et al., 2015). Surprisingly, coenzyme Q10 (CoQ) and mitoquinone (MitoQ) boosted both extracellular and subcellular lactate levels. Interestingly, we also found that two antidiabetic drugs, metformin and troglitazone, exhibited obviously distinct effects on lactate metabolism, as shown by the results that metformin treatment had minimal effects on extracellular and subcellular lactate levels, whereas troglitazone treatment decreased extracellular and mitochondrial lactate levels and increased cytosolic and nuclear lactate levels (Figures 3B and S3A; Table S3). Collectively, these data suggested that FiLa responds to subtle perturbations of various pathways of central energy metabolism or their perturbation by drug treatment.
These high-throughput data also facilitated the study of subcellular lactate interchange. Cytosolic and nuclear lactate levels were highly correlated (Pearson’s correlation coefficient r of ~0.972–0.992) (Figures 3C and S3C); however, the correlation of lactate metabolism between the cytosol/nucleus and other subcellular compartments (i.e., mitochondria, extracellular space) or between mitochondria and the extracellular space is moderate in glucose-fed conditions and lower in glucose-deprived conditions (Figure 3C). These data show that FiLa can be helpful for better understanding the central role that lactate plays in cellular metabolic activities, which is much more informative than using the extracellular lactate level as a proxy for whole-cell lactate metabolism.
To interrogate that how the NADH metabolism is linked to the lactate level, we further performed high-throughput, live-cell assays of subcellular NADH metabolism using the NADH sensors SoNar (Zhao et al., 2015) and Frex (Zhao et al., 2011) that we previous reported (Figure S3D; Table S4). The levels of lactate and NADH in the cytosol and nucleus were moderately correlated; however, the correlation between mitochondrial lactate and NADH levels was weak in glucose-fed cells (Figures S3E and S3F). It is presumably due to that the lactate is predominantlygenerated from glycolysis (Rabinowitz and Enerbäck, 2020), whereas NADH as the core coenzyme I is involved into hundreds of redox reactions (Zhao and Yang, 2016).
Imaging lactate metabolism in live mice with type 1 and type 2 diabetes mellitus
Accumulating evidence suggests that lactate is closely associated with incident diabetes (Brouwers et al., 2015; Juraschek et al., 2013). To evaluate in situ lactate metabolism in the diabetic state, we monitored lactate metabolism using the FiLa sensor in live mice with type 1 and type 2 diabetes mellitus (T1DM and T2DM). Four weeks after recombinant adeno-associated viruses (AAVs) encoding FiLa or FiLa-C via bilateral (s.c.) gastrocnemius muscle injection, T1DM mice were established by administering multiple low doses of streptozotocin (Figures 4A, S4A, and S4B). The FiLa sensors expressed well in muscle tissue of mice, and their fluorescence was visible several weeks after AAV infection. T1DM mice showed significantly elevated cytosolic lactate levels in muscle tissue compared with WT mice (~402 versus ~78 μM; Figures 4B–4D and S4C) as reported by FiLa, whereas no significant changes of FiLa-C were observed in the same mice (Figures 4C and S4D). In a type 2 diabetes model, db/db mice showed significantly elevated fasting blood glucose and body weight (Figures 4E, 4F, S4E, and S4F). Significantly elevated cytosolic lactate levels were also observed in these mice compared with WT mice, as shown by the FiLa sensor and biochemical analysis (Figures 4F-4H and S4G–S4I).
Figure 4. Imaging lactate metabolism in live mice with T1DM and T2DM.
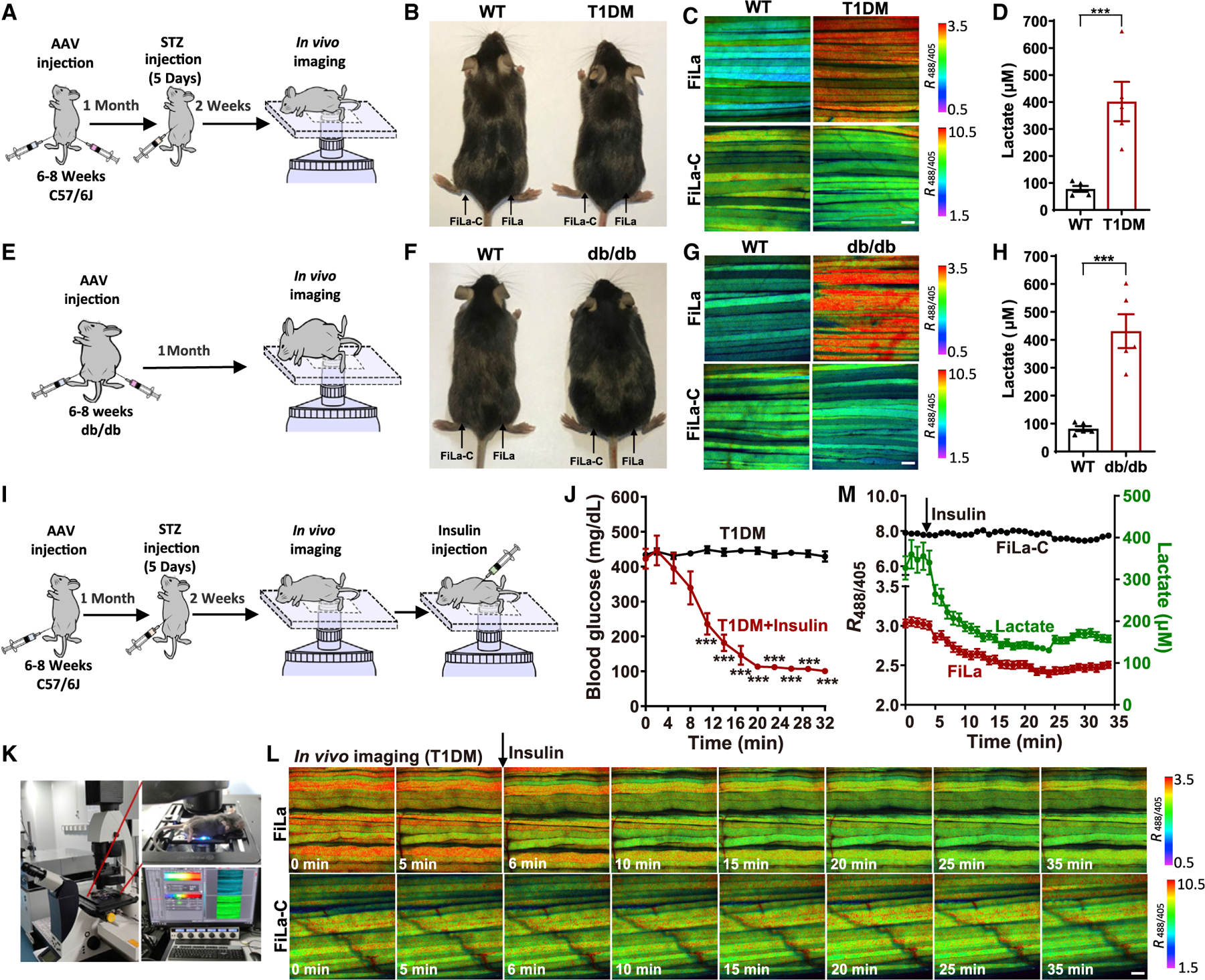
(A) General overview of the procedure for lactate imaging in muscle tissues of living T1DM mice.
(B) WT and streptozotocin-induced T1DM mice.
(C and D) In vivo fluorescence imaging (C) and lactate quantification (D) of muscle tissue expressing FiLa in living WT and T1DM mice (n = 5 mice, 10 muscle fibers per mouse).
(E) General overview of the procedure for lactate imaging in muscle tissue of living db/db mice.
(F) WT and db/db mice (genetic models of obesity-associated T2DM).
(G and H) In vivo fluorescence imaging (G) and lactate quantification (H) of muscle tissue expressing FiLa in living WT and db/db mice (n = 5 mice, 10 muscle fibers per mouse).
(I) General overview of the procedure for lactate imaging in muscle tissues of T1DM mice by insulin therapy.
(J) Insulin sensitivity within 32 min was measured by blood glucose curve. Insulin (1.25 units/kg) was injected via intraperitoneal injection and blood glucose was measured every 3 min (n = 4 mice for T1DM; n = 6 mice for T1DM + insulin).
(K–M) In vivo fluorescence imaging (K and L) and quantification (M) of FiLa in muscle tissue of living T1DM mice treated with insulin. All fluorescence images are pseudocolored by R488/405. Scale bars, 100 μm (C, G, and L). Data are the mean ± SEM (D, H, J, and M). All p values were obtained using unpaired two-tailed Student’s t test. ***p < 0.001.
See also Figure S4.
The blood glucose and lactate levels in muscle tissue were monitored over 30 min upon 1.25 units/kg insulin injection (Figures 4I–4M and S4J–S4P). The T1DM and WT mice showed a remarkable decrease of the blood glucose and lactate levels, but T2DM (db/db) mice have a minimal change of lactate, as evidenced by the FiLa sensor and biochemical assay (Figures 4J–4M and S4J–S4S). These differences may be attributed to that db/db (also known as leptin receptor deficient mice) confer susceptibility to obesity and insulin resistance (Coleman, 1978; Kim and Jung, 2019). Considering that there is a roughly synchronous decline of blood glucose and muscle lactate in 30 min upon insulin administration (Figures 4J, 4M, S4K, and S4N), it is reasonable to assume that the decrease of muscle lactate may be attributed to the change of blood glucose level. Insulin reduces blood glucose through enhancing the uptake of glucose by organs and promoting the glycogen synthesis of muscle and liver (Petersen and Shulman, 2018; Sylow et al., 2021). Thus, glycolysis and lactate production in muscle cell may be slowed down due to the upregulation of intracellular glycogen synthesis. As a control, minimal changes in fluorescence were observed in FiLa-C-expressing muscle tissues (Figures 4L, 4M, and S4M–S4P). Taken together, these data indicate that the FiLa sensor is a powerful tool for the real-time tracking of subtle fluctuation in lactate metabolism in vivo.
Rapid and simple assay of lactate level in serum and urine of patients with LADA, T2DM, and MIDD (m. 3243A>G)
We further evaluated the potential use of the FiLa-H sensor in the clinical screenings of latent autoimmune diabetes in adults (LADAs, also known as T1DM), individuals with type 2 diabetes, and patients with maternally inherited diabetes and deafness (MIDD, the mitochondria DNA 3243A>G (m.3243A>G) mutation) (Figure 5A). Fifteen patients of each disease type, as well as 15 sex-, age-, and BMI-matched healthy controls (7 females and 8 males, age 21–70 years, BMI 16–32.8 kg/m2), were enrolled in this study, whose clinical characteristics are shown in Figure S5A. The measurement takes less than 1 min and requires only 0.5 μL of serum or 2.5 μL of urine for the 96-well plate format (Figure 5B). As detected by FiLa-H, lactate levels in serum were highest in the T2DM group (3.33± 0.25 mM), followed by the m.3243A>G mutation group (2.80 ± 0.23 mM), the LADA group (2.67 ± 0.25 mM), and the control group (2.10 ± 0.20 mM) (Figure 5C). The results showed excellent correlation with the values measured in parallel by FiLa sensor (Figures S5B–S5D). As the control, FiLa-C’s fluorescence did not significantly change, excluding the possibility of interference by pH variations (Figures S5E and S5F). To validate our assay, we analyzed the samples in parallel using an ultra-high-performance liquid chromatography system coupled to a triple-quadrupole mass spectrometer (UHPLC-MS). Again, the results showed a strong correlation between the two independent methods (Figures 5D and 5E).
Figure 5. Rapid and simple assay of lactate level in serum and urine of patients with LADA, T2DM, and MIDD (m. 3243A>G).
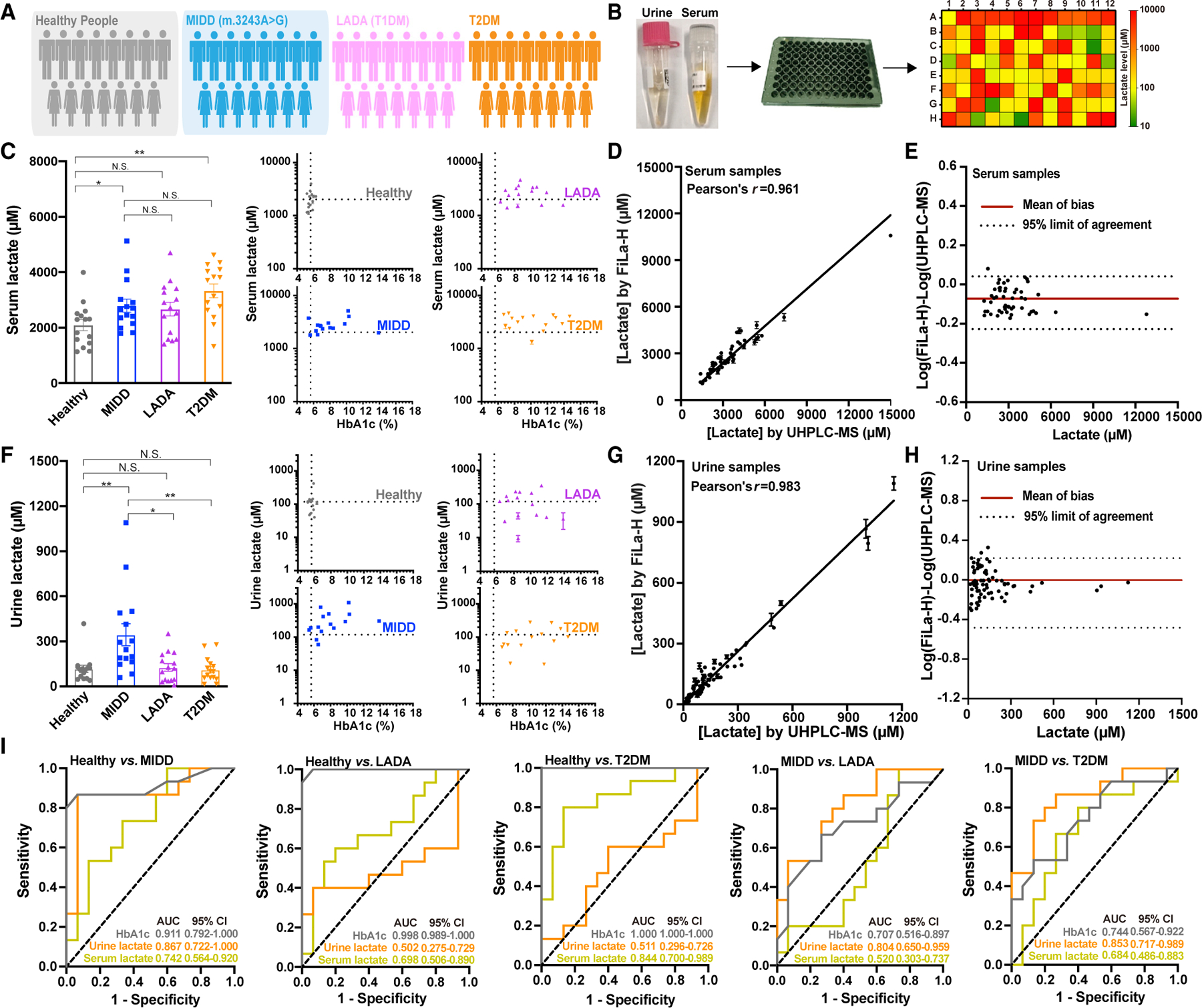
(A) Fifteen sex-, age-, and BMI-matched patients with MIDD (m. 3243A>G), LADA, and T2DM and healthy controls. Their clinical characteristics are shown in Figure S5A.
(B) General overview of the procedure for lactate assay in serum and urine samples by FiLa or FiLa-H.
(C) Serum lactate concentration of 4 groups of volunteers determined by FiLa-H (n = 15 for each group). Data are presented in bar chart or scatter plot, respectively.
(D) Quantification of lactate in serum samples. Test results obtained by FiLa-H are plotted against results obtained by UHPLC-MS. r, correlation coefficient.
(E) Bland-Altman analysis for serum samples measured by FiLa-H and UHPLC-MS.
(F) Urine lactate concentration of 4 groups of volunteers determined by FiLa-H (n = 15 for each group). Data are presented in bar chart or scatter plot.
(G) Quantification of lactate in urine samples. Test results obtained by FiLa-H are plotted against results obtained by UHPLC-MS. r, correlation coefficient.
(H) Bland-Altman analysis for urine samples measured by FiLa-H and UHPLC-MS.
(I) ROC curves of patients with MIDD, LADA, and T2DM compared with healthy controls, and MIDD compared with LADA and T2DM. Analysis was performed using Graphpad Prism 8.0. Data are the mean ± SEM (C, D, F, and G). All p values were obtained using paired two-tailed Student’s t tests. *p < 0.05, **p < 0.01. See also Figure S5.
Intriguingly, in the random spot urine test, the lactate levels in the m.3243A>G mutation group (344 ± 73 μM) were higher than the other three groups (Figure 5F); however, there were no significant differences among LADA, T2DM, and control group (127 ± 25 versus 111 ± 21 versus 117 ± 23 μM, p > 0.5) (Figure 5F). These data were consistent with the UHPLC-MS assay (Figures 5G and 5H). Thus, elevated urine lactate levels appear to be part of the clinical spectrum of patients with MIDD (m.3243A>G), an observation not previously reported. Receiver operating characteristic (ROC) analysis showed that urine lactate had reasonable discrimination comparing MIDD and healthy controls with an area under the curve (AUC) of 0.867, whereas no significant discriminatory ability of urine lactate was shown for LADA (0.502, fail score) and T2DM (0.511, fail score) versus healthy controls (Figure 5I). Patients with MIDD are frequently misdiagnosed as either T1DM or T2DM, depending on the age of onset for the patient and the clinical presentation (Murphy et al., 2008). Interestingly, we found that urine lactate but not serum lactate signature scores were useful for discriminating MIDD from LADA (urine lactate, 0.804; serum lactate, 0.520) and T2DM (urine lactate, 0.853; serum lactate, 0.684) (Figure 5I). These results suggest that increased urine lactate is associated with patients with MIDD (m.3243A>G).
DISCUSSION
Many genetically encoded sensors have been developed thus far; however, only a few of them have had significant impact. High responsiveness, high specificity, appropriate affinity, ratiometric readout, rapid response, as well as brightness are indispensable properties of a successful sensor (Ibraheem and Campbell, 2010; Palmer et al., 2011; Zhang et al., 2002, 2020). We describe herein the development of a series of genetically encoded metabolic sensors that enable specific, quantitative, and highly sensitive detection of lactate, both in vitro and in vivo. FiLa sensors exhibit several clear advantages over existing bio-sensors. First, FiLa family sensors show a 1,500%–2,700% fluorescence response in vitro, almost 75- to 130-fold greater than that of laconic (San Martín et al., 2013, 20- to 40-fold greater than that of LiLac (Koveal et al., 2022) and genetically encoded metabolic indicator for lactate (GEM-IL) (Bekdash et al., 2021) and 4- to 7-fold greater than that of Green Lindoblum (Harada et al., 2020) and eLACCO1.1 (Nasu et al., 2021), yielding unprecedented metabolite responsiveness. Second, FiLa family sensors demonstrate high selectivity to lactate over other relevant metabolites. In comparison, eLACCO1.1 sensor’s response to lactate was significantly interfered by calcium (Nasu et al., 2021). Third, FiLa has an appropriate (physiologically) affinity for lactate with a Kd of ~130 μM and displays a ~1,000% dynamic range in live cell studies, covering most physiological or pathological concentrations of lactate. By contrast, laconic, GEM-IL, LiLac, and eLACCO1.1 have a low-affinity Kd of ~830, 661–2,330, 2,680, and 3,900 μM for lactate, respectively (Bekdash et al., 2021; Koveal et al., 2022; Nasu et al., 2021; San Martín et al., 2013). Green Lindoblum has a high affinity for lactate with a Kd of 30 μM (Harada et al., 2020), making it easily saturated under normal cell culture conditions. These limitations obviously lowered laconic’s, GEM-IL’s, or Green Lindoblum’s response; in practice, they demonstrate only a ~6%–40% fluorescence change even when 10 mM lactate is present in live cells (Bekdash et al., 2021). In addition, Green Lindoblum, GEM-IL, and LiLac have only one excitation peak and one emission peak and are intensity-based sensors (Bekdash et al., 2021; Harada et al., 2020; Koveal et al., 2022). Notably, the FiLa sensors are intrinsically ratiometric sensors and provide the advantages of being quantitative and less susceptible to imaging artifacts or the degree of expression of sensors. Therefore, we conclude that FiLa is superior; it fulfills most of the requirements of a nearideal sensor, just as the NADH/NAD+ ratio sensor SoNar that we previously developed (Zhao et al., 2015).
The superior performances of FiLa pave the way for spatiotemporal detection of lactate dynamics in living cells and in vivo. Utilizing FiLa, we found unexpectedly that lactate is highly enriched in mitochondria. Nevertheless, whether or not mitochondria possess a predominant lactate pool and mitochondrial lactate metabolism remains controversial (Glancy et al., 2021), as there is no convincing evidence that reliably determines the lactate distribution in living cells in situ; contradictory results have been observed by mass spectrometric assays of isolated mitochondria, which showed mitochondrial lactate was minimal (Chen et al., 2016; Lee et al., 2019), lower (Long et al., 2020), or compared (Bayraktar et al., 2019) with cytosolic lactate. These discrepancies may be attributed to the different biochemical assays used, as isolation of mitochondria usually requires from tens of minutes to several hours (Chen et al., 2016), discerning which lactate may be either metabolized or released before the subsequent analysis. The FiLa sensor enabled us to provide the first visual evidence that lactate is highly enriched in mitochondria in different cells, never previously investigated. Identification of a specific lactate mediator is a critical step toward understanding the lactate enrichment in mitochondria. A particularly interesting area for future study will be to apply FiLa sensors to studies of mitochondrial lactate metabolism, including identifying the key mediators of lactate influx, efflux, and metabolic conversion in mitochondria.
It is well known that the lactate level is closely associated with glycolytic activity in the cytosol, but how subcellular lactate is regulated by various metabolic pathways remains to be fully elucidated. Importantly, the question can be studied using high spatiotemporal resolution of FiLa sensors, suggesting that it is possible to discriminate, for example, between changes in lactate levels in different subcellular organelles that occur concomitantly and situations where the change in one compartment is delayed relative to that of the other. In this study, we performed a head-to-head comparison study of lactate metabolism in four subcellular compartments upon chemical perturbations of key nodes in various metabolic pathways and mapped their biochemical roles in regulating subcellular lactate biology. FiLa responded well to the perturbations of these metabolic pathways, implying that subcellular lactate and cellular signaling pathways are closely interwoven and that lactate acts as a key metabolic hub sensing their interaction.
Increasing evidence indicates that lactate may serve as a universal, major circulating carbohydrate fuel in mammals, compared with glucose (Rabinowitz and Enerbäck, 2020). Since cellular and circulating lactate are readily balanced, circulating lactate becomes a key indicator of energy metabolism in the whole body, and thus, its measurement is highly diagnostically relevant. To this end, FiLa sensors enabled the ultrasensitive and rapid quantitation of lactate in body fluids, such as serum and urine. MIDD is a rare monogenic form of adult-onset diabetes caused by a mtDNA defect, and 85% of cases are caused by the m.3243A>G mutation (accounting for 1% of adult-onset diabetes) (Murphy et al., 2008; Naing et al., 2014). Recognizing these clinical manifestations (abnormal increase of urine lactate and blood glucose) of diabetes mellitus should prompt rapid screening and investigation for MIDD in some cases.
Collectively, the study presented here advances several concepts. First, we report a series of genetically encoded fluorescent sensors with excellent performances for tracking transient changes of lactate metabolism in vitro and in vivo. Second, we explore the subcellular lactate landscape, including its distribution, abundance, transport, and regulation, in living cells; identify higher lactate levels in mitochondria than in the nucleus and cytosol; and reveal that lactate is a key hub sensitively sensing various metabolic activities. Third, FiLa sensors provide a fast and convenient lactate assay, and the direct evidence that urine lactate from patients with MIDD (mtDNA 3243A>G) exhibits an abnormal increase compared with individuals with type 1 or 2 diabetes.
Limitations of the study
As with other cpYFP-based sensors, FiLa’s fluorescence excited at 485 nm is sensitive to pH. The ratio of FiLa’s fluorescence when excited at 485 and 420 nm is pH sensitive as well. Such pH effect is a limitation for all cpYFP-based sensors. Thus, one should exercise caution under conditions that alter intracellular pH. Fortunately, its fluorescence excited at 420 nm is pH resistant. When intracellular pH fluctuations do occur, FiLa fluorescence can be measured with 420-nm excitation only, at the different lactate range. FiLa could be also fused with a red fluorescent protein (i.e., mCherry), allowing the pH-resistant and ratiometric measurement of lactate levels. FiLa and FiLa-C have similar pH responses; however, only FiLa responds to lactate fluctuations. Thus, pH effects on FiLa fluorescence could be corrected by FiLa-C fluorescence measured in parallel experiments; however, it should be noted that the FiLa and FiLa-C signals could not be deconvolved in the same sample because of similar fluorescence emission spectra. For this purpose, it is also possible to generate a dual-function, genetically encoded sensor by fusing the FiLa sensor and the pH-sensitive red fluorescent protein (Tantama et al., 2011), which can be used to monitor lactate and pH simultaneously in the same sample.
To develop the point-of-care assay for lactate in body fluids, we apply FiLa sensors to the clinical screenings of individuals with diabetes. For this proof-of-concept study, the sample size of each group (n = 15) was relatively small, although the lactate assay for diagnostic application has been well established. Larger studies with indicated populations are warranted to further confirm that the increased urine lactate is associated with patients with MIDD.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yuzheng Zhao (yuzhengzhao@ecust.edu.cn).
Materials availability
Any materials generated in this study, including FiLa, FiLa-H, FiLa-C, Frex, and SoNar are available upon request.
Data and code availability
All data supporting the findings of this study are available within the article and its supplemental information files. Unprocessed data underlying the display items in the manuscript, related to Figures 1, 2, 3,4, 5, and S1–S5, are provided in Data S1.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell culture and generation of stable cell lines
H1299 cells were cultured in 1640 medium (HyClone, SH30809.01B) supplemented with 10% FBS (Biological Industries, 04–0011ACS). HEK293, HEK293T, and Hela cells were maintained in DMEM (HyClone, SH30243.01) supplemented with 10% FBS at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were plated in antibiotic-free DMEM (high glucose) supplemented with 10% FBS 16 h before DNA transfection. Then, 0.5 μg DNA with 1.5 mL of Hieff Trans™ liposomal transfection reagent were typically used for each well of a 4-chamber glass-bottom dish in accordance with the manufacturer’s protocol.
To generate the stable cell lines, the pLVX lentiviral plasmids encoding FiLa sensor was constructed. Lentivirus was produced by co-transfecting two lentiviral packaging vectors (pMD2.G and psPAX2) in HEK293T cells. Lentiviral supernatants were collected 48 and 72 h after transfection. HEK293, H1299, or HeLa cells in 6-well tissue culture plates were infected in media containing 8 μg/mL polybrene and centrifuged at 1,000 g for 1 h. After infection, the virus was removed, and cells were selected with 0.2–1 μg/mL puromycin for 1 week. For stable cell lines co-expressing SoNar or tagRFP with FiLa sensors simultaneously, two lentiviral supernatants were mixed at the ratio of 1:1 to 1:3 with the total amount fixed (SoNar-Nuc:FiLa-NES, FiLa-Nuc:SoNar-NES, or FiLa-C-Nuc:iNapc-NES=1:3; iNapc-Nuc:FiLa-C-NES, FiLa-Mit:tagRFP-Mit=1:1).
Animal studies
Six-eight-week-old male C57BL/6J (refer as ‘‘WT’’) and db/db mice were from East China Normal University, which were purchased from GemPharmatech Co., Ltd with healthy check certificate, and kept at a humidity environment in 12-hr light/dark cycles and daily health checks with ad libitum access to a standard chow diet (Shanghai JieSiJie, 0017) at room temperature. Mice were randomly assigned to experimental and control groups. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of East China Normal University and East China University of Science and Technology.
Human subjects
Human blood and urine samples were collected from sex-, age- and BMI-matched healthy controls (n=15), patients with MIDD (m.3243A>G)(n=15), latent autoimmune diabetes in adult (LADA)(n=15), and individuals with type 2 diabetes (n=15) at Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, and Shanghai East Hospital. Plasma glucose levels were assessed using the glucose-oxidase method (HITACHI 7600), and HbA1c levels were assessed using the HPLC method (Bio-rad, Variant II Turbo). Serum samples were stored at −80 °C and labeled with a unique identifier for further examinations. Urine samples were drawn on the same day as blood samples and stored at −80 °C. Detailed human sample information is listed in Figure S5A. Written informed consent was notified and obtained from all patients, and the standard procedures were approved by the Ethics Committee for Medical Research (Institutional Review Board) at Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and Shanghai East Hospital.
METHOD DETAILS
Plasmid construction
Unless stated otherwise, cloning was performed using the Hieff Clone® Plus One Step Cloning Kit. The LldR gene was amplified from Escherichia coli using Pfu polymerase and subcloned into the pCDFDuet-1 vector. Another LldR gene fragment was fused with cpYFP by overlap PCR and then inserted into pCDFDuet-LldR vector. Using the same strategy, sensors with cpYFP inserted between two complete or truncated subunits of LldR were obtained. Chimeric sensors with cpYFP inserted into the interior of LldR fragment were constructed by subjecting pCDFDuet-LldR to inverse PCR to produce DNA fragments with a split between each of the residues and ligated to cpYFP. All mutated or truncated sensors were obtained by inverse PCR using PrimeSTAR Max (TaKaRa). To construct the random mutation library, PCR was performed using primers including NNK at the residues Pro189 and Pro190 based on the M185/P189 chimera.
The coding sequences of FiLa, FiLa-C, Frex, SoNar, iNapc, and cpYFP were subcloned into the pAAV-CMV-MCS or pLVX-IRES-Puro vectors behind a Kozak sequence for mammalian expression. For mitochondrial targeting, a signal sequence from Neurospora crassa ATP synthase was fused at the N terminus of FiLa sensors. For nuclear targeting, the threefold nuclear localization sequence, (DPKKKRKV)3, was fused at the C terminus of the sensor. For nuclear-excluded targeting, the tandem MAPKK signal peptide (MALQKKLEELELDEQQRKRLEDL)2 was fused at the N terminus of the sensor. The cDNA of TagRFP was obtained by site-directed mutagenesis of T162S to tgRFPt (Addgene: #60415), and the Mit-TagRFP was constructed by fusing the mitochondrial localization signal pyruvate dehydrogenase E1α (PDHE1) (MRKMLAAVSRVLSGASQKPASRVLVASRNFANDATF) at the N terminus of TagRFP. The cDNA of lactate oxidase was synthesized and inserted into the pCDFDuet-1 vector.
Protein expression and purification
Escherichia coli BL21(DE3) cells carrying the pCDFDuet-FiLa expression plasmid were grown into 20 mL of LB medium containing 50 μg/mL streptomycin at 37 °C until the cultures reached about 0.4–0.6 OD600. The expression of sensor proteins was induced by adding 1 mM isopropyl-β-D-thiogalactoside (IPTG), and growth continued at 18 °C for 36 h. Bacteria were harvested by centrifugation at 4000 g, 4 °C for 30 min. The cell pellets were suspended in buffer A (20 mM sodium phosphate, 500 mM sodium chloride, and 10 mM imidazole, pH 7.4), and then lysed via ultrasonication. Proteins were purified using His MultiTrap 96-well filter plates. After washing with two column volumes of wash buffer (buffer A containing 50 mM imidazole), the proteins were eluted from the resin using buffer B (20 mM sodium phosphate, 500 mM sodium chloride, and 300 mM imidazole, pH 7.4). The protein preparations were then exchanged into 100 mM HEPES buffer containing 100 mM NaCl (pH 7.4) and quantified by the BCA Protein Assay Kit.
The lactate oxidase protein with a 6 × His-tag was expressed in Escherichia coli BL21(DE3) at 18 °C for 36 h with 1 μM IPTG and 1 mM FMN. The cell pellets were collected by centrifugation and sonicated in buffer A containing 1 μM FMN. The protein was purified followed the nickel-column affinity chromatography protocol. Proteins were exchanged into 100 mM HEPES, 100 mM NaCl, and 1 μM FMN, pH 7.4, before assay.
In vitro characterization of lactate sensors
Purified protein was stored at −80 °C before the experiment. For the in vitro measurement, the purified sensor protein was diluted to a final concentration of 0.5 μM with 100 mM HEPES buffer containing 100 mM NaCl (pH 7.4). Fluorescence spectroscopy was performed on a fluorescence spectrophotometer (HITACHI, F-4700, JPN). Excitation spectra were recorded at 530 nm, and emission spectra were monitored at 420 and 490 nm. Slit width was set as 10 nm bandpass for excitation and emission, and the PMT voltage was set at 500 V.
For substrate titration, the fluorescence intensity was measured by a filter-based Synergy 2 Multi-Mode microplate reader (BioTek, Synergy 2, US) using 485 BP 20 nm or 420 BP 10 nm excitation, and 528 BP 20 nm emission band pass filters. All solutions were prepared in HEPES buffer (pH 7.4). Each assay was performed in a 96-well black bottom plate using 50 μL of substrate and 50 μL of sensor protein. Fluorescence intensity was measured immediately.
For extinction coefficient determination, the sensor protein was diluted in 100 mM HEPES buffer containing 100 mM NaCl (pH 7.4) and absorption spectra were recorded from 300 nm to 650 nm on a UV-Vis spectrophotometer (Purkinje, T10S, China). For quantum yield determination, the purified standard protein EGFP and FiLa sensor protein were performed on a fluorescence spectrophotometer (HORIBA, FluoroMax-4, JPN) with an integrating sphere using a step of 1.2 nm and an integrating time of 0.1 s. Emission spectra were excited at 420 nm and recorded from 435 nm to 650 nm, or excited at 480 nm and recorded from 485 nm to 650 nm.
For calculation of quantum yield and brightness, integrated fluorescence intensity vs. absorbance was plotted for sensor protein and standard, and is the slope of the linear fit for the integrated fluorescence intensity of the sensor protein as a function of absorbance. Quantum yield of sensor protein was determined using the equation: . Extinction coefficient was determined by Beer-Lambert’s Law and brightness was defined as the product of extinction coefficient and quantum yield.
Live-cell fluorescence imaging
For fluorescence imaging, cells were plated on a 35 mm 4-chamber glass-bottom dish. The FiLa sensors were expressed in different subcellular compartments by tagging with organelle-specific signal peptides. Images were acquired using a Leica TCS SP8 SMD confocal laser-scanning microscope system with super-sensitive HyD hybrid detectors. HC Plan Apo CS2 633/1.40 NA oil objective was utilized. For dual-excitation ratio imaging, 405-nm excitation laser and 488-nm excitation laser with an emission range of 500–550 nm were used. Raw data were exported to ImageJ software as 12-bit TIF for analysis. The pixel-by-pixel ratio of the 488-nm excitation image by the 405-nm excitation image of the same cell was used to pseudocolor the images in HSB color space. The RGB value (255, 0, 255) represents the lowest ratio, the red (255, 0, 0) represents the highest ratio, and the color brightness is pro-portional to the fluorescent signals in both channels. Images were taken on random, but cells with extraordinarily strong or low expression levels were excluded for examination.
Super-resolution imaging of mitochondria
To label mitochondria, stable cell lines expressing FiLa sensors were co-expressed with mit-tagRFP or stained with MitoTracker Red (Thermo Fisher Scientific, M22425). For MitoTracker Red staining, cells were incubated with 100 nM dyes in high-glucose DMEM at 37 °C for 30 min, and then washed and imaged in HBSS buffer.
Super-resolution imaging was performed with N-SIM microscope system (Nikon). Live-cell imaging was carried out at 37 °C ina temperature-controlled and humidified live imaging chamber (Tokai Hit) on a Nikon Ti2-E microscope with CFI SR HP Apochromat TIRF 100x oil objective (NA 1.49), LU-NV laser unit with 405-, 445-, 488-, 514-, 561-, and 647-nm excitation lasers, 405/488/561/647 Laser Quad Band filter cube and ORCA-Flash 4.0 sCMOS camera (Hamamatsu Photonics K.K.). For dual-excitation ratio imaging, cells were manually identified and imaged with 488 and 561 nm lasers in time series. Deconvolution and SIM image reconstruction were completed using Nikon’s SIM software on NIS-Elements AR (NIS-A 6D and N-SIM Analysis). Reconstructed images were further processed by ImageJ for fluorescence pattern analysis.
Construction of diabetic mouse model
A type I diabetic mouse model was created as previously described (Yin et al., 2019). Briefly, 12-week-old mice were intraperitoneally administrated with multiple low doses of streptozotocin (50 mg/kg/day, 5 days) or citrate buffer after fasting for 16 h. Body weight and blood glucose concentration were monitored every 3 days using a blood glucose monitor (Johnson). Two weeks after completion of streptozotocin injection, fasted mice with hyperglycemia over 300 mg/dL were used in the following study.
To establish a genetic type II diabetic mouse model, 8-week-old male leptin receptor knockout (db/db) mice were fed with ad libitum access to a standard chow diet for 4 weeks. Blood glucose exceeding 200 mg/dL was considered as type II diabetes to use in the following study.
For insulin treatment, type I and type II diabetic mice fasting for ~4 h were intraperitoneally administrated with insulin (1.25 units/kg body weight). Plasma glucose levels were then measured at the indicated time point.
In vivo imaging of living mice
Adeno-associated virus (AAV) expressing FiLa or FiLa-C was generated and purified by Obio Technology. Serotype 9 and the CMV promoter was used to drive the sensor expression in vivo. Typical viral titers were 1 × 1013 to 3 × 1013 vg per mL. A total of 50 μL (1.2 × 1012 vg/mL) AAV diluted in phosphate-buffered saline was injected bilaterally (s.c.) in the gastrocnemius muscle, and the sensors were expressed for several weeks in vivo.
Mice were usually fasted for ~4 h for physiological synchronization. For the in vivo imaging of living mice, mice were anesthetized intraperitoneally with sodium pentobarbital at 4–6 weeks after infection. The muscle legs were depilated by a shaver to reduce the fluorescence interference of hairs and then placed on glass cover slips for imaging. Fluorescence was detected by a Leica TCS SP8 SMD confocal laser scanning microscope system with super-sensitive HyD hybrid detectors and an HC Plan APO CS2 203/0.75 NA dry objective. For dual-excitation ratio imaging, 405 nm excitation laser and 488 nm excitation laser with an emission range of 500–550 nm were used.
Biochemical analysis of lactate in muscle tissue
The gastrocnemius muscles from living mice were homogenized in 0.5 M HClO4 buffer (90 mL/mg) using Cryogenic Grinder JXFSTPRP-CL with 5.2 mm zirconium beads, 60 HZ for 2 min. After centrifugation at 12,000 g, 4 °C for 10 min, the supernatants were neutralized with 3 M NaOH, and centrifuged at 10, 000 g, 4 °C for 5 min. The supernatant was pipetted carefully to a new EP tube for assay. 50 μL diluted samples were mixed with equal volume of reaction medium containing 20 mM resazurin, 0.4 U/mL diaphorase, 1 mM NAD+, and 1 U/mL lactate dehydrogenase. Changes in fluorescence excited at 540 nm and emitted at 590 nm were measured every 20 seconds for 10 min at 37 °C by Microplate Reader.
Calibration of live-cell and in vivo lactate levels
Intracellular lactate levels can be measured after calibration of FiLa fluorescence in live cells or live mice with that of recombinant FiLa protein as previously described(Tao et al., 2017). The background values (from control cells or tissues) were subtracted from those of FiLa-expressing cells. The equation is as follows:
is the fraction of FiLa sensors bound to lactate, and is the free concentration of lactate. represents , which is the ratio of lactate at which the response of the FiLa sensor is half-maximal. and represent the minimum ratio () and the maximum ratio () of the recombinant FiLa protein corrected by the corresponding FiLa-C protein values at saturating conditions (i.e., 50 mM lactate). represents the calibrated ratio of FiLa in the live cells or live mice.
Fluorescence measurement using microplate reader
HEK293, H1299, or HeLa cells stably expressing FiLa were seeded in a 96-well or 384-well black bottom plate. After ~24 h, the cells were washed twice, incubated in HBSS buffer (pH 7.4), and then treated with different compounds at 37 °C during the measurement. Dual-excitation ratios were obtained by a Synergy Neo 2 Multi-Mode Microplate Reader (BioTek, Synergy Neo2, US) with excitation filters 420 BP 10 nm and 485 BP 20 nm, and emission filter 528 BP 20 nm. Fluorescence values were background corrected by sub-tracting the intensity of the cell samples not expressing FiLa sensors.
Mitochondria isolation and fluorescence assay
Mitochondria were isolated from HEK293, H1299, or HeLa cells stably expressing FiLa by using the mitochondria isolation kit (Beyotime, C3601), in accordance with the manufacturer’s protocol. Briefly, 10 to 20 million cells were harvested, pelleted, resuspended, and then homogenized with Dounce homogenizer in 1 mL of cold mitochondrial isolation solution. The cell fragments were removed by centrifugation at 1,000 g twice, 4 °C for 5 min. The supernatants were centrifuged at 11,000 g, 4 °C for 5 min to obtain a mitochondrial pellet. The isolated mitochondria were resuspended with buffer for further measurement. FiLa-Mit fluorescence was obtained by a Synergy 2 Multi-Mode Microplate Reader (BioTek, Synergy 2, US) with excitation filters 420 BP 10 nm and 485 BP 20 nm, and emission filter 528 BP 20 nm.
To image isolated mitochondria, the isolated mitochondria were resuspended with buffer and added into 8-chamber glass bottom dish. The suspension was sedimentated for 10 min on ice and then evenly covered with 0.2 % low melting point agarose for imaging. Fluorescence was detected by a Leica TCS SP8 SMD confocal laser scanning microscope system with super-sensitive HyD hybrid detectors and an HC Plan APO CS2 203/0.75 NA dry objective. For dual-excitation ratio imaging, 405 nm excitation laser and 488 nm excitation laser with an emission range of 500–550 nm were used.
Isolated mitochondria fluorescence was also monitored by fluorescence-activated cell sorter analysis (FACS). The measurement was performed using CytoFLEX-S flow cytometer (Beckman Coulter). Fluorescence of isolated mitochondria expressing FiLa or FiLa-C was detected after 30 minutes incubation with or without 1 mM lactate at room temperature, using laser lines at 405 nm and 488 nm. Emission filters were 525 BP 40 nm for both excitation wavelengths.
Submitochondrial fractions separation
Mitochondria were prepared from HEK293, H1299 and Hela cells using the mitochondria isolation kit (Beyotime, C3601) according to the manufacturer’s directions. Resuspended mitochondria with 100 μL cold solution A (0.25 M sucrose, 0.01 M tricine, 1 mM EDTA, 10 mM NaH2PO4, 2 mM MgCl2, pH 8.0) with 0.5% BSA. 15 μL mitochondria supernatant and 15 μL 0.15% or 0.2% digitonin (w/v) was mixed respectively and kept on ice for 10 min. Then add 90 mL solution A with 0.5% BSA to stop permeabilization and centrifuge at 20,000 g, 4 °C for 30 min. Collect the pellet and supernatant for western blotting.
Immunoblotting
For Western blot analyses, the cells were lysed in 1 × SDS sample buffer supplemented with a protease/phosphatase inhibitor cock-tail (Cell Signaling Technology). The submitochondrial fraction samples were prepared with a protease/phosphatase inhibitor cocktail (Cell Signaling Technology) and 5 × loading buffer was added before 95 °C boiling for 15 min. Equal amounts of total protein (30–50 μg) and mitochondrial protein (3–5 μg) were separated on 10% or 15% SDS-PAGE and electrotransferred onto PVDF membranes. The membranes were incubated with primary antibodies COX IV (TransGen Biotech, HL101-01), LDHA (Cell Signaling Technology, #3582), Glut1 (Cell Signaling Technology, #12939), VDAC1 (Abways Technology, CY5416), Cytochrome c (Proteintech, 10993-1-AP), Hsp60 (Santa Cruz Biotechnology, SC-57840), Flag (MBL, M185-7). Then, they were incubated with secondary anti-bodies conjugated to horseradish peroxidase, added with chemiluminescence detection mixture (YEASEN, 36208ES60), and imaged.
mtDNA copy number analysis
Total DNA was extracted from 5 x 106 cells using the TIANamp Genomic DNA Kit (DP304, TIANGEN BIOTECH). Relative and absolute mtDNA copy numbers were measured by using a real-time quantitative PCR assay (Longchamps et al., 2020). For detecting relative levels of mtDNA, a region of the mitochondrial genome encompassing a portion of ND1 and a region of the multicopy nuclear β-globin locus were used. The primers of the ND1 gene were forward, 5’-AACATACCCATGGCCAACCT-3’; and reverse, 5’-AGCGAAGG GTTGTAGTAGCCC-3’. The primers of the β-globin gene were forward, 5’-GAAGAGCCAAGGACAGGTAC-3’; and reverse, 5’-CAACTTCATCCACGTTCACC-3’. The mtDNA copy number was calculated using the 2−∆∆CT method.
Assessment of mitochondrial integrity
The integrity of isolated mitochondria was confirmed in three biochemical assays, including NADH oxidation-based oxygen consumption rate (OCR) measurement, citrate synthase activity analysis, and respiratory control ratio (RCR) detection. Seahorse XFe96 analyzer (Agilent, XFe96, US) was used to measure NADH oxidation-based OCR of freshly isolated mitochondria in the absence or presence of 1 mM NADH (Lanza and Nair, 2009; Puchowicz et al., 2004).
Citrate synthase activity is measured spectrophotometrically from the mitochondria samples using previously described methods (Lanza and Nair, 2009). Briefly, 5 μL mitochondria samples (4.6~6.1 mg protein per well) were loaded into the 96-well clear flat-bottom plate and absorbance at 412 nm was measured every 13 seconds for 10 minutes at 30 °C immediately after the addition of 100 μL 0.1 M Tris-HCl assay buffer (pH 8.0) containing 0.1 mM dithionitrobenzoic acid (DNTB), 0.05 mM acetyl-CoA and 0.25 mM oxaloacetate. Difference in citrate synthase activity between samples with and without lysis buffer represents the percentage of mitochondria with intact inner membrane.
Oxygen consumption rate of isolated mitochondria was measured by a Seahorse XFe analyzer. Isolated mitochondria were resuspended in cold mitochondrial assay solution (MAS) (70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA and 0.2% (w/v) fatty-acid-free BSA, pH was adjusted to 7.2 at 37 °C) with metabolic substrates 10 mM pyruvate and 5 mM malate. A volume of 25 μL of mitochondrial suspension was transferred to each well of the XFe cell culture microplate (resulting in 14.2~21.4 μg of mitochondria per well) and then spun down at 1, 000 g, 4 °C for 20 min. After centrifugation, 155 μL of pre-warmed MAS-containing substrates was added to each well. Then substrate (ADP, 4 mM, final) and inhibitors (oligomycin A, 3.5 μM, final; CCCP, 4 μM, final; antimycin A, 4.5 μM, final) were serially introduced to induce different respiration states. The RCR values were determined by dividing state 3 (highest point) by state 4 (lowest point).
Biochemical assay of isolated mitochondria
For mitochondrial membrane potential assay, the labeled mitochondria with JC-10 probe were treated with or without 10 mM CCCP in MAS buffer (pH 7.4), and fluorescence was measured immediately by Synergy neo2 Multi-Mode microplate reader. The ratio of the red (Ex 540 nm/Em 590 nm) and the green (Ex485 nm/Em 528 nm) fluorescence intensities was calculated to determine the membrane potential.
For lactate assay, isolated mitochondria were lysed, deproteined with 0.25 M HClO4, neutralized with 3 M KOH, and diluted with lactate assay buffer. Lactate was measured by PicoProbe™ Lactate Fluorometric Assay Kit (BioVision, K638) according to the man-ufacturer’s protocol.
Isolated mitochondria lactate uptake
To measure mitochondrial lactate uptake, isolated mitochondria were prepared from 2 x 107 cells expressing Fila-Mit/Flia-C-Mit and resuspended in MAS buffer (pH 7.4). 50 μL MAS buffer with different concentration of lactate with or without CCCP was added to 384-well black bottom plate before 10 μL resuspended mitochondrial was added into each well. Dual-excitation ratios of FiLa were obtained by a Synergy neo2 Multi-Mode microplate reader. Fluorescence values were background corrected by subtracting the intensity of the mitochondria from wild type cells. Intramitochondrial lactate levels were quantified as above. For lactate measurement outside mitochondria, mitochondria were treated in the same way in 1.5 mL centrifugal tube. Exogenous Lactate uptake was calculated from the difference of lactate level in the buffer.
FiLa-based serum and urine lactate analysis
For sensor-based lactate analysis, serum samples were diluted by a factor of 50 or 100, and urine samples were diluted 20-fold in HEPES buffer. Each assay was performed in a 96-well black bottom plate using 50 μL diluted samples and 50 μL FiLa-H protein. Alternatively, 0.5 mL of serum samples or 2.5 mL of urine samples was mixed directly with 100 mL of FiLa-H protein (0.5 mM). Fluorescence intensity was measured immediately by a Synergy neo2 Multi-Mode microplate reader using 485 BP 20 nm or 420 BP 27 nm excitation, and 532 BP 40 nm emission band-pass filters.
UHPLC-MS analysis of serum and urine lactate
Serum and urine lactate levels were measured by UHPLC (Agilent, 1290) coupled with a triple quadrupole (Agilent, 6460C) mass spectrometer as previously described(Xie et al., 2021). Briefly, 5 μL aliquots of serum or urine samples or standard solution were sequentially mixed with 25 μL of 160 mM 3NPH_HCl solution and 25 μL of 120 mM EDC. The resultants were frozen at −20 °C for 20 min and then centrifuged to obtain the supernatant for quantitative analysis.
Each 5 μL sample was injected into the Agilent 1290 UHPLC system for separation with water with 0.1% formic acid and acetonitrile/isopropanol (7:3, v/v) gradient elution. Mass analysis was performed on multiple reaction monitoring with ESI negative mode. The ion paired for quantitative of lactate was 224/137.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data are presented either as representative examples of single experiments repeated at least in triplicate or as three or more experiments. Data are expressed as mean ± SEM. P values were obtained using unpaired two-tailed Student’ s t-test or paired two-tailed Student’s t-test. In all analyses, values of p < 0.05 was considered statistically significant (*p<0.05, **p<0.01, ***p<0.001). Statistical parameters can be found in the Figures and Figure legends. No methods were used to determine whether the data met assumptions of the statistical approaches.
Correlation analysis was performed by the Pearson correlation or Spearman correlation test. Bland Altman analysis was performed to assess methods measured by sensors and by UHPLC-MS, within which 95% of the differences of measurements fall. Receiver operating characteristic (ROC) curves analysis and area under the curve (AUC) were analyzed to determine if baseline levels of urine and serum lactate may discriminate in patients with MIDD, LADA, and T2DM as compared with healthy controls. The sensitivity, specificity, and 95% confidence interval (CI) values were determined at the optimal cut-of value (threshold) from the ROC curve. Statistical analyses were performed with GraphPad Prism (version 8.0) software.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-LDH-A (C4B5) Rabbit mAb | Cell Signaling Technology | Cat# 3582; RRID: AB_2066887 |
| Anti-COX IV Mouse mAb | TransGen Biotech | Cat# HL101-01; N/A |
| Anti-Glut1 (D3J3A) Rabbit mAb | Cell Signaling Technology | Cat# 12939; RRID: AB_2687899 |
| Anti-VDAC1 Rabbit mAb | Abways Technology | Cat# CY5416; RRID: AB_2773723 |
| Anti-Cytochrome C pAb | Proteintech | Cat# 10993-1-AP; RRID: AB_2090467 |
| Anti-Hsp60 (A57-B9) Mouse mAb | Santa Cruz Biotechnology | Cat# SC-57840; RRID: AB_783868 |
| Anti-Flag mAb | MBL | Cat# M185-7;RRID: AB_2687989 |
|
Bacterial and virus strains | ||
| BL21(DE3) Chemically Competent Cell | TransGen Biotech | Cat# CD601-02 |
| Trans5α Chemically Competent Cell | TransGen Biotech | Cat# CD201-02 |
|
Biological samples | ||
| Human-derived Clinical Sample | Shanghai Jiao Tong University affiliated Sixth People’ s Hospital; Shanghai East Hospital | N/A |
|
Chemicals, peptides, and recombinant proteins | ||
| 3-Bromopyruvic Acid (3-BrPA) | Alfa Aesar | Cat# L00720 |
| Sodium Oxamate | Sigma-Aldrich | Cat# O2751 |
| Rotenone | Sigma-Aldrich | Cat# R8875 |
| Oligomycin A | MedChemExpress | Cat# HY-16589 |
| CCCP | MedChemExpress | Cat# HY-100941 |
| Antimycin A | Sigma-Aldrich | Cat# A8674 |
| AZD-3965 | TargetMol | Cat# T3210 |
| AR-C155858 | MedChemExpress | Cat# HY-13248 |
| SB-204990 | MedChemExpress | Cat# HY-16450 |
| BMS-303141 | APExBIO | Cat# B1079 |
| Rapamycin | Shanghai YEASEN | Cat# 52404ES60 |
| Octyl-(R)-2HG | Sigma-Aldrich | Cat# SML2200 |
| Octyl-(S)-2HG | J&W Pharmlab | Cat# 05R0850 |
| Ionomycin | Aladdin | Cat# I139530 |
| Compound 968 | MedChemExpress | Cat# HY-12682 |
| CB-839 | MedChemExpress | Cat# HY-12248 |
| β-Nicotinamide Mononucleotide (NMN) | Shanghai YEASEN | Cat# 60303ES80 |
| Nicotinamide Riboside (NR) | Shanghai Yuanye Bio-technology | Cat# S24896 |
| Nicotinamide (NAM) | Sigma-Aldrich | Cat# N3376 |
| Nicotinic Acid (NA) | Sigma-Aldrich | Cat# N4126 |
| FK866 Hydrochloride Hydrate | Sigma-Aldrich | Cat# F8557 |
| STF-118804 | Selleck | Cat# S7316 |
| Coenzyme Q10 (CoQ) | Sigma-Aldrich | Cat# C9538 |
| MitoQ10 Mesylate (MitoQ) | MedChemExpress | Cat# HY-100116A |
| Troglitazone | MedChemExpress | Cat# HY-50935 |
| Metformin | Ourchem | Cat# XW11157041 |
| Hieff Trans Liposomal Transfection Reagent | Shanghai YEASEN | Cat# 40802ES03 |
| DMEM Media (High Glucose) | HyClone | Cat# SH30243.01 |
| RPMI Medium Modified (1640) | HyClone | Cat# SH30809.01B |
| PBS Powder | Absin | Cat# abs9266 |
| Opti-MEM | Gibco | Cat# 31985-070 |
| Fetal Bovine Serum (FBS) | Biological Industries | Cat# 04-001-1ACS |
| Trypsin-EDTA | Gibco | Cat# 25200-072 |
| Human Fibronectin | Shanghai YEASEN | Cat# 40105ES08 |
| Streptozotocin | Sigma-Aldrich | Cat# S0130 |
| Insulin | Sigma-Aldrich | Cat# I9278 |
| Catalase | Sigma-Aldrich | Cat# C3155 |
| Sodium Pentobarbital | Sigma-Aldrich | Cat# P3761 |
| Sodium L-lactate | Sigma-Aldrich | Cat# 71718 |
| Polybrene | Shanghai YEASEN | Cat# 40804ES76 |
| Puromycin | Shanghai YEASEN | Cat# 60210ES25 |
| NAD+ | Shanghai YEASEN | Cat# 60323ES08 |
| NADH | Shanghai YEASEN | Cat# 60301ES03 |
| NADP+ | Shanghai YEASEN | Cat# 60324ES03 |
| NADPH | Shanghai YEASEN | Cat# 60302ES01 |
| ADP | Shanghai YEASEN | Cat# 60604ES03 |
| ATP | Sigma-Aldrich | Cat# A26209 |
| D-(+)-Glucose | Macklin | Cat# D810594 |
| Phosphoenolpyruvic Acid | Alfa Aesar | Cat# B20358 |
| Sodium Pyruvate | Aladdin | Cat# S104174 |
| Oxaloacetic Acid | Sigma-Aldrich | Cat# O4126 |
| Sodium Citrate | Aladdin | Cat# S189183 |
| Isocitric Acid | Sigma-Aldrich | Cat# I1252 |
| α-Ketoglutaric Acid | Sigma-Aldrich | Cat# 75892 |
| Succinic Acid | Aladdin | Cat# S108855 |
| Fumaric Acid | Aladdin | Cat# F118459 |
| L-Malic Acid | Sigma-Aldrich | Cat# 112577 |
| β-Hydroxybutyrate | MedChemExpress | Cat# HY-113378 |
| Sodium Acetate | Titan | Cat# G18900B |
| Glycine | Titan | Cat# G65953B |
| L-Isoleucine | Macklin | Cat# L811667 |
| L-Threonine | Macklin | Cat# T6272 |
| L-Glutamine | Macklin | Cat# L810391 |
| L-Alanine | Macklin | Cat# L800640 |
| L-Aspartic Acid | Macklin | Cat# A6202 |
| L-Histidine | Macklin | Cat# 811075 |
| L-Tryptophan | Macklin | Cat# L818799 |
| L-Glutamic Acid | Macklin | Cat# L810368 |
| L-Methionine | Macklin | Cat# L812760 |
| L-Serine | Macklin | Cat# S6199 |
| L-Proline | Macklin | Cat# L816039 |
| L-Lysine | Macklin | Cat# L812314 |
| L-Asparagine | Macklin | Cat# L800456 |
| L-Leucine | Macklin | Cat# L6238 |
| L-Phenylalanine | Macklin | Cat# L816180 |
| L-Arginine | Macklin | Cat# A6286 |
| L-Valine | Macklin | Cat# L820396 |
| L-Tyrosine | Macklin | Cat# T6229 |
| L-Cystenine | Macklin | Cat# L804954 |
| Dithionitrobenzoic acid (DNTB) | Alfa Aesar | Cat# A14331 |
| Acetyl-CoA | Shanghai Yuanye Bio-technology | Cat# S10019 |
| Sucrose | Energy Chemical | Cat# D100088 |
| D-Mannitol | Sigma-Aldrich | Cat# M4125 |
| EGTA | Sigma-Aldrich | Cat# E3889 |
| EDTA | GENERAL-REAGENT | Cat# G68915B |
| Resazurin | Macklin | Cat# R817239 |
| Diaphorase | Sigma-Aldrich | Cat# D5540 |
| L-Lactate Dehydrogenase | Sigma-Aldrich | Cat# L2625 |
| Digitonin | Calbiochem | Cat# 300410 |
| Low Melting Point Agarose | Shanghai YEASEN | Cat# 10214ES08 |
| MitoTracker Red | Thermo Scientific | Cat# M22425 |
| JC-10 | Shanghai YEASEN | Cat# 40707ES03 |
| 2×Hieff™ PCR Master Mix | Shanghai YEASEN | Cat# 10102ES08 |
| PrimeSTAR® Max DNA Polymerase | Takara | Cat# R045B |
| 2×Pfu PCR MasterMix | TIANGEN | Cat# KP201-02 |
| T4 Polynucleotide Kinase | Thermo Scientific | Cat# EK0032 |
| T4 DNA Ligase | Thermo Scientific | Cat# EL0012 |
| FastDigest EcoRI | Thermo Scientific | Cat# FD0274 |
| FastDigest NotI | Thermo Scientific | Cat# FD0594 |
| Super ECL Detection Reagent | Shanghai YEASEN | Cat# 36208ES60 |
| Lenti-Pac Lentivirus Concentration Solution | GeneCopoeia | Cat# LT007 |
| Protease/Phosphatase Inhibitor Cocktail | Bimake | Cat# B14001 |
|
Critical commercial assays | ||
| Cell Mitochondria Isolation Kit | Beyotime Biotechnology | Cat# C3601 |
| BCA Protein Quantification Kit | Shanghai YEASEN | Cat# 20201ES76 |
| Hieff Clone Plus One Step Cloning Kit | Shanghai YEASEN | Cat# 10911ES20 |
| PicoProbeTM Lactate Fluorometric Assay Kit | BioVision | Cat# K638 |
| Angilent Seahorse XFe96 Extracellular Flux Assay Kit | Agilent | Cat# 102418-00013 |
|
Experimental models: cell lines | ||
| HEK293T | Cell Bank of Chinese Academy of Science | GNHu17 |
| HEK293 | Cell Bank of Chinese Academy of Science | GNHu43 |
| NCI-H1299 | Cell Bank of Chinese Academy of Science | TCHu160 |
| HeLa | Cell Bank of Chinese Academy of Science | TCHu187 |
|
Experimental models: organisms/strains | ||
| C57BL/6J Mice | East China Normal University | N/A |
| Type I Diabetic Mice (T1DM) | East China Normal University | N/A |
| The db/db Mice (T2DM) | East China Normal University | N/A |
|
Oligonucleotides | ||
| Primer sequences for cloning or sequencing | This paper | See Table S1 |
|
Recombinant DNA | ||
| pAAV-CMV-MCS-FiLa | This paper | N/A |
| pAAV-CMV-MCS-FiLa-C | This paper | N/A |
| pcDNA3.1-Nuc-FiLa | This paper | N/A |
| pcDNA3.1-Nuc-FiLa-C | This paper | N/A |
| pcDNA3.1-Mit-FiLa | This paper | N/A |
| pcDNA3.1-Mit-FiLa-C | This paper | N/A |
| pLVX-FiLa | This paper | N/A |
| pLVX-FiLa-C | This paper | N/A |
| pLVX-Nuc-FiLa | This paper | N/A |
| pLVX-Nuc-FiLa-C | This paper | N/A |
| pLVX-Mit-FiLa | This paper | N/A |
| pLVX-Mit-FiLa-C | This paper | N/A |
| pLVX-NES-FiLa | This paper | N/A |
| pLVX-NES-FiLa-C | This paper | N/A |
| pLVX-Nuc-SoNar | Zhao et al., 2015 | N/A |
| pLVX-Nuc-iNapc | Tao et al., 2017 | N/A |
| pLVX-NES-SoNar | Zhao et al., 2015 | N/A |
| pLVX-NES-iNapc | Tao et al., 2017 | N/A |
| pLVX-Mit-Frex | Zhao et al., 2011 | N/A |
| pLVX-Mit-cpYFP | This paper | N/A |
| pLVX-Mit-TagRFP | This paper | N/A |
| pCDFDuet-FiLa | This paper | N/A |
| pCDFDuet-FiLa-H | This paper | N/A |
| pCDFDuet-FiLa-C | This paper | N/A |
| pCDFDuet-LOX | This paper | N/A |
| psPAX2 | Addgene | Cat# 12260 |
| pMD2.G | Addgene | Cat# 12259 |
|
Software and algorithms | ||
| ImageJ 1.50d | Fiji | http://fiji.sc/ |
| GraphPad Prism V8.0.2.263 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| Gen5 3.03 | Biotek | https://www.biotek.com/products/software-robotics/ |
| Leica Application Suite X 1.8.1.13759 | Leica | https://www.leica-microsystems.com.cn/cn/products/microscope-software/p/leica-application-suite/ |
| SnapGene 2.0.0 | GSL Biotech | https://download.cnet.com/s/snapgene/ |
| CytExpert2.3 | Beckman Coulter | https://www.mybeckman.cn/flow-cytometry/research-flow-cytometers/cytoflex/software |
| Wave Desktop | Agilent | https://www.agilent.com.cn/zh-cn/products/cell-analysis/software-download-for-wave-desktop |
|
Other | ||
| 35 mm 4-chamber Glass Bottom Dish | Cellvis | D35C4-20-1-N |
| 8 Chambered Coverglass System | Cellvis | C8-1.5H-N |
Highlights.
The lactate sensor FiLa is capable of tracking subtle lactate fluctuations
FiLa indicates that lactate is highly enriched in mammalian mitochondria
FiLa assays report lactate to be a responsive hub for various metabolic activities
FiLa enables ultrasensitive and rapid quantitation of lactate in patient samples
ACKNOWLEDGMENTS
We thank Stephanie C. Tribuna for secretarial assistance. This research is supported by National Key Research and Development Program of China (2019YFA0904800 to Y. Zhao and 2021YFA0804900 to A.W.), NSFC (32121005, 32150030, 32030065, and 92049304 to Y.Zhao; 91857202, 21937004, and 32150028 to Y.Y.; 91749203 and 82030039 to Z.J.; 82070913 to C.W.; 81770883 to Y. Zhang; 31900833 to X.L.; 32000920 to A.W.; 31901033 to T.L.; and 32201230 to Y. Zou), the Shanghai Science and Technology Commission (19YF1411400 to A.W. and 19YF1411300 to T.L.), the Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism (Y. Zhao), the Research Unit of New Techniques for Live-cell Metabolic Imaging (Chinese Academy of Medical Sciences, 2019-I2M-5-013 to Y. Zhao), the innovative research team of high-level local universities in Shanghai, the State Key Laboratory of Bioreactor Engineering, the Fundamental Research Funds for the Central Universities, US National Institutes of Health (HL119145, HL155107, HL155096, and HG007690 to J.L.), and the American Heart Association (D700382 and AHA2020CV-19 to J.L.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.cmet.2022.10.002.
DECLARATION OF INTERESTS
Y.Y., Y. Zhao, X.L., Zeyi Zhang, X.Z., and L.H. have filed a related patent by East China University of Science and Technology.
REFERENCES
- Aguilera L, Campos E, Giménez R, Badía J, Aguilar J, and Baldoma L (2008). Dual role of LldR in regulation of the lldPRD operon, involved in L-lactate metabolism in Escherichia coli. J. Bacteriol 190, 2997–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar EC, Baudrier L, Özerdem C, Lewis CA, Chan SH, Kunchok T, Abu-Remaileh M, Cangelosi AL, Sabatini DM, Birsoy K, and Chen WW (2019). MITO-Tag Mice enable rapid isolation and multimodal profiling of mitochondria from specific cell types in vivo. Proc. Natl. Acad. Sci. USA 116, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekdash R, Quejada JR, Ueno S, Kawano F, Morikawa K, Klein AD, Matsumoto K, Lee TC, Nakanishi K, Chalan A, et al. (2021). GEM-IL: a highly responsive fluorescent lactate indicator. Cell Rep. Methods 1, 100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J, Hung YP, and Yellen G (2009). A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat. Methods 6, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilan DS, Matlashov ME, Gorokhovatsky AY, Schultz C, Enikolopov G, and Belousov VV (2014). Genetically encoded fluorescent indicator for imaging NAD(+)/NADH ratio changes in different cellular compartments. Biochim. Biophys. Acta 1840, 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussouar F, and Benahmed M (2004). Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab 15, 345–350. [DOI] [PubMed] [Google Scholar]
- Brooks GA (2018). The science and translation of lactate shuttle theory. Cell Metab 27, 757–785. [DOI] [PubMed] [Google Scholar]
- Brouwers MC, Ham JC, Wisse E, Misra S, Landewe S, Rosenthal M, Patel D, Oliver N, Bilo HJ, and Murphy E (2015). Elevated lactate levels in patients with poorly regulated type 1 diabetes and glycogenic hepatopathy: a new feature of Mauriac syndrome. Diabetes Care 38. e11–e12. [DOI] [PubMed] [Google Scholar]
- Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, and Goodman RH (2016). Biosensor reveals multiple sources for mitochondrial NAD(+). Science 352, 1474–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron WD, Bui CV, Hutchinson A, Loppnau P, Gräslund S, and Rocheleau JV (2016). Apollo-NADP(+): a spectrally tunable family of genetically encoded sensors for NADP(+). Nat. Methods 13, 352–358. [DOI] [PubMed] [Google Scholar]
- Cantó C, Menzies KJ, and Auwerx J (2015). NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22, 31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WW, Freinkman E, Wang T, Birsoy K, and Sabatini DM (2016). Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166. 1324.e11–1337.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DL (1978). Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14, 141–148. [DOI] [PubMed] [Google Scholar]
- Daw CC, Ramachandran K, Enslow BT, Maity S, Bursic B, Novello MJ, Rubannelsonkumar CS, Mashal AH, Ravichandran J, Bakewell TM, et al. (2020). Lactate elicits ER-mitochondrial Mg(2+) dynamics to inte-grate cellular metabolism. Cell 183. 474.e17–489.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JR, and Cleveland JL (2013). Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest 123, 3685–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al. (2017). Lactate metabolism in human lung tumors. Cell 171. 358.e9–371.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Li H, Tai Y, Huang J, Su Y, Abramowitz J, Zhu MX, Birnbaumer L, and Wang Y (2013). Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA 110, 11011–11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glancy B, Kane DA, Kavazis AN, Goodwin ML, Willis WT, and Gladden LB (2021). Mitochondrial lactate metabolism: history and implications for exercise and disease. J. Physiol 599, 863–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Chihara T, Hayasaka Y, Mita M, Takizawa M, Ishida K, Arai M, Tsuno S, Matsumoto M, Ishihara T, et al. (2020). Green fluorescent protein-based lactate and pyruvate indicators suitable for biochemical assays and live cell imaging. Sci. Rep 10, 19562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang Guo J, et al. (2017). Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YP, Albeck JG, Tantama M, and Yellen G (2011). Imaging cytosolic NADH-NAD(+) redox state with a genetically encoded fluorescent biosensor. Cell Metab 14, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatsenko I, Boquete JP, and Lemaitre B (2018). Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase Nox and shortens drosophila lifespan. Immunity 49. 929.e5–942.e5. [DOI] [PubMed] [Google Scholar]
- Ibraheem A, and Campbell RE (2010). Designs and applications of fluorescent protein-based biosensors. Curr. Opin. Chem. Biol 14, 30–36. [DOI] [PubMed] [Google Scholar]
- Juraschek SP, Shantha GP, Chu AY, Miller ER 3rd, Guallar E, Hoogeveen RC, Ballantyne CM, Brancati FL, Schmidt MI, Pankow JS, and Young JH (2013). Lactate and risk of incident diabetes in a case-cohort of the atherosclerosis risk in communities (ARIC) study. PLoS One 8, e55113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, and Jung UJ (2019). Honokiol improves insulin resistance, hepatic steatosis, and inflammation in type 2 diabetic db/db mice. Int. J. Mol. Sci 20, 2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koveal D, Rosen PC, Meyer DJ, Díaz-García CM, Wang Y, Cai LH, Chou PJ, Weitz DA, and Yellen G (2022). A high-throughput multiparameter screen for accelerated development and optimization of soluble genetically encoded fluorescent biosensors. Nat. Commun 13, 2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, and Nair KS (2009). Functional assessment of isolated mitochondria in vitro. Methods Enzymol 457, 349–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Sohn HA, Park ZY, Oh S, Kang YK, Lee KM, Kang M, Jang YJ, Yang SJ, Hong YK, et al. (2015). A lactate-induced response to hypoxia. Cell 161, 595–609. [DOI] [PubMed] [Google Scholar]
- Lee WD, Mukha D, Aizenshtein E, and Shlomi T (2019). Spatial-fluxomics provides a subcellular-compartmentalized view of reductive glutamine metabolism in cancer cells. Nat. Commun 10, 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobas MA, Tao R, Nagai J, Kronschläger MT, Borden PM, Marvin JS, Looger LL, and Khakh BS (2019). A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat. Commun 10, 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NP, Min JE, Anh NH, Kim SJ, Park S, Kim HM, Yoon SJ, Lim J, Lee SJ, and Kwon SW (2020). Isolation and metabolic assessment of cancer cell mitochondria. ACS Omega 5, 27304–27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longchamps RJ, Castellani CA, Yang SY, Newcomb CE, Sumpter JA, Lane J, Grove ML, Guallar E, Pankratz N, Taylor KD, et al. (2020). Evaluation of mitochondrial DNA copy number estimation techniques. PLoS One 15. e0228166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, and Allaman I (2018). Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci 19, 235–249. [DOI] [PubMed] [Google Scholar]
- Marin E, Bouchet-Delbos L, Renoult O, Louvet C, Nerriere-Daguin V, Managh AJ, Even A, Giraud M, Vu Manh TP, Aguesse A, et al. (2019). Human tolerogenic dendritic cells regulate immune responses through lactate synthesis. Cell Metab 30 1075.e8–1090.e8. [DOI] [PubMed] [Google Scholar]
- Marunouchi T, Abe Y, Murata M, Inomata S, Sanbe A, Takagi N, and Tanonaka K (2013). Changes in small heat shock proteins HSPB1, HSPB5 and HSPB8 in mitochondria of the failing heart following myocardial infarction in rats. Biol. Pharm. Bull 36, 529–539. [DOI] [PubMed] [Google Scholar]
- Murphy R, Turnbull DM, Walker M, and Hattersley AT (2008). Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet. Med 25, 383–399. [DOI] [PubMed] [Google Scholar]
- Naing A, Kenchaiah M, Krishnan B, Mir F, Charnley A, Egan C, and Bano G (2014). Maternally inherited diabetes and deafness (MIDD): diagnosis and management. J. Diabetes Complications 28, 542–546. [DOI] [PubMed] [Google Scholar]
- Nasu Y, Murphy-Royal C, Wen Y, Haidey JN, Molina RS, Aggarwal A, Zhang S, Kamijo Y, Paquet ME, Podgorski K, et al. (2021). A genetically encoded fluorescent biosensor for extracellular L-lactate. Nat. Commun 12, 7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginuma M, Moncuquet P, Xiong F, Karoly E, Chal J, Guevorkian K, and Pourquié O (2017). A gradient of glycolytic activity coordinates FGF and Wnt signaling during elongation of the body axis in amniote embryos. Dev. Cell 40. 342.e10–353.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AE, Qin Y, Park JG, and McCombs JE (2011). Design and application of genetically encoded biosensors. Trends Biotechnol 29, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MC, and Shulman GI (2018). Mechanisms of insulin action and insulin resistance. Physiol. Rev 98, 2133–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchowicz MA, Varnes ME, Cohen BH, Friedman NR, Kerr DS, and Hoppel CL (2004). Oxidative phosphorylation analysis: assessing the integrated functional activity of human skeletal muscle mitochondria–case studies. Mitochondrion 4, 377–385. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JD, and Enerbäck S (2020). Lactate: the ugly duckling of energy metabolism. Nat. Metab 2, 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martín A, Ceballo S, Ruminot I, Lerchundi R, Frommer WB, and Barros LF (2013). A genetically encoded FRET lactate sensor and its use to detect the Warburg effect in single cancer cells. PLoS One 8, e57712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, and Alberini CM (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylow L, Tokarz VL, Richter EA, and Klip A (2021). The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab 33, 758–780. [DOI] [PubMed] [Google Scholar]
- Tantama M, Hung YP, and Yellen G (2011). Imaging intracellular pH in live cells with a genetically encoded red fluorescent protein sensor. J. Am. Chem. Soc 133, 10034–10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Zhao Y, Chu H, Wang A, Zhu J, Chen X, Zou Y, Shi M, Liu R, Su N, et al. (2017). Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat. Methods 14, 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velentzas PD, Zhang L, Das G, Chang TK, Nelson C, Kobertz WR, and Baehrecke EH (2018). The proton-coupled monocarboxylate transporter Hermes is necessary for autophagy during cell death. Dev. Cell 47. 281.e4–293.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, Menk AV, Rittenhouse NL, DePeaux K, Whetstone RD, et al. (2021). Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Wang L, Chen T, Zhou K, Zhang Z, Li J, Sun B, Guo Y, Wang X, Wang Y, et al. (2021). A metabolite array technology for precision medicine. Anal. Chem 93, 5709–5717. [DOI] [PubMed] [Google Scholar]
- Xu K, Yin N, Peng M, Stamatiades EG, Shyu A, Li P, Zhang X, Do MH, Wang Z, Capistrano KJ, et al. (2021). Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science 371, 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Yang L, Mou L, Dong K, Jiang J, Xue S, Xu Y, Wang X, Lu Y, and Ye H (2019). A green tea-triggered genetic control system for treating diabetes in mice and monkeys. Sci. Transl. Med 11, eaav8826. [DOI] [PubMed] [Google Scholar]
- Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al. (2019a). Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Campbell RE, Ting AY, and Tsien RY (2002). Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol 3, 906–918. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang G, Xu ZG, Tu H, Hu F, Dai J, Chang Y, Chen Y, Lu Y, Zeng H, et al. (2019b). Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 178. 176.e15–189.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cheng X, Zhao Y, and Yang Y (2020). Lighting up live-cell and in vivo central carbon metabolism with genetically encoded fluorescent sensors. Annu. Rev. Anal. Chem. (Palo Alto. Calif) 13, 293–314. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hu Q, Cheng F, Su N, Wang A, Zou Y, Hu H, Chen X, Zhou HM, Huang X, et al. (2015). SoNar, a highly responsive NAD+/NADH sensor, allows High-throughput metabolic screening of anti-tumor agents. Cell Metab 21, 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Jin J, Hu Q, Zhou HM, Yi J, Yu Z, Xu L, Wang X, Yang Y, and Loscalzo J (2011). Genetically encoded fluorescent sensors for intracellular NADH detection. Cell Metab 14, 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang A, Zou Y, Su N, Loscalzo J, and Yang Y (2016). In vivo monitoring of cellular energy metabolism using SoNar, a highly responsive sensor for NAD(+)/NADH redox state. Nat. Protoc 11, 1345–1359. [DOI] [PubMed] [Google Scholar]
- Zhao Y, and Yang Y (2015). Profiling metabolic states with genetically encoded fluorescent biosensors for NADH. Curr. Opin. Biotechnol 31, 86–92. [DOI] [PubMed] [Google Scholar]
- Zhao Y, and Yang Y (2016). Real-time and high-throughput analysis of mitochondrial metabolic states in living cells using genetically encoded NAD+/ NADH sensors. Free Radic. Biol. Med 100, 43–52. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang Z, Zou Y, and Yang Y (2018). Visualization of nicotine adenine dinucleotide redox homeostasis with genetically encoded fluorescent sensors. Antioxid. Redox Signal 28, 213–229. [DOI] [PubMed] [Google Scholar]
- Zou Y, Wang A, Huang L, Zhu X, Hu Q, Zhang Y, Chen X, Li F, Wang Q, Wang H, et al. (2020). Illuminating NAD(+) metabolism in live cells and in vivo using a genetically encoded fluorescent sensor. Dev. Cell 53. 240.e7–252.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Wang A, Shi M, Chen X, Liu R, Li T, Zhang C, Zhang Z, Zhu L, Ju Z, et al. (2018). Analysis of redox landscapes and dynamics in living cells and in vivo using genetically encoded fluorescent sensors. Nat. Protoc 13, 2362–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the article and its supplemental information files. Unprocessed data underlying the display items in the manuscript, related to Figures 1, 2, 3,4, 5, and S1–S5, are provided in Data S1.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


